1. Introduction
Eimeria is a protozoan parasite genus that belongs to the phylum Apicomplexa. Most of the microorganisms in this phylum are zoonotic or cause an economic impact on livestock animals. Parasites of the genus
Eimeria are closely related to other coccidian genera such as
Cyclospora,
Toxoplasma,
Neospora,
Hammondia,
Cystoisospora, and
Sarcocystis. They are more distant to
Cryptosporidium and
Plasmodium [
1].
Chicken coccidiosis is caused by seven species of
Eimeria. It is a highly widespread enteric disease, causing economic losses to the poultry industry worldwide. In 2016, the global impact was estimated at approximately €12 billion [
2]. There are extensive studies focused on economic impact and epidemiology in the intensive poultry industry; however, little is known about small-scale productions (i.e., family household or backyard poultry). Household poultry production provides a valuable source of income in small communities and is recognised as an important contribution to food security and to decreasing rural poverty. According to the Food and Agriculture Organization (FAO) of the United Nations and a few reports from different part of the world [
3,
4,
5], rural women are mainly dedicated to chicken husbandry and exposed to greater risks, but have little participation in decision-making and access to resources. However, when they market their productions, this activity constitutes an empowering tool for African women in rural communities [
6,
7]. Reports of coccidiosis in backyard productions stated a prevalence of
Eimeria spp. ranging from 25.8 to 85.7% [
8,
9,
10,
11,
12,
13].
Species that affect chickens are
E. acervulina,
E. brunetti,
E. maxima,
E. mitis,
E. necatrix,
E. praecox, and
E. tenella. Depending on the parasite species involved, infective doses, chicken immune status, and the breed line of the chickens, infections can vary from low to severe forms. Chicken
Eimeria spp. that cause haemorrhagic disease are
E. brunetti,
E. necatrix, and
E. tenella, and species that cause malabsorption are
E. acervulina,
E. maxima,
E. mitis, and
E. praecox. Broiler diets are formulated in accordance with the digestible and ideal amino acids (AA) concepts. There is an optimal ratio of AA to provide the exact balance needed for best performance and growth [
14,
15]. However, this ratio might change during a broiler’s lifespan due to age or physiological state [
15]. Malabsorptive species alter the ideal AA profile due to biochemical and physiological changes in the intestinal epithelium, which impact the productive parameters (feed conversion, weight gain, or egg-laying). On the other hand, haemorrhagic species produce destruction of the intestinal mucosa, causing haemorrhages and severe diarrhoea that can lead to the death of the animal [
2]. The most frequent species are
E. acervulina,
E. maxima, and
E. tenella [
12,
13,
16] but there may be differences between the type of production and geographical areas [
13,
17]. Infections with mixed species are common, but single infections have also been reported [
12]. In addition to the seven chicken species, new cryptic species known as operational taxonomic units OTUx, OTUz, and OTUy have been recently described. They appeared to be geographically restricted, but new studies show a greater spatial distribution [
17,
18,
19]. These OTUs have recently shown sufficient genetic and biological diversity to be considered new and distinct species and were named
E. lata,
E. nagambie, and
E. zaria, respectively [
20]. It is important to highlight that these new species could limit the effectiveness of current live vaccines.
Chicken coccidiosis is controlled by good husbandry measures, chemoprophylaxis, and/or live vaccination. The application of anticoccidial drugs usually added to food or drinking water has been used for more than sixty years and has led to the appearance of resistant strains for all
Eimeria spp. over the world [
21,
22,
23,
24]. In addition, their use is under pressure worldwide, given the social influence in favour of the consumption of food free of chemicals, such as that produced in agroecological systems. Therefore, chemoprophylaxis faces new global regulations that limit or even ban its use. Live wild-type or attenuated vaccines have been effective in controlling the disease. However, wild-type vaccines can have safety issues and are not licensed for use in Europe, and attenuated vaccines possess a low reproductive index, increasing production costs, and limiting production capacity to meet worldwide demand [
25]. To acquire full protection, chickens have to be vaccinated against each of the seven species of the parasite and in some cases with multiple strains, since they only confer specific immunity to homologous strains, making the manufacturing process highly demanding and stringent [
26]. Therefore, the drawbacks inherent to live vaccination, and the emergence of resistant strains to traditional anticoccidials have raised concerns. The One Health paradigm has an integrated vision of health, considering animals, humans, and the environment. Antimicrobial resistance, food safety and security, and environmental contamination, among others, are issues concerning One Health; therefore, developing new cost-effective control strategies is crucial. This is especially relevant for small-scale poultry producers who have limited access to live vaccination given their high costs. Although the first live vaccines against chicken coccidiosis were developed in the 1950s [
27], only two non-live vaccines have reached the market, these are Coxabic
® (Abic, Israel) [
28] and Vac COX
® (Vetanco S.A) [
29], this last one only commercially available in Argentina. One main barrier in protozoal vaccinology relates to the complex life cycles [
30]. Furthermore, coccidiosis is caused by multiple species infections, and developing a universal vaccine that protects against heterologous species is not a simple goal to achieve. Effective vaccines against chicken coccidiosis may confer protection against multiple species, which differ according to the different types of flocks. Therefore, new multivalent designs should include protective proteins from—at least—the most frequent and pathogenic species to confer cross-protection. Numerous studies have demonstrated various levels of protection against vaccination with recombinant antigens, DNA, or live-vectored vaccines against coccidiosis. These results open the way to the pursuit of new prophylactic alternatives, for which the search for new candidates is paramount.
Surface proteins are of particular interest for studies on pathogen-host interactions as they are exposed on the outer membranes of parasites and establish interaction with host cells prior to invasion. They are exposed to the immune systems and could, therefore, be potential targets for developing protective immunity [
31]. The rapid expansion of bioinformatics, genomics, transcriptomics, and immunoproteomics conducted in
Eimeria spp. has increased the knowledge of protein function, fillings gaps in fundamental processes such as invasion and improving the first genomic annotations for these species [
32]. All Apicomplexa contain in their genomes genes encoding for proteins localised on the surface of invasive stages, playing a key role in attachment, invasion, and elicitation of host immune responses. Antigens from the asexual reproduction stage, i.e., sporozoites, first generation schizonts and merozoites, have been related to immunoprotection against secondary infections [
33,
34]. In contrast, very few antigens from the sexual stages have been studied and be included in vaccine trials. From these stages, only GAM56 and GAM82, which have an important role in oocyst wall biogenesis and are components of the Coxabic
® vaccine, have been evaluated as vaccine candidates [
35].
2. Life Cycle of Eimeria spp.
The
Eimeria life cycle has been reviewed before [
36]. Nevertheless, a schematic representation is shown in
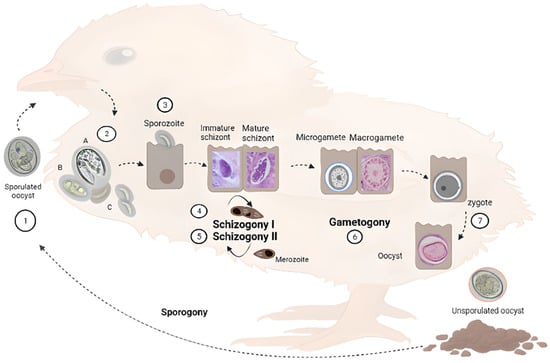
Figure 1 to introduce readers to the different parasitic stages that are mentioned. Different
Eimeria species have different intestinal predilection sites and pathogenicity. This obligated intracellular parasite develops in the intestine of a single animal species, e.g., chicken (monoxenous), and comprises an asexual and a sexual cycle referred to as schizogony and gametogony, respectively. In addition, the parasite encompasses an exogenous (environmental) phase, necessary to become infective, termed sporogony. Oocysts are excreted and spread in the environment in a non-sporulated (non-infective) form. Sporogony initiates in suitable conditions of oxygen, temperature and humidity. Four sporocysts develop inside, each containing two sporozoites.
Figure 1. Schematic representation of Eimeria spp. life cycle in chickens. Parasites are transmitted among chickens via the faecal-oral route through water or food contaminated with sporulated oocysts (1). Upon ingestion, sporozoites are released in the gastrointestinal tract from sporocysts contained in the oocysts (2). Sporozoites invade intestinal epithelial cells—in different locations depending on the species—(3) and develop into the parasitophorous vacuole (PV) where they undergo schizogony (4), forming trophozoites that become immature and mature schizonts. After two to four rounds of schizogony (5), merozoites invade new cells undergoing gametogony (6). Zygote forms after fertilization of macrogametes by microgametes, which then develop into unsporulated oocysts (7) that are excreted with faces. In the environment, they undergo sporogony, restarting the life cycle. A, B and C are micrographs of freshly hatched parasites, i.e., oocysts, sporocysts and sporozoites, respectively, under a magnification of 400×. Immature and mature schizonts, macrogametes, and oocysts are micrographs of histological sections of intestines of experimentally infected birds stained with haematoxylin and eosin at 1000× magnification.
Sporulated oocysts disseminate in the environment, contaminating food and/or water, allowing coccidiosis transition by the faecal-oral route. When infective oocysts are ingested by chickens, sporozoites (Figure 1, step 2) are released in the gastrointestinal tract in a process called excystation, invading the intestinal host cells.
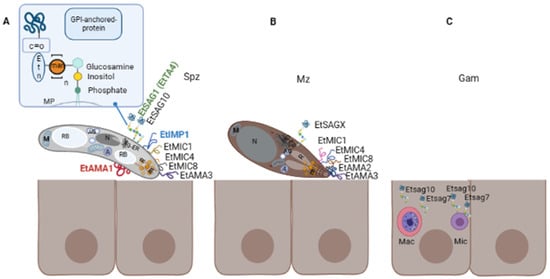
Sporozoites possess classical eukaryotic organelles such as the nucleus, mitochondrion, endoplasmic reticulum (ER), and Golgi apparatus; they also contain typical structures of the phylum, i.e., conoid, apicoplast, micronemes, rhoptries, and refractile bodies (the last are specific of the genus) [
37] (
Figure 2A). The sporozoite cell surface is structured in a triple-layered pellicle, composed by the plasma membrane and the inner membrane complex, beneath, an array of sub-pellicular microtubules is found. In apicomplexan parasites, the host cell invasion process can be divided into attachment, apical re-orientation, formation of moving-junction structure (MJ), active invagination, and formation of the parasitophorous vacuole (PV) [
37,
38]. After invasion, intracellular sporozoites initiate schizogony, by growing inside the PV, forming trophozoites that later become multinucleated schizonts. Merozoites share characteristics of the sporozoites such as the triple-layered pellicle, nucleus, amylopectin granules, ER, Golgi, and apical complex, but they do not contain refractile bodies. Additionally, rod-shaped mitochondria and a vacuole are present in new merozoites [
37].
Figure 2. Surface proteins in three different life stages of E. tenella (Et). (A) Extracellular sporozoite possesses an apical complex composed of structural elements, rhoptries (R) and micronemes (m) that are involved in the invasion process, which comprises attachment, apical reorientation, formation of moving-junction (MJ) and formation of the PV. Eukaryotic organelles are depicted (nucleus (N), Golgi apparatus (G), endoplasmic reticulum (ER) mitochondria (M)), as well as organelles exclusive of the genus and the phylum or genus (refractile bodies (RB), apicoplast (A) and amylopectin granules (AG)). Studied surface proteins involved in these steps are EtSAG1 (TA4), EtIMP1, EtMIC4, EtMIC8, EtAMA1, EtAMA3, and EtSAG10. Proteins identified in other chicken species are indicated in different colours: green SAG1 identified in E. acervulina and E. necatrix; blue IMP1 identified in E. maxima and red AMA1 identified in E. brunetti and E. maxima. A representative scheme of the GPI anchor of SAG proteins is amplified in the upper right corner, this is composed of a molecule of glucosamine, n-mannoses (man), and an ethanolamine phosphate (etn). (B) Extracellular merozoite also possesses an apical complex. Identified surface proteins at this stage, including the first and second-generation merozoites, are EtSAG 2- 23 (referred to as EtSAGX), EtMIC1, EtMIC4, and EtMIC8 and EtAMA2. (C) Intracellular gametocytes. Transcriptomic analysis has identified two sag genes, Etsag7 and Etsag10, which are upregulated at this sexual stage in E. tenella, they are represented in macrogametocytes (Mac) and microgametocytes (Mic); however, identification of the protein has not been reported.
The life cycle progresses with the formation of gametocytes that could become male and female gametes (micro and macrogametes) in a process termed gametogony. The diploid zygote is formed by the fertilization of haploid macrogametes by microgametes, which develops into an unsporulated oocyst. These oocysts are excreted with faeces and become infective in the environment, producing the consequent reinfection of chickens. The duration of the complete life cycle is variable, depending on the specific strains, usually lasting from five to seven days. Oocysts can persist in the environment for long periods, given their high resistance structure [
36,
39].
3. Surface Proteins of Eimeria spp.
Proteins located at the parasite surface are one of the first-acting proteins that are exposed to the host immune system, they recognise the host cell ligands and interact with them prior to invasion. In consequence, they could be interesting targets for immunoprophylaxis. In apicomplexan parasites, most of them are anchored to the parasite surface by a GPI anchor [
40]. Importantly, GPIs of related parasites mediate strong immunomodulatory effects on the host immune system [
41]. For example, recognition of GPI in malaria parasites by host Toll-like receptors 2 and/or 4, mediates a strong pro-inflammatory cytokine response from host cells in vitro [
42]. Activation of the same receptors by
Toxoplasma gondii GPIs seems to be critical to elicit an innate immune response [
43]. Additionally, increasing evidence also suggests a role of GPI-proteins in host immune evasion in related parasites [
44].
In the
Eimeria genus, one of the most important and studied surfaces antigens are known as surface antigens anchored to the membrane by GPI (SAG), these are present in invasive stages (sporozoite and merozoite), interacting with the host before the invasion, and they are usually differentially expressed in different stages of the life cycle [
45]. According to Reid et al., 2014 [
32] there are 559
sag genes and 357 pseudogenes in the seven chicken species;
sag genes are aggregated in three subfamilies:
sagA,
sagB and
sagC. Whereas the former is present in all chicken species,
sagB is only found in
E. tenella and
E. necatrix, with
sagC restricted to the other five species. The three subfamilies possess a signal peptide at the N-terminal and a sequence for the GPI anchor at the C-terminal of the protein and an extracellular domain with six conserved cysteines, except for
sagC which contains four instead [
32]. They play an important role in the early recognition of the host, adhesion, and invasion as well as immune modulation [
46,
47].
In addition to SAG proteins, there are other proteins secreted from specialised organelles also involved in the host cell recognition and initiation of the invasion process: micronemes and rhoptries. They are present in the apical complex of Apicomplexa (
Figure 2A) and all play essential biological functions for the invasion and development of the PV. Proteins secreted from micronemes (MICs) have been studied in different protozoan parasites, including
Eimeria [
48]. MICs secretion is upregulated through a calcium-mediated release pathway [
49,
50]. During the invasion, they display adhesive domains and are involved in gliding motility, migration, adhesion to the surface [
51,
52], and potentially in later egress from host cells after intracellular replication [
53]. Some coccidian MICs harbour specific microneme adhesive repeat regions (MAR) domains that are involved in binding sialylated glycans [
54]. It has been demonstrated that many of them, but not all, possess a transmembrane domain, among other relevant motifs, providing a dual localisation, i.e., in micronemes and surface membranes after secretion. This is the case for the
E. tenella (Et) EtMIC1, EtMIC4 [
52,
55], and EtMIC8 [
56], reasons why they are included in the current review. Apical Membrane Antigens (AMAs) are another well-known family of proteins secreted from micronemes and exported to the surface of the parasite. These were first identified in
Plasmodium knowlesi [
57] and
T. gondii, which have been recognised as important vaccine candidates. Together with proteins from the neck of the rhoptries (RONs), AMA1 is involved in the formation of the moving junction (MJ) complex, a structure created between parasite and host to allow active parasite internalization and PV formation [
58]. AMA1 from the apicomplexan parasites
Babesia,
Toxoplasma,
Neospora, and
Plasmodium falciparum has proven immunoprotective properties [
50,
59,
60,
61].
Immune-mapped protein-1 (IMP-1) is a highly conserved antigen located at the parasite surface. This was first identified in
E. maxima [
62] and later it was found in
E. tenella [
63] and other related Apicomplexa:
T. gondii [
64] and
N. caninum [
65]. Vaccination with IMP-1 induced a strong specific immune response [
66,
67].
Surface proteins studied for each chicken
Eimeria spp. which have been considered therapeutic targets have been compiled in this section (
Figure 2). No surface proteins have been described so far for
E. praecox. Information related to constitutively expressed proteins of
Eimeria spp. can be found in Olajide et al. [
66].
3.1. Eimeria acervulina
Sporozoite and merozoite immunodominant proteins, with the ability to activate T- cells in vitro when expressed in
E. coli fused to β-galactosidase, were identified in the early 90 s [
67,
68]. They were termed: cSZ-1, which encoded for a sporozoite surface antigen that appeared to be minority; cMZ-8, which was localised in a concentric pattern over the surface of merozoites, and whose recombinant form is recognised by sera from infected chicken; EAMZp30-47, containing a fragment in common in other surface and rhoptry proteins from
E. acervulina merozoites; and MA1, a 22 kDa protein found in sporozoites. A few years later, Laurrent et al. [
69] demonstrated that the protein cSZ1 initially identified by Jenkins et al. was a 19 kDa protein localised in the cytoplasm. The authors speculated that the iodination method used in the previous assays may alter the antigenic properties of the proteins; in consequence, the previous identification of these tentative surface antigens should be further confirmed.
Protein 3-1E is a profilin expressed at the surface of merozoites and sporozoites stages. This protein is highly conserved among
Eimeria spp. [
70,
71] and plays a significant role in host-parasite interaction. Although profilins have usually a cytoplasmic location, the authors reported a surface localisation for
E. acervulina. This superfamily of proteins prevents the polymerization of actin into filaments and, in certain circumstances, promotes actin polymerization. It has been demonstrated that a baculovirus-expressed recombinant 3-1E could induce the expression of IFN-ɣ in in vitro splenocytes stimulated with the recombinant protein, isolated from chickens challenged with
E. acervulina; suggesting a cellular-mediated response. Immunoprotection against coccidiosis led by this antigen was also evidenced. Chickens vaccinated with a chimeric DNA vaccine, comprising cDNA encoding for 3-1E and mature chicken IL-15, have shown elevated levels of IFN-ɣ and IL-2 mRNAs [
71,
72]. These data indicate that Protein 3-1E is a suitable candidate antigen to be included in recombinant vaccines. Furthermore, this protein has been co-expressed with the Actin-depolymerizing factor, an actin-binding protein highly conserved in all eukaryotic cells; however, the immunoprotective properties of this combination have not been evaluated [
73].
Few
mic genes of
E. acervulina (EaMIC2, EaMIC3, and EaMIC5) [
56,
74,
75,
76] have been identified and their function has been investigated. EaMIC3 has a key role in parasite survival since it inhibited cell apoptosis [
74]. They all were found in the apical complex in sporozoites, but EaMIC2 and EaMIC5 are located diffused at both poles of merozoites. It was suggested that MICs might be translocated to the sporozoite surface when it comes in contact with the host cell [
75]. The predicted transmembrane domain in EaMIC2 [
77] overlapped with the signal peptide, suggesting that it is actually a hydrophobic region of the signal peptide rather than a transmembrane region. However, experimental confirmation is needed [
51].
A proteomic analysis of
E. acervulina sporozoites was conducted by Zhang et al., 2015 [
78] in which chicken duodenal cells were co-cultured with soluble proteins of
E. acervulina sporozoites. Upon electrophoresis, immunoblotting, and analysis by mass spectrometry, fifteen proteins were identified, including profilin 14-3-3, MIC, ROP, and a SAG (EAH_00011660). The results suggested that the identified proteins, including EaSAG, could bind to chicken duodenal, providing insight into the molecules and mechanisms involved in the parasite invasion process.
3.2. Eimeria brunetti
There are only two antigens reported for
E. brunetti, EbMIC2 [
101] and EbAMA1 [
80] (
Figure 2B). EbMIC2 has 77 and 71% similarity in their amino acids sequence with MIC2 of
E. maxima and
E. tenella, respectively. EbAMA1 was cloned and expressed in
E. coli and its immunogenicity was evaluated in immunised chickens.
3.3. Eimeria maxima
An immunoprotective surface antigen has been recently identified through the construction of a cDNA expression library that was later screened through different rounds in an
E. maxima-challenged model in chicken [
84]. Upon the last round of immunisation, six individual clones of cDNA were identified corresponding to three hypothetical proteins, a rhomboid, a SAG (EmSAG), and a CAMP-dependent protein kinase regulatory subunit. An ORF of 708 bp encoded EmSAG, with a predicted molecular mass of 24.73 kDa and 13 predicted T-cell epitopes [
84]. EmSAG proved to be an effective vaccine candidate against homologous challenges [
85].
By using a combination of parasite genetics and selective barriers with population-based genetic fingerprinting, the protective antigens EmAMA1 and EmIMP1 were identified [
62]. The former is involved in host-parasite interaction and is a single-pass type I membrane protein. EmIMP1 is recognised as a vaccine candidate and is highly conserved among apicomplexan parasites such as
N. caninum and
T. gondii [
64].
N. caninum IMP1 may be involved in parasite invasion [
65]. The C-terminal derivative of EmIMP1 could be used as a potent immunogenic candidate in the development of recombinant vaccines [
82]. IMP1 transcripts were increased at the initial hours of sporulation between 6 and 12 h, and upon 18 h it was downregulated. Multiple sequence alignment has revealed seven single nucleotide polymorphisms, of which one of them could alter the IMP1 secondary structure. Additionally, indirect immunofluorescence with serum anti-IMP1 recognised
E. maxima sporozoites but not merozoites, and immunohistochemical staining of
E. maxima-infected chicken ileum tissue with specific sera, recognised intracellular parasite stages at up to 48 h post-infection. The authors suggest that these results may explain the immunoprotective effect previously observed [
83].
3.4. Eimeria mitis
E. mitis is a frequent species found in backyard productions [
13,
102]. This species can enhance the pathogenicity of
E. tenella and
E. necatrix infections [
103]. However, studies on this species are limited and only a few reports have included proteins from this species in vaccine formulation. One of the immunogenic antigens identified in this species was EmiMIC3 [
86]. This protein is expressed on the surface of sporozoites and merozoites and more specifically in the anterior end of the sporozoites. EmiMIC3 possesses 9 MARs, with seven cysteine residues, in contrast to the 7 MARs of EtMIC3. The Type I MAR domain motif LxxY is present in MAR1, MAR2, MAR3, and MAR8 of EmiMIC3, as well as the coordinating binding motifs HxT [
86]. It was suggested that the motifs in MARs of EmiMIC3 could contribute to site specificity during invasion and tissue tropisms. Immunogenicity of the native protein was demonstrated since it was recognised by rat polyclonal antibodies raised against the recombinant form (rEmiMIC3) produced in
E. coli. Chicken immunised intramuscularly with rEmiMIC3 showed changes in T lymphocyte subpopulation, serum cytokines, and IgY levels, specifically increasing the proportions of CD4+ and CD8 + T lymphocytes, and the level of IFN-γ [
86]; consequently, MIC3 from
E. mitis was considered a good vaccine candidate to be included in multivalent vaccines.
3.5. Eimeria necatrix
A comparative transcriptomic study from merozoites performed by Su et al., 2017 [
104], demonstrated that 2053 genes were differentially transcribed between
E. necatrix second and third-generation merozoites (Mz-2 and Mz-3, respectively). From these, 99 transcripts corresponded to
sag genes found to be differently expressed in Mz-2 and Mz-3. Sixty-four genes were upregulated only in Mz-3, which may indicate that they could be related to the different intestinal locations of both stages given that Mz-2 migrate from the mid-intestine to the caeca where they infect and develop into MZ-3. Additionally, an immunoproteomic analysis recently performed by Qu et al., 2022 [
88] identified 50 immunogenic proteins from sporozoites, which were divided into nine groups according to their structure, function, and localisation. Group 9 comprises two SAGs antigens (ENH_00010300, and ENH_00078390), suggesting that they are good vaccine candidates as their
T. gondii or eimerian counterparts.
The antigen NA4 (
Figure 2A) is a member of the sporozoite TA4 surface antigen family and showed 85% of identity with EtSAG1 (EtTA4, ETH_00010835) according to protein BLAST [
105]. This antigen can induce a strong protective effect against
E. necatrix [
87].
3.6. Eimeria tenella
According to Reid et al., 2014 [
32],
E. tenella contains a total of 89
sag genes and 23 pseudogenes. One of the first and better-studied antigens of this species is TA4 also known as EtSAG1 (
Figure 2A). This is a 25 kDa protein that is cleaved into 17 kDa and 8 kDa polypeptides, contains disulphide bonds, both transcript, and protein appear between 10- and 20-h post-sporulation, and the sequence contains conformational B- epitopes [
106]. EtSAG1 can bind epithelial cells in vitro, playing an important role in parasite attachment to the host cell surface before invasion, and it can also induce protective immunity against homologous challenge (
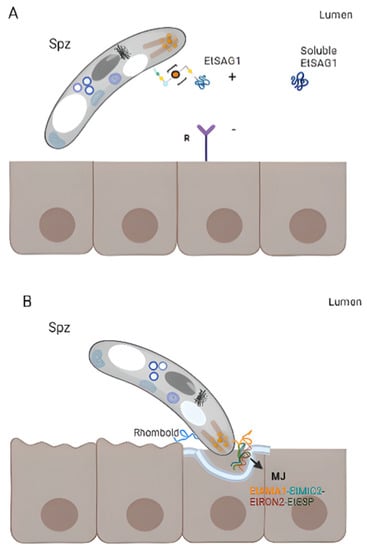
Figure 3A) [
32,
46,
87]. EtSAG1 is localised on the surface of extracellular sporozoites as well as after invasion into human hepatocyte cells in in vitro cultures. Antigen shedding outside and inside the infected cells was suggested [
46]. Interestingly, a positively charged patch in the outward-facing surface of EtSAG1 seems to attach to negatively charged sulphated proteoglycans on the surface of the host cell, initiating host invasion (
Figure 3A) [
46]; resembling
T. gondii TgSAG1 role [
107]. Recently, the genetic diversity of this antigen—along with EtMIC2—was analysed among 231
E. tenella- positive isolates from China and India, and compared with Korean isolates. The study demonstrated that this antigen is highly conserved since little variability was found among isolates [
108].
Etsag1 in these isolates was classified into four different haplotypes and showed limited amino acid polymorphism. Seven amino acid changes with low frequencies were found and, interestingly, amino acid changes identified in Korean and Chinese sequences were located in a putative B-cell epitope, suggesting the importance of determining the genetic heterogeneity and evolution of genes to design improved
Eimeria-control strategies. Tabarés et al., 2004 [
45] identified 37 EtSAGs, from which 23 were clustered in two multi-gen families: family
sagA including EtSAGs 1–12, showing a mosaic pattern with conserved and variable regions; and family
sagB, including EtSAGs 13–23, exhibiting a more biased pattern with variation predominantly in the N-terminal. Several
sags are differentially expressed in sporozoites and merozoites. While EtSAG1 is expressed only in sporozoites, seven SAGs were found to be expressed in both stages, with the remaining 29 expressed only in merozoites, demonstrating a wide repertoire. In addition, surface localisation was demonstrated for EtSAG1, EtSAG4, and EtSAG13; GPI- anchoring was shown for EtSAG8, EtSAG17, and EtSAG23. Chow et al., 2011 [
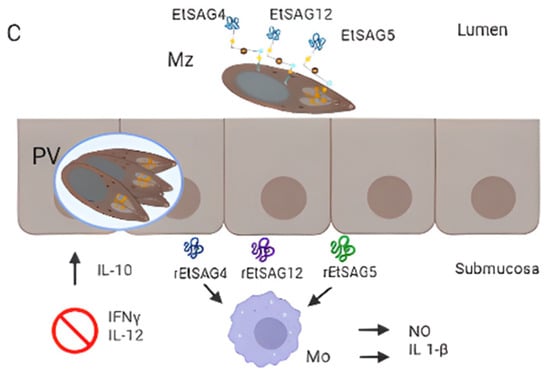
93] studied the inflammatory responses of eight SAGs by producing their recombinant forms and measuring inflammatory mediators in chicken macrophages. From them, EtSAG4, EtSAG5, and EtSAG12 induced high levels of nitric oxide and IL-1β. Moreover, the three recombinant forms have altered the expression of interleukins, suggesting a weakening of cellular-mediated immunity (suppression of IL-12 and IFN-γ, with elevation of IL-10), (
Figure 3C) [
93].
Figure 3. Surface antigens involved in host-parasite interaction. (A) Attachment. EtSAG1 at the surface of an extracellular sporozoite with a patch positively charged in the outward-facing (indicated with +), which might attach to negatively charged sulphated proteoglycans on the surface of the host cell (indicated with a −). The soluble form produced by the cleavage of EtSAG1 is also represented. (B) Formation of the moving junction (MJ) during invasion. EtAMA1 is represented in the apical region of a sporozoite (after being secreted by micronemes), interacting with an Eimeria-specific protein (EtESP), EtMIC2, and EtRON2 (grey). (C) Immunogenicity of EtSAG4, EtSAG5 and EtSAG12 in intra and extracellular merozoites. The recombinant forms of the three SAGs showed that they elicited pro-inflammatory cytokine IL-1b and nitric oxide (NO) produced by macrophages, inhibiting INF-ɣ and IL-12 and increased IL-10.
A study focusing on genes differentially expressed between stages of
E. tenella has identified genes encoding for EtSAG1 (TA4), EtSAG13, and EtSAG23, in addition to genes encoding for MICs, ROPs, small heat-shock proteins, and calcium-dependent protein kinase ROPs. Whereas EtSAG23 was found in early sporulation and second-generation merozoites, EtSAG1 and EtSAG13 were expressed in sporulated oocysts and sporozoite stages [
91]. Thirty-six immunogenic proteins were identified in second-generation merozoites, including three surface antigens: EtSAG2, EtSAG4, and EtSAG12 [
109].
A transcriptomic analysis performed in
E. tenella gametocytes (the sexual stages) has identified 863 genes that were upregulated in gametocytes compared with merozoites and sporozoites (asexual stages) [
96]. Besides the abundance of transcripts encoding for hypothetical proteins containing PAN domains that are involved in adhesion, oocyst wall proteins, and a macrophage migration inhibitory factor were also found upregulated in gametocytes; transcripts encoding for SAG7 and SAG10 were also detected [
96]. Authors identified genes encoding for oocysts wall proteins, subtilisin, and oxidoreductase on macrogametocytes and a microgamete-specific fusion protein, shedding light on the sexual biology of
E. tenella. Another study found that EtSAG10 localised in the surface of sporozoites, merozoites, and the initial asexual stages (trophozoites, and immature schizonts), and importantly, in the PV membrane. Polyclonal antibodies anti-rEtSAG10 diminished the capacity of sporozoites to invade chicken embryo fibroblast (DF-1) cells in vitro.
Etsag10 gene was downregulated and contained from one to ten mutations in strains resistant to anticoccidial drugs (maduramicin and diclazuril) [
47]. Thereby, EtSAG10 might also be involved in host cell invasion, pathogenesis, and immune evasion [
47]. A recent study conducted by Xie et al., 2020 [
23] has performed a comparative transcriptomic analysis of
E. tenella strains sensitive and resistant to diclazuril and maduramicin. The study showed 1070 differentially expressed genes (DEG) involved in the peroxisome, biosynthesis of unsaturated fatty acids, and fatty acid metabolism, and some DEGs coded for surface antigens, which were downregulated in the two drug-resistant strains studied. Specifically, EtSAG10 and EtSAG13 are differentially expressed between drug-resistant and drug-sensitive strains, being downregulated in the two resistant strains.
The tri-dimensional structure of EtSAG19 has been recently solved [
97]. The authors have demonstrated that the protein fold is a three-layer αβα sandwich, which resembles the structure of the CAP superfamily, present in unrelated eukaryotes such as insects or plants. Members of this superfamily are involved in defence mechanisms such as antifungal [
110] or systemic acquired resistance in plants [
111] or in blocking cardiac and skeletal human ryanodine receptors in lizard venom [
112]; accordingly, a role in modulating host-immune system in defence of the parasite life was suggested for EtSAG19.
E. tenella EtAMA1 is secreted by sporozoites micronemes and is critical for host cell invasion by the formation of the MJ, in association with rhoptry neck protein 2 (EtRON2), EtMIC2 and
Eimeria-specific protein (EtESP) [
98,
113]. The latter is a secreted protein and it has been demonstrated that antibodies anti-EtAMA1 or combined with its associated protein significantly impair sporozoite invasion (
Figure 3B) [
98,
113]. In addition, authors showed that host transcripts involved in cell signalling, regulation of metabolic processes, and cytoskeletal reorganization were downregulated, whereas transcripts implied in cytoskeletal organization, cell migration, and movement, were upregulated upon the overexpression of EtAMA1 in the DF-1 cell line, providing relevant data related to the molecular mechanism of this protein during invasion. The paralog EtAMA2 has been identified in merozoites. In vitro assays demonstrated that it did not impair the parasite invasion and did not show a protective effect when used as a vaccinogen [
81]. EtAMA3 was recently identified [
98]. It is a type I integral membrane with cysteine residues in domains I and II, as other apicomplexan orthologues; however, an amino acid variance was observed in domain III. It is localised in the apical region of sporozoites and is involved in host cell invasion as AMA1.
Interestingly,
E. tenella IMP1 homologue can elicit specific antibodies and IFN-Ɣ responses in chickens, conferring partial protection against homologous challenge [
62].
Among the eight MIC proteins identified in
E. tenella so far, three (EtMIC1, EtMIC4, and EtMIC8) contain a transmembrane domain [
56,
100] and proved to be crucial during parasite invasion. EtMIC8 is expressed in sporozoites and merozoites, it is involved in host cell attachment and confers moderate levels of immunoprotection [
56]. The analysis of the coding sequence has revealed a signal peptide at the N-terminal, low-complexity fragments, four tandemly arranged EGF-like domains with an incomplete EGF-like domain and a transmembrane domain at the C-terminal.
This entry is adapted from the peer-reviewed paper 10.3390/life13061295