Sjögren’s Syndrome (SS) is a chronic autoimmune disorder characterized by focal mononuclear cell infiltrates that surround the ducts of the exocrine glands, impairing the function of their secretory units. Compared to other autoimmune disorders, SS is associated with a notably high incidence of non-Hodgkin lymphoma (NHL) and more frequently mucosa associated lymphoid tissue (MALT) lymphoma, leading to increased morbidity and mortality rates. High risk features of lymphoma development include systemic extraepithelial manifestations, low serum levels of complement component C4 and mixed type II cryoglobulinemia. The discrimination between reactive and neoplastic lymphoepithelial lesion (LEL) is challenging, probably reflecting a continuum in the evolution from purely inflammatory lymphoid infiltration to the clonal neoplastic evolution. Early lesions display a predominance of activated T cells, while B cells prevail in severe histologic lesions. This strong B cell infiltration is not only a morphologic phenomenon, but it is also progressively associated with the presence of ectopic germinal centers (GCs).
Thanks so much for your check. We sincerely hope you may create this entry, since you are the expert in this academic research. You can click the “submit” button to upload it after revision. We will help you layout after you submit it. Moreover, we will link your article at the entry, and more scholars and students can look through it.
1. Introduction
Sjögren’s Syndrome (SS) is benign autoimmune disease characterized by organ specific as well as systemic manifestations. Of interest this benign autoimmune disease can potentially lead to lymphomagenesis. A major complication in the progression of SS is lymphoma development, leading to increased mortality of SS patients that develop lymphoma in the course of their disease [
1,
2].
B cells displaying a marginal zone (MZ) phenotype are detected in the majority of lymphomas developing in the setting of SS. Mucosa associated lymphoid tissue (MALT) lymphomas account for 65% of SS related lymphomas, followed by diffuse large B cell lymphomas (DLBCL) and nodal MZ lymphomas [
3,
4]. Of note, most cases of DLBCL are probably the evolution of low-grade MZ lymphomas [
5,
6]. Since MALT lymphomas account for the majority of SS associated lymphomas, the pathophysiologic mechanisms described in this review mainly refer to this type of lymphoma.
Compared to patients with other autoimmune diseases, namely systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), patients with primary SS (pSS) present a higher risk for lymphoma development [
7]. Several clinical and laboratory parameters have been proposed to distinguish patients at this high risk [
3,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24].
SS is characterized by lymphocytic infiltration of the exocrine glands. Lymphoepithelial sialadenitis (LESA), a notable histological feature of SS, is characterized by the presence of lymphoid populations surrounding and infiltrating the salivary ducts, along with disorganization and proliferation of the ductal epithelial cells [
25]. The spectrum of histopathologic features of LESA ranges from a fully benign lymphoid infiltrate, sometimes associated with lymphoid follicular structures, that does not display immunoglobulin (Ig) light chain restriction in B-cells, to lymphoproliferative lesions with presence of centrocyte-like cells with or without areas of Ig light chain restriction in B cells [
26]. B-cell clones are found in approximately 50% of LESAs without morphological or clinical evidence of lymphoma [
26,
27]. Monoclonal B cell expansion does not necessarily constitute lymphoma. The evolution from persistent polyclonal B cell activation to monoclonal B cell expansion and thereafter lymphoma development is a multistep process.
2. Summarized Model of Lymphomagenesis in SS
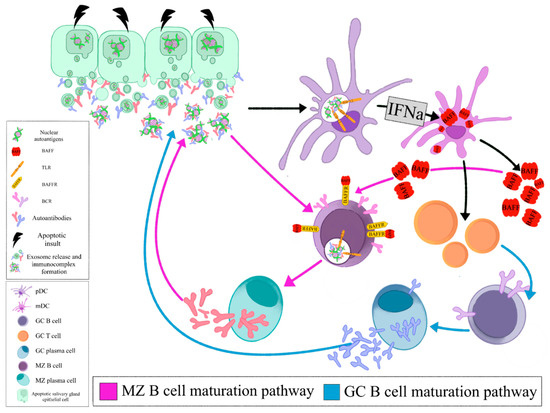
The local auto- antigen expression by the epithelium of the LEL in SGs of SS patients drives the emergence of autoreactive B-cell clones bearing an RF reacting BCR. Opsonized epithelial apoptotic particles and ICs containing nuclear autoantigens bind to plasmacytoid DCs (pDCs) and RF B cells via TLRs and Fc receptors, respectively. TLR activation in pDCs leads to IFNa release that subsequently stimulates myeloid DCs (mDCs) to produce BAFF. The same apoptotic particles and ICs also bind to RF B cells via TLR and BCR, respectively. TLR activation in B cells results in enhanced BCR mediated signaling and upregulation of BAFF-R. BAFF, secreted by mDCs, further upregulates TLR expression, favors B cell survival, promotes immunoglobulin class-switching and plasma cell differentiation (, Z B cell maturation pathway). The activated mDCs also act as antigen presenting cells to T cells that help B cell responses (, GC B cell maturation pathway). Therefore, the combined signals of BCR, TLR, BAFF and IFNa drive the propagated production of autoantibodies and lead to expansion of RF B cells () [
139].
Figure 1. Opsonized epithelial apoptotic particles and ICs containing nuclear autoantigens bind to plasmacytoid DCs (pDCs) and RF B cells via TLRs and Fc receptors, respectively. TLR activation in pDCs leads to IFNa release that subsequently stimulates myeloid DCs (mDCs) to produce BAFF. The same apoptotic particles and ICs also bind to RF B cells via TLR and BCR, respectively. TLR activation in B cells results in enhanced BCR mediated signaling and upregulation of BAFF-R. BAFF, secreted by mDCs, further upregulates TLR expression, favors B cell survival, promotes immunoglobulin class- switching and plasma cell differentiation (MZ B cell maturation pathway). The activated mDCs also act as antigen presenting cells to T cells that help B cell responses (GC B cell maturation pathway). (pDC: plasmacytoid dendritic cell, mDC: myeloid dendritic cell, GC: germinal center, MZ: marginal zone, BAFF: B cell activating factor, TLR: Toll like receptor, BAFFR: BAFF receptor, BCR: B cell receptor, IFNa: interferon a).
Selection of these RF-expressing B cells offers them a proliferative advantage, leading to their malignant transformation and eventually MALT lymphoma development. Autoreactive MZ B cells have been shown to be negatively selected in healthy individuals. RF-expressing, autoreactive MZ like B cells in SS bypass the peripheral checkpoint against autoreactivity and proceed to proliferation and differentiation through T cell independent pathways [
140].
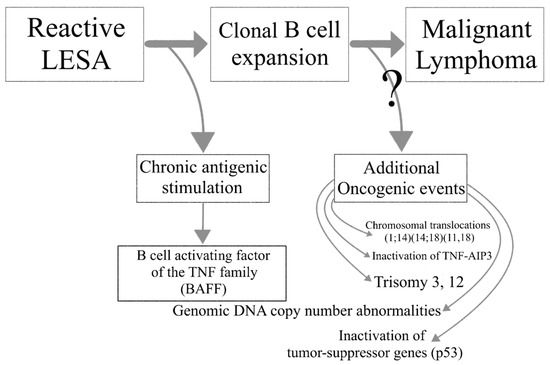
Lymphoma development in the setting of SS is associated with increased overall disease mortality. A multistep process leads to the transition of reactive LESA to lymphomagenesis. Chronic antigenic stimulation leads to abnormal B cell activation in the SG glands of SS patients and the emergence of autoreactive B cell clones. Loss of immune control, ectopic GC formation and oncogenic events further drive the malignant transformation to lymphoma ().
Figure 2. The transition from lymphoepithelial sialadenitis to malignant lymphoma.
This entry is adapted from the peer-reviewed paper 10.3390/jcm9123794