Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The use of naturally derived drugs in anti-cancer therapies has grown exponentially in recent years. Among natural compounds, polyphenols have shown potential therapeutic applications in treatment due to their protective functions in plants, their use as food additives, and their excellent antioxidant properties, resulting in beneficial effects on human health. Building more efficient cancer therapies with fewer side effects on human health can be achieved by combining natural compounds with conventional drugs, which are typically more aggressive than natural chemicals with polyphenols.
- natural health products
- polyphenols
- flavonoids
- oncology
1. Introduction
Cancer is a group of diseases that involve the unusual growth of malignant cells with the potential to invade or metastasize to other parts of the body. Lifestyle has a big influence on the causes of cancer and may lead to habits that are fundamental to the development of lifestyle diseases. In addition, pollution, exposure to dangerous chemicals, radiation, stress, smoking, and alcohol consumption can lead to the development of cancer [1]. However, the initiation and development of cancer is not limited to lifestyle causes, but can result from changes in the human genome.
Over the past few years, preclinical and clinical cancer research has identified various collections of developmentally important genes that remain relatively quiescent in normal tissues [2].
Under normal circumstances, the body’s immune system can identify and eliminate cancer cells; however, cancer cells have an “immune escape” mechanism that allows them to evade recognition and attack by the immune system in various ways, allowing them to multiply in the body and prevent elimination [3].
Research to date has made tremendous progress in the prevention, detection, and treatment of cancer, leading to a decline in mortality rates. The use of conventional cancer treatment procedures, such as chemotherapy and radiation therapy, often causes harmful side effects. Therefore, the current goals of cancer research are related to the development of new therapies that are less harmful to the human body. Natural compounds can be very useful in this respect [4][5].
Natural compounds derived from plants or phytochemicals have been used in traditional medicine for centuries. Phytochemicals are chemical compounds that are produced by plants; they are usually involved in the growth of plants or in the process of protecting them against predators or pathogens [6][7]. Currently, the use of phytochemicals, especially polyphenols, as alternative anticancer drugs is a promising alternative to conventional therapies [5][8]. In addition, the human body develops resistance to the conventional drugs that are involved in cancer therapy [9].
2. Polyphenols
Polyphenols are secondary metabolites that are produced by plants, and they are characterized by the presence of numerous phenolic rings [10]. The main sources of polyphenols are blueberries, grapes, olive oil, cocoa, nuts, peanuts, and other fruits and vegetables that contain up to 200–300 mg of polyphenols per 100 g fresh weight [11]. Figure 1 shows the classification of the main groups of polyphenols.
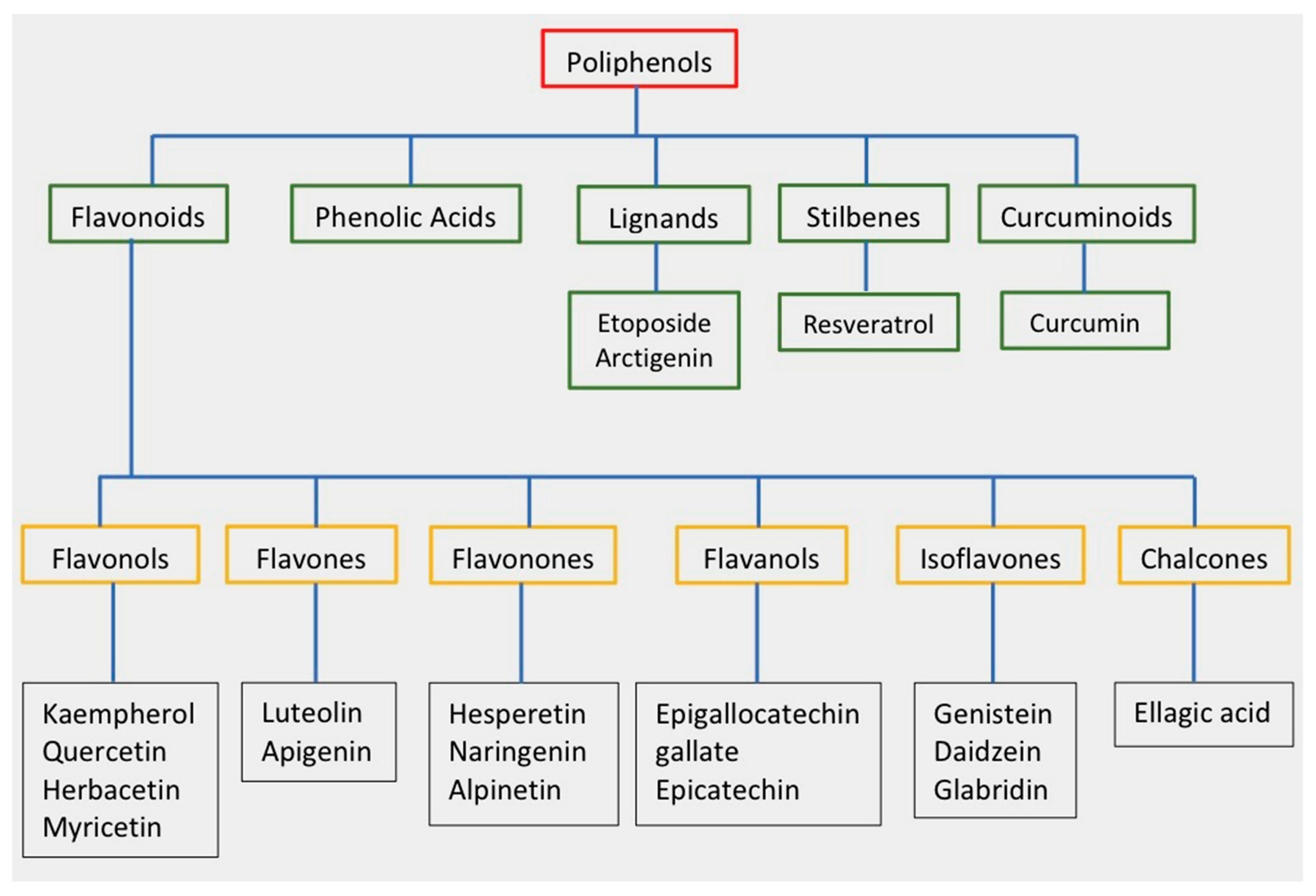
Figure 1. Classification of polyphenols and flavonoids. Examples of each subgroup with antitumor activity are listed.
Polyphenols can be extracted by simple and ecological techniques, including ultrasonically assisted extraction. T After extraction, polyphenols retain most of their properties. This characteristic facilitates research into the use of these compounds as potential anticancer drugs [12][13].
2.1. Flavonoids
The most important group of polyphenols the family. Flavonoids consist of over 6000 molecules that have been identified and isolated. Flavonoids are found in abundance in colorful vegetables and fruits such as blueberries, apples, grapes, oranges, strawberries, plums, and in some common foods and drinks, including dark chocolate, nuts, red wine, tea, soybeans and soybean derivatives, spinach [14].
2.2. Phenolic Acids
Another subgroup of polyphenols that can be found in several plants, especially in dried fruit, is phenolic acids. These compounds are characterized by the presence of a phenolic ring and have the function of an organic carboxylic acid [15].
Phenolic acid (p-coumaric acid) has shown medicinal properties that make it a likely candidate for the treatment of cancer.
P-coumaric acid (or 4-hydroxycinnamic acid) is an organic compound that is derived from cinnamic acid, which can be found in many different edible plants (tomatoes, carrots, garlic, mushrooms, white beans, and other plants). Moreover, p-coumaric acid that is contained in pollen is a component of honey [16].
In the last decade, several studies have been published that confirm the antitumor activity of p-coumaric acid in breast and gastric cancer cells [17][18][19].
2.3. Lignans
Lignans are diphenolic compounds found in a wide variety of plants, including broccoli, beans, soybeans, rye, sesame seeds, pumpkin seeds, linseed, and some berries in very small amounts [20]. Lignans are one of the two main groups of phytoestrogens that are known for their own good antioxidant properties [21]. Numerous lignans can be considered possible anticancer drugs. Among them are etoposide, arctigenin, and magnolol, the main lignans that are studied in medicine. In addition, etoposide is a commercial lignan, belonging to the podophyllotoxin subfamily, that is used to treat various types of cancer such as lung cancer and breast cancer [22][23]. However, etoposide chemotherapy has several side effects, including low blood cell counts, vomiting, diarrhea, fever, loss of appetite, and alopecia.
Arctigenin Some plants produce arctigenin, especially in the seeds of the greater burdock (Arctium lappa). Current studies have shown that arctigenin inhibits the growth of various cancer cells, including cells in the stomach, lung, liver, and colon, as well as leukocytes [24]. At the same time, the addition of arctigenin enhances the action of caspase-3, a protein that plays a key role in the death of cancer cells. Huang et al. showed that the treatment of OVCAR3 and SKOV3 ovarian cancers with arctygenin causes apoptosis of neoplastic cells in vitro [25].
Lee et al. investigated the effect of arctigenin (ATG) on doxorubicin-induced cell death (DOX) using human breast cancer cells MDA-MB-231. The results showed that DOX-induced cell death was enhanced by concurrent treatment with ATG/DOX in a concentration-dependent manner and that this was associated with increased DOX uptake and the suppression of multidrug-resistance-associated protein 1 (MRP1) gene expression in MDA-MB cells-231 [26].
2.4. Resveratrol
Resveratrol is a natural polyphenol from the stilbene family. Resveratrol is produced by several plants (grapes, almonds, beans, blueberries, raspberries, mulberries, peanuts, etc.) in response to infections and injuries, or as a defense against various attacks by pathogens such as fungi and bacteria [27]. In addition, red wine contains significant amounts of resveratrol.
In 1997, Jang et al. were the first investigators to report the inhibition of skin cancer development in mice with resveratrol [28]. Since then, many studies have suggested that resveratrol is able to prevent or delay the onset of cancer [29][30][31].
In fact, studies have shown that resveratrol is active in vitro against many human cancers, including cancers of the breast, skin, ovary, stomach, prostate, colon, liver, pancreas, and cervix, as well as thyroid cancer cells, lymphoid carcinoma cells, and myeloid carcinoma cells [32].
Resveratrol has been shown to be beneficial at various stages of neoplastic disease (tumor initiation, promotion, and progression). For example, resveratrol protects DNA against reactive oxygen species (ROS) and traps the hydroxyls, superoxides, and free radicals produced in cells (events that are usually associated with tumor initiation) [33].
In addition, human clinical trials with resveratrol have been conducted with satisfactory results [34][35][36].
2.5. Curcuminoids
Curcuminoids are natural polyphenols that contain two phenolic units linked by a linear diarylheptanoid. Among them, curcumin is one of the best known and researched structures, with high potential as a drug. Nevertheless, the poor solubility of curcumin in water of acidic and physiological pH requires a variety of alternatives to avoid losing the efficacy of curcumin as a drug [37].
Curcumin has been used in anticancer therapies for various types of cancer: lung, cervical, prostate, breast, bone, and liver [38]. Nevertheless, the administration of free curcumin has some drawbacks, including poor water solubility, instability under water conditions, and low bioavailability [39].
Many different clinical trials have been conducted on the use of curcumin as an anticancer drug. Recently, various research groups have reported that the combination of curcumin with gemcitabine-based chemotherapy is safe and that its use is possible in pancreatic cancer patients [40][41][42].
Overall, gemcitabine adjuvant therapy with curcumin phytosome complex is not only safe, but it also effectively translates into a good first-line response rate for advanced pancreatic cancer.
Table 1 shows the most important studies on the molecular mechanisms of anticancer activity of the main polyphenols and flavonoids.
Table 1. Mechanisms of anticancer activity of selected polyphenols and flavonoids.
| Flavonoids | Cancer Model | Mechanisms | Ref |
|---|---|---|---|
| Apigenin | Hepatocellular carcinoma doxorubicin-resistant cell BEL-7402/ADM Nude mice |
Sensitizes drug-resistant cells to doxorubic through suppressing miR-520b/ATG7 axis. | [43] |
| Breast cancer T47D, MDA-MB-231 |
Induction of protective autophagy and apoptosis. | [44] | |
| Colorectal cancer HCT116 |
Autophagy inhibitor significantly enhanced the apoptosis. | [45] | |
| Hepatocellular carcinoma Hepg2 |
Increases levels of Caspase-3, PARP cleavage, and Bax/Bcl-2 ratios. | [46] | |
| Non-small cell lung cancer EGFR-TKIs-resistant NCI-H1975 (Apigenin + Gefitinib) |
Inhibits the AMPK pathway and autophagy flux, leading to enhanced apoptotic cell death. Inhibits multiple oncogenic drivers such as c-Myc, HIF-1α, and EGFR, and reduces Gluts and MCT1 protein expression. Downregulates Cyclin D1, CDK4, E-cadherin, MMP2, and MMP9, and induces G0/G1 cell cycle arrest and cell metastasis. |
[47] | |
| Colorectal cancer cisplatin-resistant cell HT-29 |
Induces autophagic cell death and inhibits the growth of cells by targeting the m-TOR/PI3K/AKT signaling pathway. Autophagy inhibits the occurrence of MDR. |
[48] | |
| Breast cancer (MDA-MB-468), prostate cancer (PC3), | The investigated compounds cause intracellular copper mobilization and ROS production, resulting in cancer cell death. | [49] | |
| Baicalein | Prostate cancer PC-3, DU145 Breast cancer MDA-MB-231 |
Activation of AMPK and ULK1 and downregulation of mRNA level of mTOR/Raptor induces autophagic cell death. Upregulates the expression of Beclin1, Atg5, Atg7, ULK1, and LC3B-II. Induction of autophagic cell death. |
[50] |
| Breast cancer MCF-7, MDA-MB-231 |
Induces apoptosis and autophagy by inhibiting the PI3K/AKT pathway. | [51] | |
| Non-small cell lung cancer A549, H1299 |
Induces the loss of mitochondrial membrane potential and the release of cyto-c and apoptosis inducing factor into the cytoplasm. Induces autophagy and activates autophagy flux. |
[52] | |
| Human glioblastoma U87 and U251 cell lines |
Maturation of microtubule-associated protein 1A/1B-LC3B indicated the activation of autophagy potentially through the PI3K/Akt/mTOR pathway, and inhibition of autophagy by 3-methyladenine decreased the apoptotic cell ratio. | [53] | |
| Quercetin | Glioblastoma multiforme T98G (quercetin + temozolomide) Anaplastic astrocytoma MOGGCCM (quercetin + temozolomide) |
Activates ER stress, increases the level of caspase 12 expression, and changes the shape of nuclei. Inhibition of HSP expression results in severe apoptosis and no obvious signs of autophagy, which decreases mitochondrial membrane potential, and increases level of cyto-c in the cytoplasm and the activation of caspase 3 and caspase 9. |
[54] |
| Glioblastoma U251, U87 |
T-AUCB induces overexpression of Atg7 and regulates autophagy-related gene expression. | [55] | |
| Glioblastoma multiforme T98G (quercetin + sorafenib) |
In T98G cells, sorafenib mainly initiated autophagy, resulting in an increased number of autophagic cells with quercetin. | [56] | |
| Glioblastoma U373MG |
Activates JNK signal, increases the expression and translocation of p53 to the mitochondria, and causes the release of cyto-c into the cytoplasm. | [57] | |
| Melanoma (B16-F10) | Inhibits Akt/PI3 K and MEK-ERK signaling while augmenting UVB-induced nuclear translocation of NF-κb. | [58] | |
| Galangin | Laryngeal carcinoma TU212, HEP-2 |
Modulates apoptosis through caspase-3, caspase-9, and PARP cleavage activation and bcl-2 downregulation. Regulates apoptosis and autophagy by p38 and AKT/NF-κB/mTOR pathways. |
[59] |
| Epigallocatechin gallate |
Non-small cell lung cancer A549 (gefitinib-resistant cell)/ | Inhibits autophagy induced by gefitinib and promotes cell death. | [60] |
| Colorectal cancer HCT-116 |
The combined effect of epigallocatechin Gallate and quercetin caused cell cycle arrest at the G1 phase. | [61] | |
| Chalcone | Breast cancer Epirubicin-resistant cell MCF-7/ADR |
Induction of autophagy and G2/M checkpoint block and downregulation of ABCG2 expression, but no induction of apoptosis. Induces autophagic cell death through inhibition of miR-25 and upregulation of ULK1 expression. |
[62] |
| Breast cancer MCF-7 cells | Licochalcone A inhibits PI3K/Akt/mTOR activation and promotes autophagy and apoptosis in MCF-7 cells | [63] | |
| Malignant melanoma | Cell-cycle arrest at the G2/M phase was associated with modulation of expression or phosphorylation of specific cell cycle-associated proteins (cyclin B1, p21, and ChK1) and tubulins. | [64] | |
| Human uterine sarcoma | Induces A375 cells to differentiate and lose their pluripotency by inhibiting the expression of Notch1, β-catenin, and Oct-3/4 and targeting members of the key signals PI3K/Akt and MEK-ERK pathways. | [65] | |
| Ovarian cancer OVCAR5 and ES-2 | Isoliquiritigenin induced G2/M phase arrest. Furthermore, the expression of cleaved PARP, cleaved caspase-3, Bax/Bcl-2 ratio, LC3B-II, and Beclin-1 levels were increased in Western blot analysis. | [66] | |
| Human breast cancer | Cell-cycle arrest at G2/M phase and induced apoptosis and autophagy in human breast cancer cells. Interruption of the PI3K/AKT/mTOR/p70S6K/ULK signaling pathway. | [67] |
This entry is adapted from the peer-reviewed paper 10.3390/molecules28104080
References
- Willett, W.C. World Cancer Report 2014; Stewart, B.W., Wild, C.P., Eds.; International Agency for Research on Cancer: Lyon, France, 2014.
- Manzo, G. Similarities Between Embryo Development and Cancer Process Suggest New Strategies for Research and Therapy of Tumors: A New Point of View. Front. Cell Dev. Biol. 2019, 7, 20.
- Zheng, J.; Zhou, X.; Fu, Y.; Chen, Q. Advances in the Study of Hyperprogression of Different Tumors Treated with PD-1/PD-L1 Antibody and the Mechanisms of Its Occurrence. Cancers 2023, 15, 1314.
- Smyth, M.J.; Hayakawa, Y.; Takeda, K.; Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer 2002, 2, 850–861.
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Pawliszak, W.; Giamberini, M.; Mocek-Płóciniak, A.; Tylkowski, B. Current Perspectives of the Applications of Polyphenols and Flavonoids in Cancer Therapy. Molecules 2020, 25, 3342.
- Surh, Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780.
- Zeng, X.; Li, H.; Jiang, W.; Li, Q.; Xi, Y.; Wang, X.; Li, J. Phytochemical compositions, health-promoting properties and food applica-tions of crabapples: A review. Food Chem. 2022, 386, 132789.
- Usman, M.; Khan, W.R.; Yousaf, N.; Akram, S.; Murtaza, G.; Kudus, K.A.; Ditta, A.; Rosli, Z.; Rajpar, M.N.; Nazre, M. Exploring the Phy-tochemicals and Anti-Cancer Potential of the Members of Fabaceae Family: A Comprehensive Review. Molecules 2022, 27, 3863.
- Mitra, T.; Bhattacharya, R. Phytochemicals modulate cancer aggressiveness: A review depicting the anticancer efficacy of dietary polyphenols and their combinations. J. Cell. Physiol. 2020, 235, 7696–7708.
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181.
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621.
- Montané, X.; Bajek, A.; Roszkowski, K.; Montornés, J.M.; Giamberini, M.; Roszkowski, S.; Kowalczyk, O.; Garcia-Valls, R.; Tylkowski, B. Encapsulation for Cancer Therapy. Molecules 2020, 25, 1605.
- Ammendola, M.; Haponska, M.; Balik, K.; Modrakowska, P.; Matulewicz, K.; Kazmierski, L.; Lis, A.; Kozlowska, J.; Garcia-Valls, R.; Giamberini, M.; et al. Stability and anti-proliferative properties of biologically active compounds extracted from Cistus L. after sterilization treatments. Sci. Rep. 2020, 10, 6521.
- Williams, C.A.; Grayer, R.J. Anthocyanins and Other Flavonoids. Nat. Prod. Rep. 2004, 21, 539–573.
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513.
- Stojkovic, D.; Petrovic, J.; Sokovic, M.; Glamoclija, J.; Kukic-Markovic, J.; Petrovic, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208.
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Karthikkumar, V.; Wang, M.-H. Smart drug delivery of p-Coumaric acid loaded aptamer conjugated starch nanoparticles for effective triple-negative breast cancer therapy. Int. J. Biol. Macromol. 2022, 195, 22–29.
- Sun, Q.; Yuan, M.; Wang, H.; Zhang, X.; Zhang, R.; Wang, H.; Chen, X.; Zhu, M.; Liu, S.; Wu, J. PKM2 Is the Target of a Multi-Herb-Combined Decoction During the Inhibition of Gastric Cancer Progression. Front. Oncol. 2021, 11, 767116.
- Jang, M.G.; Ko, H.C.; Kim, S.-J. Effects of p-coumaric acid on microRNA expression profiles in SNU-16 human gastric cancer cells. Genes Genom. 2020, 42, 817–825.
- Crosby, G.A. Lignans in food and nutrition. Food. Technol. 2005, 59, 32–36.
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, 14264.
- Voutsadakis, I.A. A Systematic Review and Pooled Analysis of Studies of Oral Etoposide in Metastatic Breast Cancer. Eur. J. Breast Health 2018, 14, 10–16.
- Jiang, S.; Huang, L.; Zhen, H.; Jin, P.; Wang, J.; Hu, Z. Carboplatin versus cisplatin in combination with etoposide in the first-line treatment of small cell lung cancer: A pooled analysis. BMC Cancer 2021, 21, 1308.
- He, Y.; Fan, Q.; Cai, T.; Huang, W.; Xie, X.; Wen, Y.; Shi, Z. Molecular mechanisms of the action of Arctigenin in cancer. Biomed. Pharmacother. 2018, 108, 403–407.
- Huang, K.; Li, L.A.; Meng, Y.G.; You, Y.Q.; Fu, X.Y.; Song, L. Arctigenin Promotes Apoptosis in Ovarian Cancer Cells via the iN-OS/NO/STAT3/Survivin Signalling. Basic Clin. Pharmacol. Toxicol. 2014, 115, 507–511.
- Lee, K.-S.; Lee, M.-G.; Kwon, Y.-S.; Nam, K.-S. Arctigenin Enhances the Cytotoxic Effect of Doxorubicin in MDA-MB-231 Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 2997.
- Fremont, L. Minireview-Biological effects of resveratrol. Life Sci. 2000, 66, 663–673.
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.W.; Fong, H.H.S.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer Chemopreventive Activity of Resveratrol, a Natural Product Derived from Grapes. Science 1997, 275, 218–220.
- Singh, C.K.; Ndiaye, M.A.; Ahmad, N. Resveratrol and cancer: Challenges for clinical translation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1178–1185.
- Yang, R.; Dong, H.; Jia, S.; Yang, Z. Resveratrol as a modulatory of apoptosis and autophagy in cancer therapy. Clin. Transl. Oncol. 2022, 24, 1219–1230.
- Robertson, I.; Hau, T.W.; Sami, F.; Ali, S.; Badgujar, V.; Murtuja, S.; Hasnain, S.; Khan, A.; Majeed, S.; Ansari, M.T. The science of resveratrol, formulation, pharmacokinetic barriers and its chemotherapeutic potential. Int. J. Pharm. 2022, 618, 121605.
- Berretta, M.; Bignucolo, A.; Di Francia, R.; Comello, F.; Facchini, G.; Ceccarelli, M.; Iaffaioli, R.V.; Quagliariello, V.; Maurea, N. Resveratrol in Cancer Patients: From Bench to Bedside. Int. J. Mol. Sci. 2020, 21, 2945.
- Leonard, S.S.; Xia, C.; Jiang, B.-H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026.
- Popat, R.; Plesner, T.; Davies, F.; Cook, G.; Cook, M.; Elliott, P.; Jacobson, E.; Gumbleton, T.; Oakervee, H.; Cavenagh, J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2013, 160, 714–717.
- Kjaer, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Jørgensen, J.O.L.; Hougaard, D.M.; Cohen, A.S.; Neghabat, S.; Richelsen, B.; Pedersen, S.B. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate 2015, 75, 1255–1263.
- Howells, L.M.; Berry, D.P.; Elliot, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I Ran-domized, Double-Blind Pilot Study of Micronized Resveratrol (SRT501) in Patients with Hepatic Metastases-Safety, Pharmacokinetics, and Pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425.
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854.
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M.C. Curcumin and Health. Molecules 2016, 21, 264.
- Panahi, Y.; Darvishi, B.; Ghanei, M.; Jowzi, N.; Beiraghdar, F.; Varnamkhasti, B.S. Molecular mechanisms of curcumins suppressing effects on tumorigenesis, angio-genesis and metastasis, focusing on NF-κB pathway. Cytokine Growth Factor Rev. 2016, 28, 21–29.
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and Gemcitabine in Patients with Advanced Pancreatic Cancer. Nutr. Cancer 2010, 62, 1137–1141.
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164.
- Pastorelli, D.; Fabricio, A.S.; Giovanis, P.; D’Ippolito, S.; Fiduccia, P.; Soldà, C.; Buda, A.; Sperti, C.; Bardini, R.; Da Dalt, G.; et al. Phytosome complex of curcumin as complementary therapy of advanced pan-creatic cancer improves safety and efficacy of gemcitabine: Results of a prospective phase II trial. Pharmacol. Res. 2018, 132, 72–79.
- Lee, Y.; Sung, B.; Kang, Y.J.; Kim, D.H.; Jang, J.-Y.; Hwang, S.Y.; Kim, M.; Lim, H.S.; Yoon, J.-H.; Chung, H.Y.; et al. Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int. J. Oncol. 2014, 44, 1599–1606.
- Yang, J.; Pi, C.; Wang, G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed. Pharmacother. 2018, 103, 699–707.
- Chen, X.; Xu, H.; Yu, X.; Wang, X.; Zhu, X.; Xu, X. Apigenin inhibits in vitro and in vivo tumorigenesis in cisplatin-resistant colon cancer cells by inducing autophagy, programmed cell death and targeting m-TOR/PI3K/Akt signalling pathway. J. BUON 2019, 24, 488–493.
- Chen, Z.; Tian, D.; Liao, X.; Zhang, Y.; Xiao, J.; Chen, W.; Liu, Q.; Chen, Y.; Li, D.; Zhu, L.; et al. Apigenin Combined with Gefitinib Blocks Autophagy Flux and Induces Apoptotic Cell Death Through Inhibition of HIF-1α, c-Myc, p-EGFR, and Glucose Metabolism in EGFR L858R+T790M-Mutated H1975 Cells. Front. Pharmacol. 2019, 10, 260.
- Yi, S.; Liu, G.; Wu, Y.; Liang, Q.; Li, L. Baicalein suppresses the growth of the human thyroid cancer cells by inducing mitotic ca-tastrophe, apoptosis and autophagy via NF-kB signaling pathway. J. BUON 2020, 25, 389–394.
- Lv, S.-X.; Qiao, X. Isovitexin (IV) induces apoptosis and autophagy in liver cancer cells through endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 2018, 496, 1047–1054.
- Khan, H.Y.; Zubair, H.; Faisal, M.; Ullah, M.F.; Farhan, M.; Sarkar, F.H.; Ahmad, A.; Hadi, S.M. Plant polyphenol induced cell death in human cancer cells involves mobilization of intracellular copper ions and reactive oxygen species generation: A mechanism for cancer chemopreventive action. Mol. Nutr. Food Res. 2013, 58, 437–446.
- Yan, W.; Ma, X.; Zhao, X.; Zhang, S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des. Dev. Ther. 2018, 12, 3961–3972.
- Han, Z.; He, J.; Zou, M.; Chen, W.; Lv, Y.; Li, Y. Small Interfering RNA Target for Long Non-coding RNA PCGEM1 Increases Sensi-tivity of LNCaP Cells to Baicalein. Anat. Rec. 2020, 303, 2077–2085.
- Su, G.; Chen, H.; Sun, X. Baicalein suppresses non-small cell lung cancer cell proliferation, invasion and Notch signaling pathway. Cancer Biomark. 2018, 22, 13–18.
- Zhu, Y.; Fang, J.; Wang, H.; Fei, M.; Tang, T.; Liu, K.; Niu, W.; Zhou, Y. Baicalin suppresses proliferation, migration, and invasion in human glioblastoma cells via Ca-dependent pathway. Drug Des. Dev. Ther. 2018, 12, 3247–3261.
- Jakubowicz-Gil, J.; Langner, E.; Badziul, D.; Wertel, I.; Rzeski, W. Quercetin and sorafenib as a novel and effective couple in programmed cell death induction in human gliomas. Neurotox. Res. 2014, 26, 64–77.
- Liu, Y.; Gong, W.; Yang, Z.Y.; Zhou, X.S.; Gong, C.; Zhang, T.R.; Wei, X.; Ma, D.; Ye, F.; Gao, Q.L. Quercetin induces protective autophagy and apoptosis through ER stress via the p-STAT3/Bcl-2 axis in ovarian cancer. Apoptosis 2017, 22, 544–557.
- Granato, M.; Rizzello, C.; Gilardini Montani, M.S.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J. Nutr. Biochem. 2017, 41, 124–136.
- Wen, M.; Wu, J.; Luo, H.; Zhang, H. Galangin Induces Autophagy through Upregulation of p53 in HepG2 Cells. Pharmacology 2012, 89, 247–255.
- Rafiq, R.A.; Quadri, A.; Nazir, L.A.; Peerzada, K.; Ganai, B.A.; Tasduq, S.A. A Potent Inhibitor of Phosphoinositide 3-Kinase (PI3K) and Mitogen Activated Protein (MAP) Kinase Signalling, Quercetin (3, 3’, 4’, 5, 7-Pentahydroxyflavone) Promotes Cell Death in Ultraviolet (UV)-B-Irradiated B16F10 Melanoma Cells. PLoS ONE 2015, 10, e0131253.
- Wang, H.X.; Tang, C. Galangin suppresses human laryngeal carcinoma via modulation of caspase-3 and AKT signaling path-ways. Oncol Rep. 2017, 38, 703–714.
- Meng, J.; Chang, C.; Chen, Y.; Bi, F.; Ji, C.; Liu, W. EGCG overcomes gefitinib resistance by inhibiting autophagy and augmenting cell death through targeting ERK phosphorylation in NSCLC. OncoTargets Ther. 2019, 12, 6033–6043.
- Al-Ghamdi, M.A.; Al-Enazy, A.; Huwait, E.; Albukhari, A.; Harakeh, S.; Moselhy, S.S. Enhancement of Annexin V in response to combination of epigallocatechin gallate and quercetin as a potent arrest the cell cycle of colorectal cancer. Braz. J. Biol. 2023, 83, e248746.
- Wang, Z.; Wang, N.; Liu, P.; Chen, Q.; Situ, H.; Xie, T.; Chen, J. MicroRNA-25 regulates chemoresistance associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget 2014, 5, 7013–7026.
- Xue, L.; Zhang, W.J.; Fan, Q.X.; Wang, L.X. Licochalcone A inhibits PI3K/Akt/mTOR signaling pathway activation and promotes autophagy in breast cancer cells. Oncol. Lett. 2018, 15, 1869–1873.
- Gazdova, M.; Michalkova, R.; Kello, M.; Vilkova, M.; Kudlickova, Z.; Baloghova, J.; Mirossay, L.; Mojzis, J. Chalcone-Acridine Hybrid Suppresses Melanoma Cell Progression via G2/M Cell Cycle Arrest, DNA Damage, Apoptosis, and Modulation of MAP Kinases Activity. Int. J. Mol. Sci. 2022, 23, 12266.
- Lin, L.-C.; Wu, C.-H.; Shieh, T.-M.; Chen, H.-Y.; Huang, T.-C.; Hsia, S.-M. The licorice dietary component isoliquiritigenin chemosensi-tizers human uterine sarcoma cells to doxorubicin and inhibits cell growth by inducing apoptosis and autophagy via inhibition of m-TOR signaling. J. Funct. Foods 2017, 33, 332–344.
- Chen, H.-Y.; Huang, T.-C.; Shieh, T.-M.; Wu, C.-H.; Lin, L.-C.; Hsia, S.-M. Isoliquiritigenin Induces Autophagy and Inhibits Ovarian Cancer Cell Growth. Int. J. Mol. Sci. 2017, 18, 2025.
- Zhang, H.-W.; Hu, J.-J.; Fu, R.-Q.; Liu, X.; Zhang, Y.-H.; Li, J.; Liu, L.; Li, Y.-N.; Deng, Q.; Luo, Q.-S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 11255.
This entry is offline, you can click here to edit this entry!
