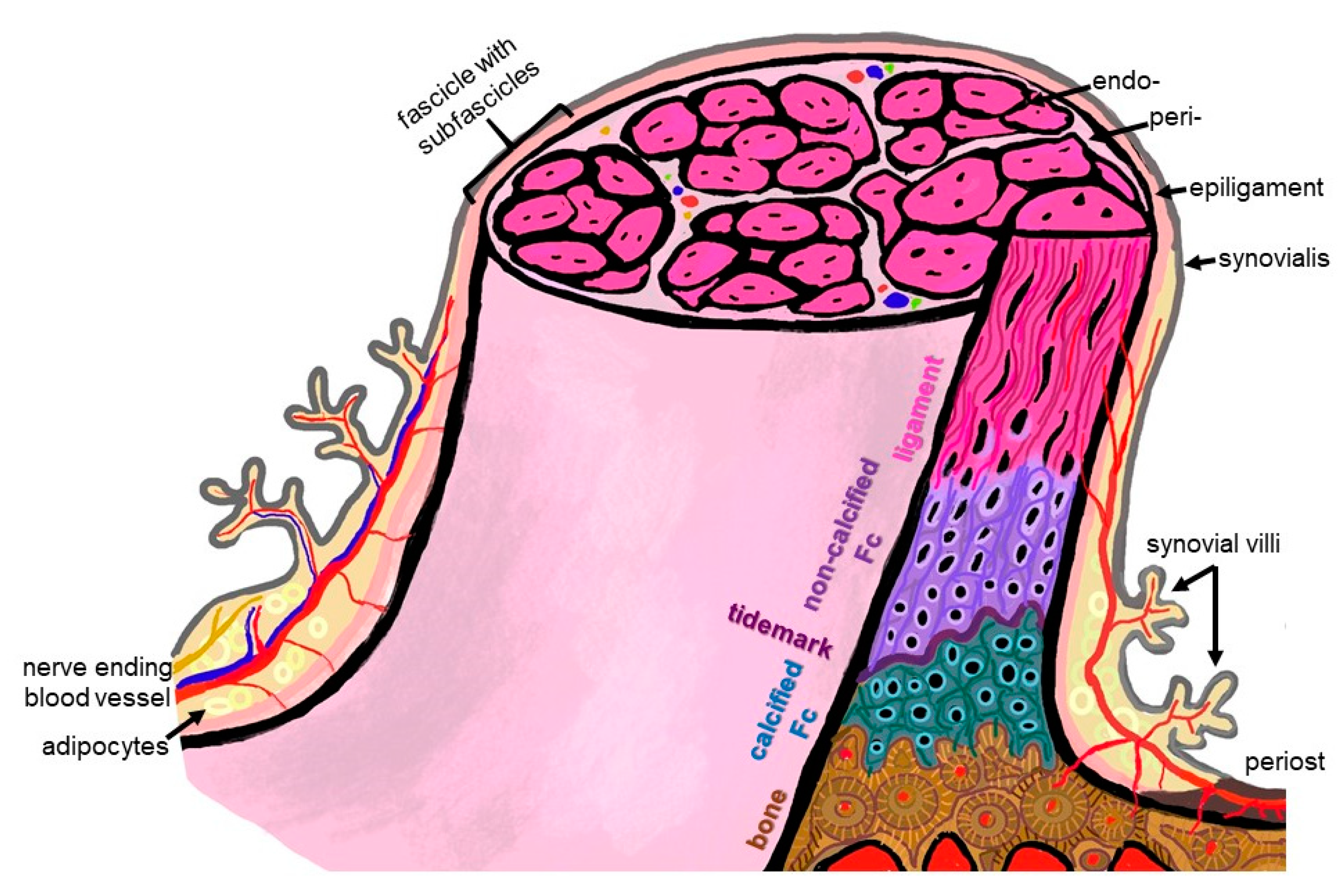
The firm integration of anterior cruciate ligament (ACL) grafts into bones remains the most demanding challenge in ACL reconstruction, since graft loosening means graft failure. For a functional-tissue-engineered ACL substitute to be realized in future, robust bone attachment sites (entheses) have to be re-established. The latter comprise four tissue compartments (ligament, non-calcified and calcified fibrocartilage, separated by the tidemark, bone) forming a histological and biomechanical gradient at the attachment interface between the ACL and bone. The ACL enthesis is surrounded by the synovium and exposed to the intra-articular micromilieu.
- ACL
- enthesis
- ligament
- synovioentheseal complex knee
- tissue engineering
- triphasic and graded scaffold
- fibrocartilage
- bone—ligament interface
- zonality
- tidemark
1. Introduction
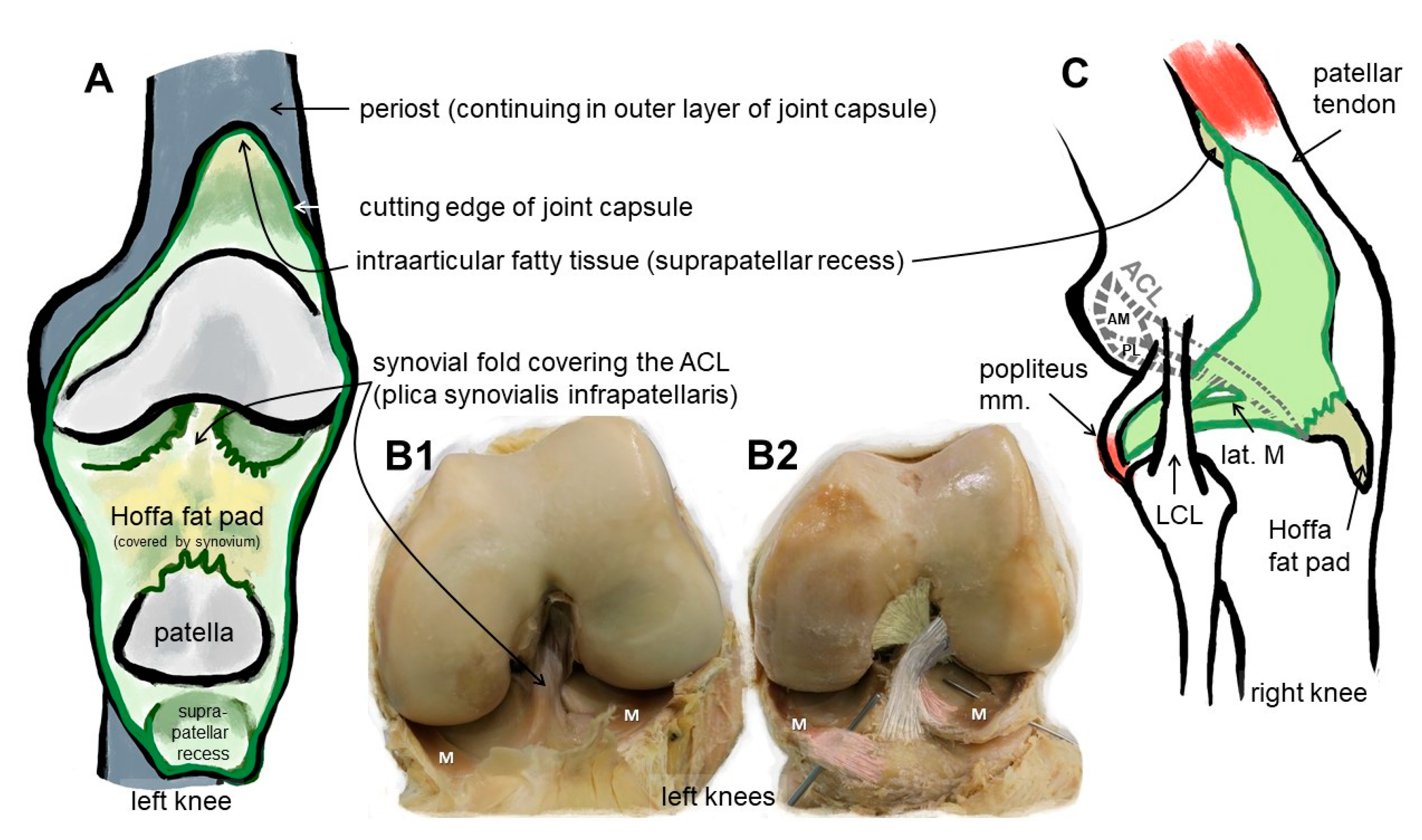
2. Anatomy: The ACL Entheses
3. Differences between the Femoral and Tibial ACL Entheses
4. ACL and Enthesis Development
5. Histoarchitecture, Cellular and Biochemical Pecularities
6. Sex- and Training-Dependent Individual Aspects
7. ACL Animal Models
8. In Silico ACL Modeling
9. Strategies for Multiphasic Enthesis Scaffolds for ACL Tissue Engineering
10. Biochemical Instruction of Cells Used for ACL Tissue Engineering
11. Ligamentogenic/Entheseal Differentiation of Cells for ACL Enthesis Tissue Engineering
12. Co-Cultures for Zonal ACL Tissue Engineering and Mechanical Training of Cells
This entry is adapted from the peer-reviewed paper 10.3390/ijms24119745
References
- Beaulieu, M.L.; Carey, G.E.; Schlecht, S.H.; Wojtys, E.M.; Ashton-Miller, J.A. On the heterogeneity of the femoral enthesis of the human ACL: Microscopic anatomy and clinical implications. J. Exp. Orthop. 2016, 3, 14.
- Chen, C.H. Strategies to enhance tendon graft–bone healing in anterior cruciate ligament reconstruction. Chang. Gung Med. J. 2009, 32, 483–493.
- Lu, H.; Chen, C.; Xie, S.; Tang, Y.; Qu, J. Tendon Healing in Bone Tunnel after Human Anterior Cruciate Ligament Reconstruction: A Systematic Review of Histological Results. J. Knee Surg. 2019, 32, 454–462.
- Tabuchi, K.; Soejima, T.; Kanazawa, T.; Noguchi, K.; Nagata, K. Chronological changes in the collagen-type composition at tendon-bone interface in rabbits. Bone Joint Res. 2012, 1, 218–224.
- Lin, K.M.; Boyle, C.; Marom, N.; Marx, R.G. Graft Selection in Anterior Cruciate Ligament Reconstruction. Sports Med. Arthrosc. Rev. 2020, 28, 41–48.
- Hoher, J.; Moller, H.D.; Fu, F.H. Bone tunnel enlargement after anterior cruciate ligament reconstruction: Fact or fiction? Knee Surg. Sports Traumatol. Arthrosc. 1998, 6, 231–240.
- Fahey, M.; Indelicato, P.A. Bone tunnel enlargement after anterior cruciate ligament replacement. Am. J. Sports Med. 1994, 22, 410–414.
- Brandt, K.D. Insights into the natural history of osteoarthritis provided by the cruciate-deficient dog. An animal model of osteoarthritis. Ann. N. Y. Acad. Sci. 1994, 732, 199–205.
- Benjamin, M.; McGonagle, D. Entheses: Tendon and ligament attachment sites. Scand. J. Med. Sci. Sports 2009, 19, 520–527.
- Lyons, T.J.; McClure, S.F.; Stoddart, R.W.; McClure, J. The normal human chondro-osseous junctional region: Evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Musculoskelet. Disord. 2006, 7, 52.
- Arkill, K.P.; Winlove, C.P. Fatty acid transport in articular cartilage. Arch. Biochem. Biophys. 2006, 456, 71–78.
- Pan, J.; Zhou, X.; Li, W.; Novotny, J.E.; Doty, S.B.; Wang, L. In situ measurement of transport between subchondral bone and articular cartilage. J. Orthop. Res. 2009, 27, 1347–1352.
- Gongadze, L.R. Basophilic line of the articular cartilage in normal and various pathological states. Arkh. Anat. Gistol. Embriol. 1987, 92, 52–57.
- Beaulieu, M.L.; Carey, G.E.; Schlecht, S.H.; Wojtys, E.M.; Ashton-Miller, J.A. Quantitative comparison of the microscopic anatomy of the human ACL femoral and tibial entheses. J. Orthop. Res. 2015, 33, 1811–1817.
- Dienst, M.; Burks, R.T.; Greis, P.E. Anatomy and biomechanics of the anterior cruciate ligament. Orthop. Clin. N. Am. 2002, 33, 605–620.
- Kohn, L.; Rembeck, E.; Rauch, A. Anterior cruciate ligament injury in adults: Diagnostics and treatment. Orthopade 2020, 49, 1013–1028.
- Zhao, L.; Lee, P.V.S.; Ackland, D.C.; Broom, N.D.; Thambyah, A. Microstructure Variations in the Soft-Hard Tissue Junction of the Human Anterior Cruciate Ligament. Anat. Rec. 2017, 300, 1547–1559.
- Smigielski, R.; Zdanowicz, U.; Drwiega, M.; Ciszek, B.; Ciszkowska-Lyson, B.; Siebold, R. Ribbon like appearance of the midsubstance fibres of the anterior cruciate ligament close to its femoral insertion site: A cadaveric study including 111 knees. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3143–3150.
- Qu, D.; Subramony, S.D.; Boskey, A.L.; Pleshko, N.; Doty, S.B.; Lu, H.H. Compositional mapping of the mature anterior cruciate ligament-to-bone insertion. J. Orthop. Res. 2017, 35, 2513–2523.
- Dyment, N.A.; Breidenbach, A.P.; Schwartz, A.G.; Russell, R.P.; Aschbacher-Smith, L.; Liu, H.; Hagiwara, Y.; Jiang, R.; Thomopoulos, S.; Butler, D.L.; et al. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev. Biol. 2015, 405, 96–107.
- Thomopoulos, S.; Genin, G.M.; Galatz, L.M. The development and morphogenesis of the tendon-to-bone insertion—What development can teach us about healing. J. Musculoskelet. Neuronal. Interact. 2010, 10, 35–45.
- Milz, S.; Boszczyk, B.M.; Boszczyk, A.A.; Putz, R.; Benjamin, M. The enthesis. Physiological morphology, molecular composition and pathoanatomical alterations. Orthopade 2005, 34, 526–532.
- Bellemans, J.; Carpentier, K.; Vandenneucker, H.; Vanlauwe, J.; Victor, J. The John Insall Award: Both morphotype and gender influence the shape of the knee in patients undergoing TKA. Clin. Orthop. Relat. Res. 2010, 468, 29–36.
- Guy, S.P.; Farndon, M.A.; Sidhom, S.; Al-Lami, M.; Bennett, C.; London, N.J. Gender differences in distal femoral morphology and the role of gender specific implants in total knee replacement: A prospective clinical study. Knee 2012, 19, 28–31.
- Beaulieu, M.L.; Lamontagne, M.; Xu, L. Gender differences in time-frequency EMG analysis of unanticipated cutting maneuvers. Med. Sci. Sports Exerc. 2008, 40, 1795–1804.
- Beaulieu, M.L.; McLean, S.G. Sex-dimorphic landing mechanics and their role within the noncontact ACL injury mechanism: Evidence, limitations and directions. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2012, 4, 10.
- Rodas, G.; Caceres, A.; Ferrer, E.; Balague-Dobon, L.; Osaba, L.; Lucia, A.; Gonzalez, J.R. Sex Differences in the Association between Risk of Anterior Cruciate Ligament Rupture and COL5A1 Polymorphisms in Elite Footballers. Genes 2022, 14, 33.
- Little, D.; Thompson, J.W.; Dubois, L.G.; Ruch, D.S.; Moseley, M.A.; Guilak, F. Proteomic differences between male and female anterior cruciate ligament and patellar tendon. PLoS ONE 2014, 9, e96526.
- Liu, S.H.; Al-Shaikh, R.A.; Panossian, V.; Finerman, G.A.; Lane, J.M. Estrogen affects the cellular metabolism of the anterior cruciate ligament. A potential explanation for female athletic injury. Am. J. Sports Med. 1997, 25, 704–709.
- Liu, X.; Luo, Z.P. Combined effects of estrogen and mechanical loading on anterior cruciate ligament fibroblast biosynthesis. ScientificWorldJournal 2005, 5, 5–8.
- Lee, H.; Petrofsky, J.S.; Daher, N.; Berk, L.; Laymon, M.; Khowailed, I.A. Anterior cruciate ligament elasticity and force for flexion during the menstrual cycle. Med. Sci. Monit. 2013, 19, 1080–1088.
- Beaulieu, M.L.; DeClercq, M.G.; Rietberg, N.T.; Li, S.H.; Harker, E.C.; Weber, A.E.; Ashton-Miller, J.A.; Wojtys, E.M. The Anterior Cruciate Ligament Can Become Hypertrophied in Response to Mechanical Loading: A Magnetic Resonance Imaging Study in Elite Athletes. Am. J. Sports Med. 2021, 49, 2371–2378.
- Beaulieu, M.L.; Nowak, E.K.; Beynnon, B.D.; Ashton-Miller, J.A.; Sturnick, D.R.; Wojtys, E.M. Clinical-Grade MRI-Based Methods to Identify Combined Anatomic Factors That Predict ACL Injury Risk in Male and Female Athletes. Am. J. Sports Med. 2021, 49, 2615–2623.
- Hohmann, E.; Bryant, A.; Reaburn, P.; Tetsworth, K. Is there a correlation between posterior tibial slope and non-contact anterior cruciate ligament injuries? Knee Surg. Sports Traumatol. Arthrosc. 2011, 19 (Suppl. S1), S109–S114.
- Mengsteab, P.Y.; Conroy, P.; Badon, M.; Otsuka, T.; Kan, H.M.; Vella, A.T.; Nair, L.S.; Laurencin, C.T. Evaluation of a bioengineered ACL matrix’s osteointegration with BMP-2 supplementation. PLoS ONE 2020, 15, e0227181.
- Lu, D.; Yang, C.; Zhang, Z.; Xiao, M. Enhanced tendon-bone healing with acidic fibroblast growth factor delivered in collagen in a rabbit anterior cruciate ligament reconstruction model. J. Orthop. Surg. Res. 2018, 13, 301.
- Jiang, Q.; Wang, L.; Liu, Z.; Su, J.; Tang, Y.; Tan, P.; Zhu, X.; Zhang, K.; Ma, X.; Jiang, J.; et al. Canine ACL reconstruction with an injectable hydroxyapatite/collagen paste for accelerated healing of tendon-bone interface. Bioact. Mater. 2023, 20, 1–15.
- Bascunan, A.L.; Biedrzycki, A.; Banks, S.A.; Lewis, D.D.; Kim, S.E. Large Animal Models for Anterior Cruciate Ligament Research. Front. Vet. Sci. 2019, 6, 292.
- Schulze-Tanzil, G.; Silawal, S.; Hoyer, M. Anatomical feature of knee joint in Aachen minipig as a novel miniature pig line for experimental research in orthopaedics. Ann. Anat. 2020, 227, 151411.
- Shi, Q.; Wang, H.; He, K.; Tao, M.; Cheng, C.K. Comparison of the morphology of the anterior cruciate ligament and related bony structures between pigs and humans. Front. Vet. Sci. 2022, 9, 1045785.
- Erdemir, A.; Guess, T.M.; Halloran, J.; Tadepalli, S.C.; Morrison, T.M. Considerations for reporting finite element analysis studies in biomechanics. J. Biomech. 2012, 45, 625–633.
- Kiapour, A.; Kiapour, A.M.; Kaul, V.; Quatman, C.E.; Wordeman, S.C.; Hewett, T.E.; Demetropoulos, C.K.; Goel, V.K. Finite element model of the knee for investigation of injury mechanisms: Development and validation. J. Biomech. Eng. 2014, 136, 011002.
- Pena, E.; Calvo, B.; Martinez, M.A.; Palanca, D.; Doblare, M. Finite element analysis of the effect of meniscal tears and meniscectomies on human knee biomechanics. Clin. Biomech. 2005, 20, 498–507.
- Benos, L.; Stanev, D.; Spyrou, L.; Moustakas, K.; Tsaopoulos, D.E. A Review on Finite Element Modeling and Simulation of the Anterior Cruciate Ligament Reconstruction. Front. Bioeng. Biotechnol. 2020, 8, 967.
- Catani, F.; Innocenti, B.; Belvedere, C.; Labey, L.; Ensini, A.; Leardini, A. The Mark Coventry Award: Articular contact estimation in TKA using in vivo kinematics and finite element analysis. Clin. Orthop. Relat. Res. 2010, 468, 19–28.
- Harris, M.D.; Cyr, A.J.; Ali, A.A.; Fitzpatrick, C.K.; Rullkoetter, P.J.; Maletsky, L.P.; Shelburne, K.B. A Combined Experimental and Computational Approach to Subject-Specific Analysis of Knee Joint Laxity. J. Biomech. Eng. 2016, 138, 0810041–0810048.
- Readioff, R.; Geraghty, B.; Comerford, E.; Elsheikh, A. A full-field 3D digital image correlation and modelling technique to characterise anterior cruciate ligament mechanics ex vivo. Acta Biomater. 2020, 113, 417–428.
- Chokhandre, S.; Schwartz, A.; Klonowski, E.; Landis, B.; Erdemir, A. Open Knee(s): A Free and Open Source Library of Specimen-Specific Models and Related Digital Assets for Finite Element Analysis of the Knee Joint. Ann. Biomed. Eng. 2023, 51, 10–23.
- Currey, J.D. Bones: Structure and Mechanics; Princeton University Press: Princeton, NJ, USA, 2002.
- Chandrashekar, N.; Mansouri, H.; Slauterbeck, J.; Hashemi, J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. J. Biomech. 2006, 39, 2943–2950.
- Thomopoulos, S.; Marquez, J.P.; Weinberger, B.; Birman, V.; Genin, G.M. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J. Biomech. 2006, 39, 1842–1851.
- Ristaniemi, A.; Tanska, P.; Stenroth, L.; Finnila, M.A.J.; Korhonen, R.K. Comparison of material models for anterior cruciate ligament in tension: From poroelastic to a novel fibril-reinforced nonlinear composite model. J. Biomech. 2021, 114, 110141.
- Tits, A.; Ruffoni, D. Joining soft tissues to bone: Insights from modeling and simulations. Bone Rep. 2021, 14, 100742.
- Spalazzi, J.P.; Boskey, A.L.; Pleshko, N.; Lu, H.H. Quantitative mapping of matrix content and distribution across the ligament-to-bone insertion. PLoS ONE 2013, 8, e74349.
- Armitage, O.E.; Oyen, M.L. Indentation across interfaces between stiff and compliant tissues. Acta Biomater. 2017, 56, 36–43.
- Ruffoni, D.; Fratzl, P.; Roschger, P.; Phipps, R.; Klaushofer, K.; Weinkamer, R. Effect of temporal changes in bone turnover on the bone mineralization density distribution: A computer simulation study. J. Bone Miner. Res. 2008, 23, 1905–1914.
- Dai, C.; Guo, L.; Yang, L.; Wu, Y.; Gou, J.; Li, B. Regional fibrocartilage variations in human anterior cruciate ligament tibial insertion: A histological three-dimensional reconstruction. Connect. Tissue Res. 2015, 56, 18–24.
- Mallett, K.F.; Arruda, E.M. Digital image correlation-aided mechanical characterization of the anteromedial and posterolateral bundles of the anterior cruciate ligament. Acta Biomater. 2017, 56, 44–57.
- Luetkemeyer, C.M.; Cai, L.; Neu, C.P.; Arruda, E.M. Full-volume displacement mapping of anterior cruciate ligament bundles with dual MRI. Extrem. Mech. Lett. 2018, 19, 7–14.
- Luetkemeyer, C.M.; Marchi, B.C.; Ashton-Miller, J.A.; Arruda, E.M. Femoral entheseal shape and attachment angle as potential risk factors for anterior cruciate ligament injury. J. Mech. Behav. Biomed. Mater. 2018, 88, 313–321.
- Luetkemeyer, C.M.; Scheven, U.; Estrada, J.B.; Arruda, E.M. Constitutive modeling of the anterior cruciate ligament bundles and patellar tendon with full-field methods. J. Mech. Phys. Solids 2021, 156, 104577.
- Rafieyan, S.; Vasheghani-Farahani, E.; Baheiraei, N.; Keshavarz, H. MLATE: Machine learning for predicting cell behavior on cardiac tissue engineering scaffolds. Comput. Biol. Med. 2023, 158, 106804.
- Seidi, A.; Ramalingam, M.; Elloumi-Hannachi, I.; Ostrovidov, S.; Khademhosseini, A. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater. 2011, 7, 1441–1451.
- Cai, J.; Wang, J.; Sun, C.; Dai, J.; Zhang, C. Biomaterials with stiffness gradient for interface tissue engineering. Biomed. Mater. 2022, 17, 064103.
- Klabukov, I.; Tenchurin, T.; Shepelev, A.; Baranovskii, D.; Mamagulashvili, V.; Dyuzheva, T.; Krasilnikova, O.; Balyasin, M.; Lyundup, A.; Krasheninnikov, M.; et al. Biomechanical Behaviors and Degradation Properties of Multilayered Polymer Scaffolds: The Phase Space Method for Bile Duct Design and Bioengineering. Biomedicines 2023, 11, 745.
- Paxton, J.Z.; Grover, L.M.; Baar, K. Engineering an in vitro model of a functional ligament from bone to bone. Tissue Eng. Part A 2010, 16, 3515–3525.
- Hurley-Novatny, A.; Arumugasaamy, N.; Kimicata, M.; Baker, H.; Mikos, A.G.; Fisher, J.P. Concurrent multi-lineage differentiation of mesenchymal stem cells through spatial presentation of growth factors. Biomed. Mater. 2020, 15, 055035.
- Font Tellado, S.; Chiera, S.; Bonani, W.; Poh, P.S.P.; Migliaresi, C.; Motta, A.; Balmayor, E.R.; van Griensven, M. Heparin functionalization increases retention of TGF-beta2 and GDF5 on biphasic silk fibroin scaffolds for tendon/ligament-to-bone tissue engineering. Acta Biomater. 2018, 72, 150–166.
- Fan, J.; Sun, L.; Chen, X.; Qu, L.; Li, H.; Liu, X.; Zhang, Y.; Cheng, P.; Fan, H. Implementation of a stratified approach and gene immobilization to enhance the osseointegration of a silk-based ligament graft. J. Mater. Chem. B 2017, 5, 7035–7050.
- Ferguson, M.W.; O’Kane, S. Scar-free healing: From embryonic mechanisms to adult therapeutic intervention. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 839–850.
- Zou, G.; Song, E.; Wei, B. Effects of tendon-bone healing of anterior cruciate ligament reconstruction by osteoprotegerin combined with deproteinized bovine bone. Muscles Ligaments Tendons J. 2017, 7, 256–262.
- Nakase, J.; Kitaoka, K.; Matsumoto, K.; Tomita, K. Facilitated tendon-bone healing by local delivery of recombinant hepatocyte growth factor in rabbits. Arthroscopy 2010, 26, 84–90.
- Iannucci, L.E.; Boys, A.J.; McCorry, M.C.; Estroff, L.A.; Bonassar, L.J. Cellular and Chemical Gradients to Engineer the Meniscus-to-Bone Insertion. Adv. Healthc. Mater. 2019, 8, e1800806.
- Mutsuzaki, H.; Kuwahara, K.; Nakajima, H. Influence of periostin on the development of fibrocartilage layers of anterior cruciate ligament insertion. Orthop. Traumatol. Surg. Res. 2022, 103215.
- Mutsuzaki, H.; Yoshida, Y.; Nakajima, H. Periostin Contributes to Fibrocartilage Layer Growth of the Patella Tendon Tibial Insertion in Mice. Medicina 2022, 58, 957.
- Cai, L.; Brophy, R.H.; Tycksen, E.D.; Duan, X.; Nunley, R.M.; Rai, M.F. Distinct expression pattern of periostin splice variants in chondrocytes and ligament progenitor cells. FASEB J. 2019, 33, 8386–8405.
- Miescher, I.; Rieber, J.; Calcagni, M.; Buschmann, J. In Vitro and In Vivo Effects of IGF-1 Delivery Strategies on Tendon Healing: A Review. Int. J. Mol. Sci. 2023, 24, 2370.
- Sha, Y.; Afandi, R.; Zhang, B.; Yang, L.; Lv, Y. MGF E peptide pretreatment improves collagen synthesis and cell proliferation of injured human ACL fibroblasts via MEK-ERK1/2 signaling pathway. Growth Factors 2017, 35, 29–38.
- Sha, Y.; Yang, L.; Lv, Y. MGF E peptide improves anterior cruciate ligament repair by inhibiting hypoxia-induced cell apoptosis and accelerating angiogenesis. J. Cell. Physiol. 2019, 234, 8846–8861.
- Eagan, M.J.; Zuk, P.A.; Zhao, K.W.; Bluth, B.E.; Brinkmann, E.J.; Wu, B.M.; McAllister, D.R. The suitability of human adipose-derived stem cells for the engineering of ligament tissue. J. Tissue Eng. Regen. Med. 2012, 6, 702–709.
- Schwarz, S.; Goegele, C.; Ondruschka, B.; Hammer, N.; Kohl, B.; Schulze-Tanzil, G. Migrating Myofibroblastic Iliotibial Band-Derived Fibroblasts Represent a Promising Cell Source for Ligament Reconstruction. Int. J. Mol. Sci. 2019, 20, 1972.
- Benjamin, M.; Ralphs, J.R. Fibrocartilage in tendons and ligaments—An adaptation to compressive load. J. Anat. 1998, 193 Pt 4, 481–494.
- Schulze-Tanzil, G.G.; Delgado-Calcares, M.; Stange, R.; Wildemann, B.; Docheva, D. Tendon healing: A concise review on cellular and molecular mechanisms with a particular focus on the Achilles tendon. Bone Joint Res. 2022, 11, 561–574.
- Schulze-Tanzil, G. Intraarticular Ligament Degeneration Is Interrelated with Cartilage and Bone Destruction in Osteoarthritis. Cells 2019, 8, 99.
- Ruschke, K.; Meier, C.; Ullah, M.; Krebs, A.C.; Silberreis, K.; Kohl, B.; Knaus, P.; Jagielski, M.; Arens, S.; Schulze-Tanzil, G. Bone morphogenetic protein 2/SMAD signalling in human ligamentocytes of degenerated and aged anterior cruciate ligaments. Osteoarthr. Cartil. 2016, 24, 1816–1825.
- Fang, F.; Xiao, Y.; Zelzer, E.; Leong, K.W.; Thomopoulos, S. A mineralizing pool of Gli1-expressing progenitors builds the tendon enthesis and demonstrates therapeutic potential. Cell Stem Cell 2022, 29, 1669–1684.e6.
- Schwartz, A.G.; Galatz, L.M.; Thomopoulos, S. Enthesis regeneration: A role for Gli1+ progenitor cells. Development 2017, 144, 1159–1164.
- Buschmann, J.; Gao, S.; Harter, L.; Hemmi, S.; Welti, M.; Werner, C.M.; Calcagni, M.; Cinelli, P.; Wanner, G.A. Yield and proliferation rate of adipose-derived stromal cells as a function of age, body mass index and harvest site-increasing the yield by use of adherent and supernatant fractions? Cytotherapy 2013, 15, 1098–1105.
- Matsumoto, T.; Sato, Y.; Kobayashi, T.; Suzuki, K.; Kimura, A.; Soma, T.; Ito, E.; Kikuchi, T.; Kobayashi, S.; Harato, K.; et al. Adipose-Derived Stem Cell Sheets Improve Early Biomechanical Graft Strength in Rabbits After Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2021, 49, 3508–3518.
- Bianchi, E.; Faccendini, A.; Del Favero, E.; Ricci, C.; Caliogna, L.; Vigani, B.; Pavesi, F.C.; Perotti, C.; Domingues, R.M.A.; Gomes, M.E.; et al. Topographical and Compositional Gradient Tubular Scaffold for Bone to Tendon Interface Regeneration. Pharmaceutics 2022, 14, 2153.
- Font Tellado, S.; Bonani, W.; Balmayor, E.R.; Foehr, P.; Motta, A.; Migliaresi, C.; van Griensven, M. (*) Fabrication and Characterization of Biphasic Silk Fibroin Scaffolds for Tendon/Ligament-to-Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 859–872.
- Teuschl, A.; Heimel, P.; Nuernberger, S.; van Griensven, M.; Redl, H.; Nau, T. A Novel Silk Fiber-Based Scaffold for Regeneration of the Anterior Cruciate Ligament: Histological Results From a Study in Sheep. Am. J. Sports Med. 2016, 44, 1547–1557.
- Teuschl, A.H.; Tangl, S.; Heimel, P.; Schwarze, U.Y.; Monforte, X.; Redl, H.; Nau, T. Osteointegration of a Novel Silk Fiber-Based ACL Scaffold by Formation of a Ligament-Bone Interface. Am. J. Sports Med. 2019, 47, 620–627.
- Tremblay, P.; Cloutier, R.; Lamontagne, J.; Belzil, A.M.; Larkin, A.M.; Chouinard, L.; Chabaud, S.; Laverty, S.; Lussier, B.; Goulet, F. Potential of skin fibroblasts for application to anterior cruciate ligament tissue engineering. Cell Transplant. 2011, 20, 535–542.
- He, P.; Ng, K.S.; Toh, S.L.; Goh, J.C. In vitro ligament-bone interface regeneration using a trilineage coculture system on a hybrid silk scaffold. Biomacromolecules 2012, 13, 2692–2703.
- Harris, E.; Liu, Y.; Cunniffe, G.; Morrissey, D.; Carroll, S.; Mulhall, K.; Kelly, D.J. Biofabrication of soft tissue templates for engineering the bone-ligament interface. Biotechnol. Bioeng. 2017, 114, 2400–2411.
- Li, H.; Fan, J.; Sun, L.; Liu, X.; Cheng, P.; Fan, H. Functional regeneration of ligament-bone interface using a triphasic silk-based graft. Biomaterials 2016, 106, 180–192.
- Xiong, J.; Wang, H.; Lan, X.; Wang, Y.; Wang, Z.; Bai, J.; Ou, W.; Cai, N.; Wang, W.; Tang, Y. Fabrication of bioinspired grid-crimp micropatterns by melt electrospinning writing for bone-ligament interface study. Biofabrication 2022, 14, 025008.
- Schulze-Tanzil, G.; Arnold, P.; Goegele, C.; Hahn, J.; Breier, A.; Meyer, M.; Kohl, B.; Schropfer, M.; Schwarz, S. SV40 Transfected Human Anterior Cruciate Ligament Derived Ligamentocytes-Suitable as a Human in Vitro Model for Ligament Reconstruction? Int. J. Mol. Sci. 2020, 21, 593.
- Hoyer, M.; Meier, C.; Breier, A.; Hahner, J.; Heinrich, G.; Drechsel, N.; Meyer, M.; Rentsch, C.; Garbe, L.A.; Ertel, W.; et al. In vitro characterization of self-assembled anterior cruciate ligament cell spheroids for ligament tissue engineering. Histochem. Cell Biol. 2015, 143, 289–300.
- Goegele, C.; Hahn, J.; Elschner, C.; Breier, A.; Schroepfer, M.; Prade, I.; Meyer, M.; Schulze-Tanzil, G. Enhanced Growth of Lapine Anterior Cruciate Ligament-Derived Fibroblasts on Scaffolds Embroidered from Poly(l-lactide-co-epsilon-caprolactone) and Polylactic Acid Threads Functionalized by Fluorination and Hexamethylene Diisocyanate Cross-Linked Collagen Foams. Int. J. Mol. Sci. 2020, 21, 1132.
- Goegele, C.; Konrad, J.; Hahn, J.; Breier, A.; Schroepfer, M.; Meyer, M.; Merkel, R.; Hoffmann, B.; Schulze-Tanzil, G. Maintenance of Ligament Homeostasis of Spheroid-Colonized Embroidered and Functionalized Scaffolds after 3D Stretch. Int. J. Mol. Sci. 2021, 22, 8204.
- Zahn, I.; Braun, T.; Goegele, C.; Schulze-Tanzil, G. Minispheroids as a Tool for Ligament Tissue Engineering: Do the Self-Assembly Techniques and Spheroid Dimensions Influence the Cruciate Ligamentocyte Phenotype? Int. J. Mol. Sci. 2021, 22, 11011.
- Zahn, I.; Stoebener, D.D.; Weinhart, M.; Goegele, C.; Breier, A.; Hahn, J.; Schroepfer, M.; Meyer, M.; Schulze-Tanzil, G. Cruciate Ligament Cell Sheets Can Be Rapidly Produced on Thermoresponsive poly(glycidyl ether) Coating and Successfully Used for Colonization of Embroidered Scaffolds. Cells 2021, 10, 877.
- Hahner, J.; Hoyer, M.; Hillig, S.; Schulze-Tanzil, G.; Meyer, M.; Schroepfer, M.; Lohan, A.; Garbe, L.A.; Heinrich, G.; Breier, A. Diffusion chamber system for testing of collagen-based cell migration barriers for separation of ligament enthesis zones in tissue-engineered ACL constructs. J. Biomater. Sci. Polym. Ed. 2015, 26, 1085–1099.
