Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Steelmaking is a water-intensive process. The mean water intake against each ton of steel manufactured is ascertained as between 2 and 20 m3. Primarily, the stated requirement is in the form of make-up water to compensate for evaporation and mechanical losses and does not contribute to wastewater generation. Conversely, unit operations, such as rolling, continuous casting, pickling, etc., generate highly complex wastewater rich in polycyclic aromatic hydrocarbons (PAH), cyanide, ammonia, non-consumed acids, benzene, toluene, xylene, oil, grease, etc.
- steel industry
- water
1. Introduction
Ironmaking and steel production are vital industrial processes that involve the transformation of iron ore into molten iron and its subsequent conversion into various grades of steel, supporting diverse sectors worldwide. The production process is complex and incorporates a series of operations in the following order: manufacturing of pig iron in the blast furnace (BF), steel manufacturing, shaping through rolling mills, finishing, and polishing [1]. The production of carbon steel involves a synergistic use of both BF and the basic oxygen furnace (BOF). In the BF, iron ore is melted and purified, yielding molten iron. Subsequently, the molten iron is carefully transferred to the basic oxygen furnace, where a controlled supply of oxygen is blown through it, eliminating the excess carbon and impurities. This meticulous process yields superior-quality carbon steel. The production of stainless steel using the electric arc furnace (EAF) process in conjunction with refining methods, such as argon oxygen decarburization (AOD) or vacuum oxygen decarburization (VOD), involves several key steps. Initially, a mixture of carbon steel and stainless steel scrap is charged into the EAF, where the electric arc generates high temperatures to melt the scrap into a molten state [1][2]. The other two primary alloying elements used in fusion with iron (Fe) are Chromium (Cr) and Nickel (Ni). The mix ratio varies between 7.8:1.6:0.6 and 5.2:2.6:2.2 for Fe, Cr, and Ni, respectively. The quality of the final output depends mainly on the optimal proportionate factor. Subsequently, the molten metal may undergo further refining in either an AOD or VOD vessel. In the AOD process, a mixture of argon gas and oxygen is injected to reduce the carbon and impurity content, while the VOD process utilizes a vacuum environment and oxygen to achieve decarburization. The refined stainless steel is then solidified through continuous casting, resulting in various shapes such as slabs, blooms, or billets, which can be further processed into sheets, bars, or coils. This integrated approach offers a comprehensive method for efficiently converting carbon steel or scrap stainless steel into high-quality stainless steel products with the desired composition and properties. The addition of iron yields better strength and durability but impacts it adversely through rusting and pitting [3]. Adding a significant amount of Cr and Ni hampers the strength but improves the ductility and corrosion resistance [2][3]. Based on the Fe, Cr, and Ni mixing ratio, stainless steel can be classified into three categories: austenitic, ferritic, and duplex. Austenitic stainless steel (face-centered cubic structure) with a higher percentage of Ni (8–12%) and low carbon iron provides enhanced corrosion resistance and ductility. The ferritic variant with a higher percentage of chromium (11–30%) and a minuscule portion of nickel (<0.5%) (body-centered cubic structure) stands better against stress but is tough to weld. Duplex, the equal combination of both, offers superior strength and resistance against corrosion and cracking [1][4]. The steel manufacturing process is subdivided into multiple operations, summarized in Figure 1 [1][2][5][6].
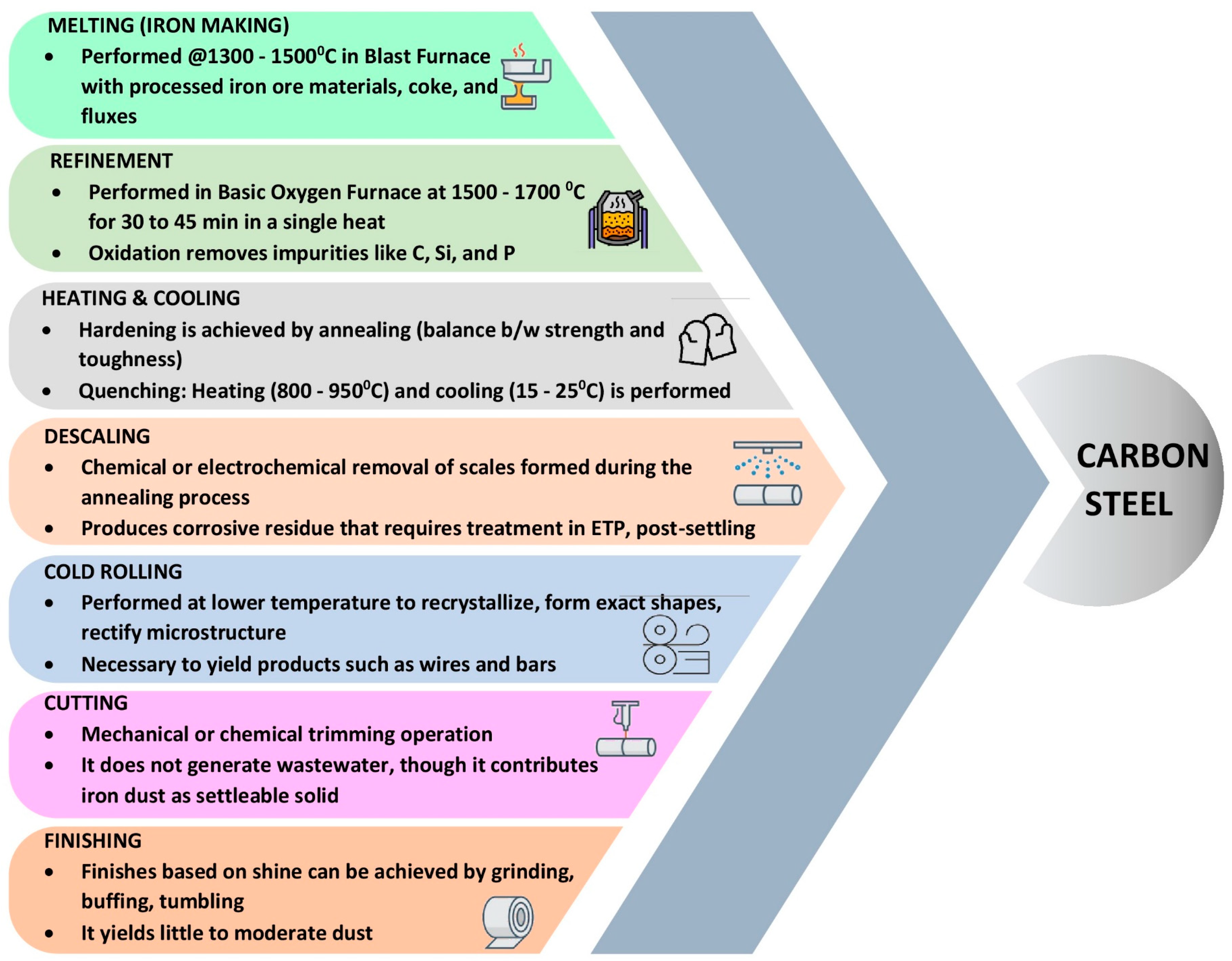
Figure 1. Process flow diagram for carbon steel manufacturing.
2. The Requirement for Water
The comprehensive water demand in steel plants is the summation of the individual requirements for activities such as cooling (make-up water to compensate for evaporation and mechanical loss in operations such as quenching, BF shell, continuous casting, and hot rolling), cleaning (BF, basic oxygen furnace (BOF), coke oven, etc.) descaling (hot rolling and continuous casting), chemical and electrochemical treatments (tin-plating and galvanization), and scrubbing (particulate suppression). Further, the micro-scale water demand can be dissected as direct and indirect cooling (BF oven and other machinery) related requirements, gas washing related requirements in BF, and combined requirements for chemical operations, such as degreasing, pickling, rinsing, etc. Figure 2 delineates water demand, and possible wastewater characteristics contributed to the sequence of each unit operation [7].
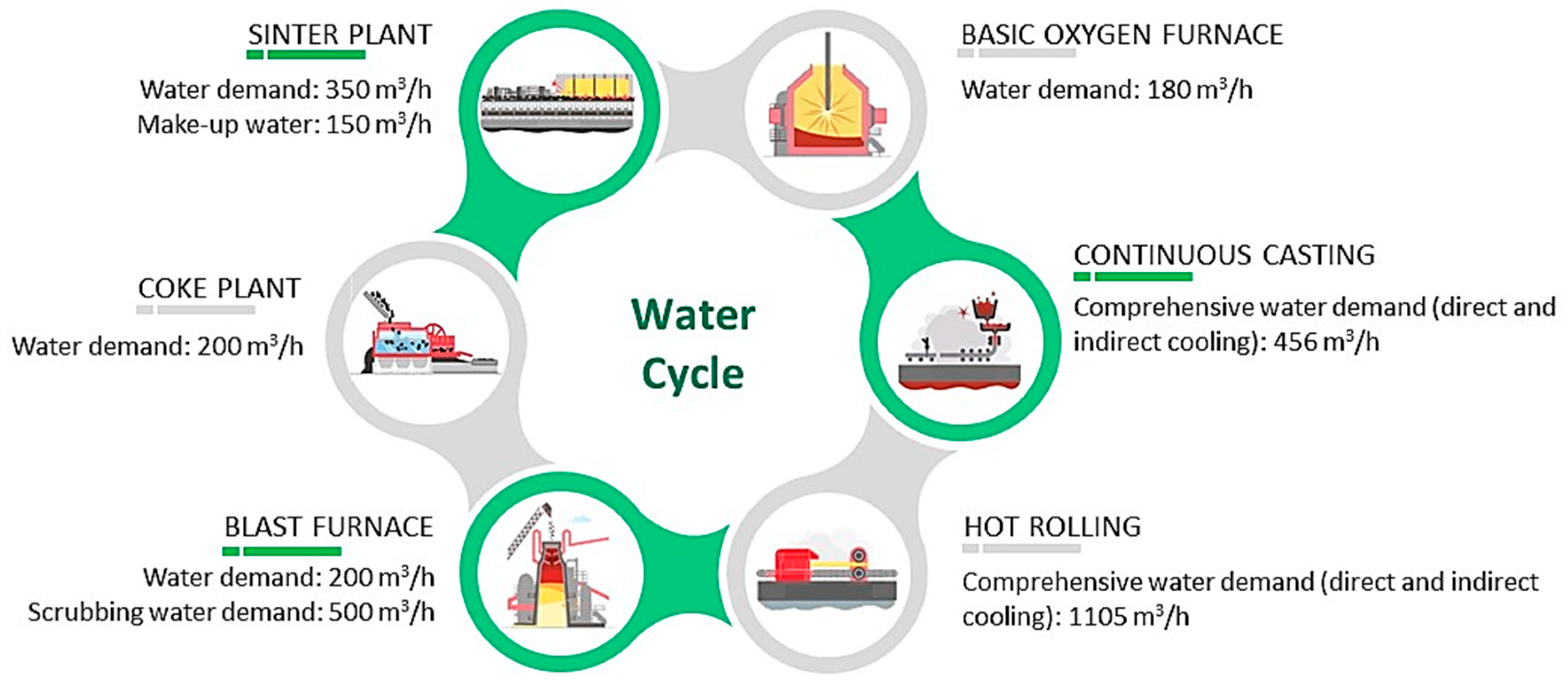
Figure 2. Qualitative and quantitative assessment of water requirements for unit operations involved in carbon steel manufacturing.
2.1. Water Cycle
Water consumption is a crucial aspect of iron and carbon steelmaking processes, playing a fundamental role in their production. The utilization of cooling systems is of paramount importance as they rely on water to regulate temperatures in equipment and machinery, including blast furnaces, converters, and continuous casting machines. Water is efficiently circulated through cooling jackets and spray systems to effectively absorb heat and prevent overheating. An essential operation in BF operations is stave cooling, where water-cooled staves act as protective barriers against the elevated temperatures and chemical reactions occurring within the furnace’s inner walls. Furthermore, water is instrumental in gas cleaning systems, which purify hot gases by effectively removing impurities. Within the steelmaking processes, water serves the critical purpose of cooling during operations such as those performed in the basic oxygen furnace and electric arc furnace. Water-cooled panels and systems successfully disperse the heat generated during these reactions. Continuous casting also relies on water, as it solidifies molten steel by guiding it through water-cooled molds and spray cooling systems. Consequently, these water-consuming operations are indispensable for ensuring the integrity and efficiency of carbon steel- and ironmaking processes. The water footprint and associated information of major unit operations related to iron and carbon steelmaking are delineated below [7][8].
Sinter plant: Freshwater is essential for serving as a coolant in the sinter machines, ignition hood, and fan within the sinter plant. The intake demand consists of 350 m3/h of raw water and 150 m3/h of make-up water. In a sinter plant, water serves multiple functions, primarily encompassing the cooling of the sinter machines, the ignition hood, and the fan. During these operations, the water inevitably interfaces with the sintering process, thereby exposing it to potential impurities, such as suspended solids, iron oxides, and chemicals employed in the overall procedure. As a consequence, the cooling and cleaning activities produce wastewater as an inevitable by-product.
Coke plant: Freshwater is required to function as a coolant for wet quenching, as well as direct and indirect cooling in the coke plant. The intake demand for the plant is 200 m3/h of freshwater, which compensates for mechanical losses, evaporation, and blowdown. As the coking process generates intense heat, the resulting hot gases undergo a cooling phase, leading to condensation and the generation of wastewater. This wastewater encompasses a range of impurities, including ammonia, phenol, and cyanide compounds. Moreover, in order to mitigate air pollution, the gases released from the coke ovens undergo a series of scrubbing and cleaning procedures. Water serves as a medium to effectively eliminate particulate matter, sulfur compounds, and various other pollutants from the gases.
Blast furnace: The blast furnace operation necessitates 200 m3/h of freshwater for the indirect cooling of the BF body, valve, and tuyere. A small quantity of wastewater is generated from the direct cooling of BF slag. Additionally, 500 m3/h of water is indispensable for the wet scrubbing of BF gas, although the resulting hot wastewater contains a high concentration of suspended solids.
Basic oxygen furnace: Water plays a crucial role in the indirect cooling of lances, hoods, and side tuyere in the steelmaking process. Additionally, a raw water intake demand of 180 m3/h is necessary for the wet scrubbers used in BOF gas cleaning.
Continuous casting: The casting process requires a total water supply of 456 m3/h for effective cooling. This includes 6 m3/h of demineralized water for the indirect cooling of the casting mold, along with 300 m3/h of coolant water. Additionally, there is a need for 150 m3/h of water supply to directly cool the continuous casting machines, resulting in the generation of wastewater containing high concentrations of hydrocarbon and metal oxides.
Hot rolling: In the hot rolling process, efficient cooling is crucial, and it involves three distinct water requirements.
Firstly, for the indirect cooling of the furnace, a continuous supply of 5 m3/h of softened mixed water is necessary. This water aids in maintaining the optimal temperature and preventing overheating.
Secondly, to compensate for various losses and ensure effective cooling tower operation, approximately 100 m3/h of mixed water is required. The cooling towers play a vital role in dissipating the excess heat and maintaining the overall efficiency of the system.
Lastly, mitigating direct cooling losses is essential for the hot rolling operation. To achieve this, a substantial supply of approximately 1000 m3/h of mixed water is needed. This water is utilized to cool hot run out tables, steel products, and to facilitate scale breaking, among other related processes.
Most of the unit operations associated with steelmaking are water-intensive, and the total water requirement per hour of steel manufactured is around 180 m3 for BOF at a rate of 0.6 to 0.9 m3/t. Mostly the water in direct and indirect cooling applications in steel plants records thermal losses and seeks make-up water. The heat-intensive application requires demineralized water to evade corrosion-related issues, and pasteurization of the coolant is mandatory after certain cycles in the semi-closed circuit. Disinfection is generally performed using a combination of sodium hypochlorite and biocide. For a few occasions, pH adjustment is also required prior to the recirculation. However, the blowdown can primarily be reused within the loop as make-up water without any prominent treatment. The comprehensive water consumption stands at nearly 10% against the intake volume, including evaporation loss, mechanical loss, and other miscellaneous losses in the ETP.
Presently, most steel plants across the globe operate on the zero liquid discharge ideology. Usually, a combination of electrolytic precipitation, biological digestion, and membrane filtration yields more than 90% of the recovery. Recovery and reuse of treated wastewater are ideal options from both environmental and economic perspectives. However, the hazardous sludge generated from the treatment process has to be handled according to regulatory norms [1][7][9][10][11].
2.2. Wastewater Scenario
The quantity of wastewater discharge is estimated to be 25–26 m3/ton of steel produced, which signifies the quantity of water consumption is not more than 3–4 m3/ton and the rest is lost due to evaporation. The discharge water has a diverse range of pollutants based on the point of generation. The wastewater and effluent generated from hot rolling and coking operations tend to have higher concentrations compared to those generated from cold rolling and scrubbing. In hot rolling, the wastewater contains elevated levels of scale particles, oil, acids, and various contaminants. Similarly, wastewater from coke plants includes pollutants, such as ammonia, phenols, cyanides, PAHs, sulfides, and heavy metals. On the other hand, cold rolling operations typically utilize water-based lubricants or emulsions that have lower environmental impacts. In scrubbing processes, the wastewater is less concerning but still carries a higher concentration of particulate matter and sulfur compounds [7][8][11][12][13]. Source segregation and separate pre-treatment are mandatory for each category mentioned above.
2.3. Sources and Characteristics
The major sources of wastewater generation in the steel processing industry include BF, Electrostatic Precipitator (ESP), and rolling mills. Wastewater generated from the BF is primarily from the washing activity, which includes the washing of gas containers. Thus, the primary pollutant existing in the waste stream is suspended solids. The concentration varies between 1000 and 8000 mg/L, based on factors such as furnace size, mode of operation, blast rate, and optimum pressure exerted. Further, a slip in the furnace can cause an additional solid burden of up to 25,000 mg/L. Further, sometimes semi-colloidal ferromanganese particles can be generated from the furnace that are to be co-flocculated and removed, impinging on additional operational cost. Additionally, washing flue gas containers contributes to the finest dust (particle size: 8–10 microns), adding color to the waste stream [8][11][12]. The activated carbon filter is widely used to mitigate the above issue. ESP generates a lesser volume of wastewater with higher concentrations of hard-to-settle finer particles, which can only be removed by advanced filtration.
The rolling mill’s operation causes scale (higher oxides of iron) formation, and water is used to remove the same from the surface of the steel. Further, water is used as a coolant and transport media for rolled items and scales, respectively. The freshwater consumption usually varies between 450 and 1600 m3/h, depending upon factors such as the rolling process (hot rolling consumes more water than cold rolling) and operational design [7][14]. The characteristics of the wastewater that contains scales vary significantly with the rolled products. Production of slabs and blooms yields scales of coarser nature. Most particles are more than 200 mm and can easily be removed by a coarse screen, followed by sedimentation. Manufacturing billets, tube rolls, and rods contribute to finer scales, complicating the wastewater treatment. The specific gravity of the scale is approximately 4, and the same can easily cause clogging of physical treatment units. If the scales are not effectively removed from the wastewater, they settle at the base of the receiving streams, leading to a decrement in depth and volume [8][11].
Milling operation also involves processes such as pickling and rinsing. The processes are primarily descaling- and finishing-oriented mechanisms which involve extensive acid usage at elevated temperature (~70 °C) [13][15], which generates a corrosive process residue. Though both the effluents have a similar ratio of raw acid and ferrous salt, the pickling water is highly concentrated (four to five times greater than rinse water). The quality of pickling water further varies based on the mode of operation. The continuous mode of stripping operation constitutes about 15% of ferrous salt and 7% of raw acid (H2SO4, HCL, HNO3, H3PO4, and HF) [16]. Contrarily, acid concentration is reduced drastically to ~1% and salt percentage increases to 20% for batch operation, which is relevant from a salt recovery perspective. Despite encountering higher temperatures (75–95 °C), the wastewater stream generated from rinsing does not carry any combined acids, so the treatment is hassle-free [11].
Other prominent contaminants from the rolling operations are soluble and insoluble oils, grease, and other lubricants. The presence of oil both in free and emulsified forms is non-tolerable. Even the smallest volume may cause elevation of the COD level, the formation of film, and anoxic conditions, leading to the failure of the biological treatment unit. The operations of cold rolling, electrolytic lining, and mechanical workshops are the primary sources of soluble oils. These oils are a complex amalgamation of palm oil and synthetic substances, which at higher temperatures can cause emulsion. The treatment becomes challenging when the emulsion blends with detergents and kerosene. The degree of pollution varies proportionally with the recirculation count of the process water. A typical single-used effluent comprises around 150 mg/L of mixed oil with >30% solubility. A significant proportion (~40%) of ETP influent load comes from the cold rolling mill, and the elevated temperature (~120 °C) of it further makes it difficult to treat [5][8][12][13].
Steelmaking is a water-intensive process. Non-intermittent supply of freshwater is a mandate for direct and indirect cooling, scrubbing, and washing. The cooling process generally does not contribute to wastewater generation and only seeks make-up water regularly. Scrubbing and washing yields effluent rich in suspended solids, oils, metals, and other hazardous chemical compounds. The present study identified coke oven (CO) processing as the most hydro-intensive operation with the highest wastewater generation. Further, BF, quenching, chilling and scrubbing of CO gas, and separation of the by-products are identified as the unit operations with ample water and wastewater footprint. An old BF can consume up to 7.6 m3 of freshwater to yield 1 mg of ingot steel. The ingot steel is further processed in the steel-melting shop through the continuous casting mechanism that generates hot wastewater with elevated concentrations of suspended and emulsified solids, oils, and grease. In addition, subsequent rolling operations contribute to effluent rich in scales (100–200 mg/L) and oils (10–25 mg/L). Finally, the spent pickle liquor (SPL) generated from the pickling process with a higher concentration of ferrous salts, heavy metals such as Zn, Ni, Cu, Cr, etc., and highly acidic waters impact the overall effluent quality. The composite process effluent is toxic and carries critical impurities, such as cyanides, heavy metals, phenol, oil and grease, ammonia, etc. SPL is primarily responsible for metal-rich sludge generation from steel mill ETPs. Figure 3 summarizes the wastewater cycle of the steel industry [8].
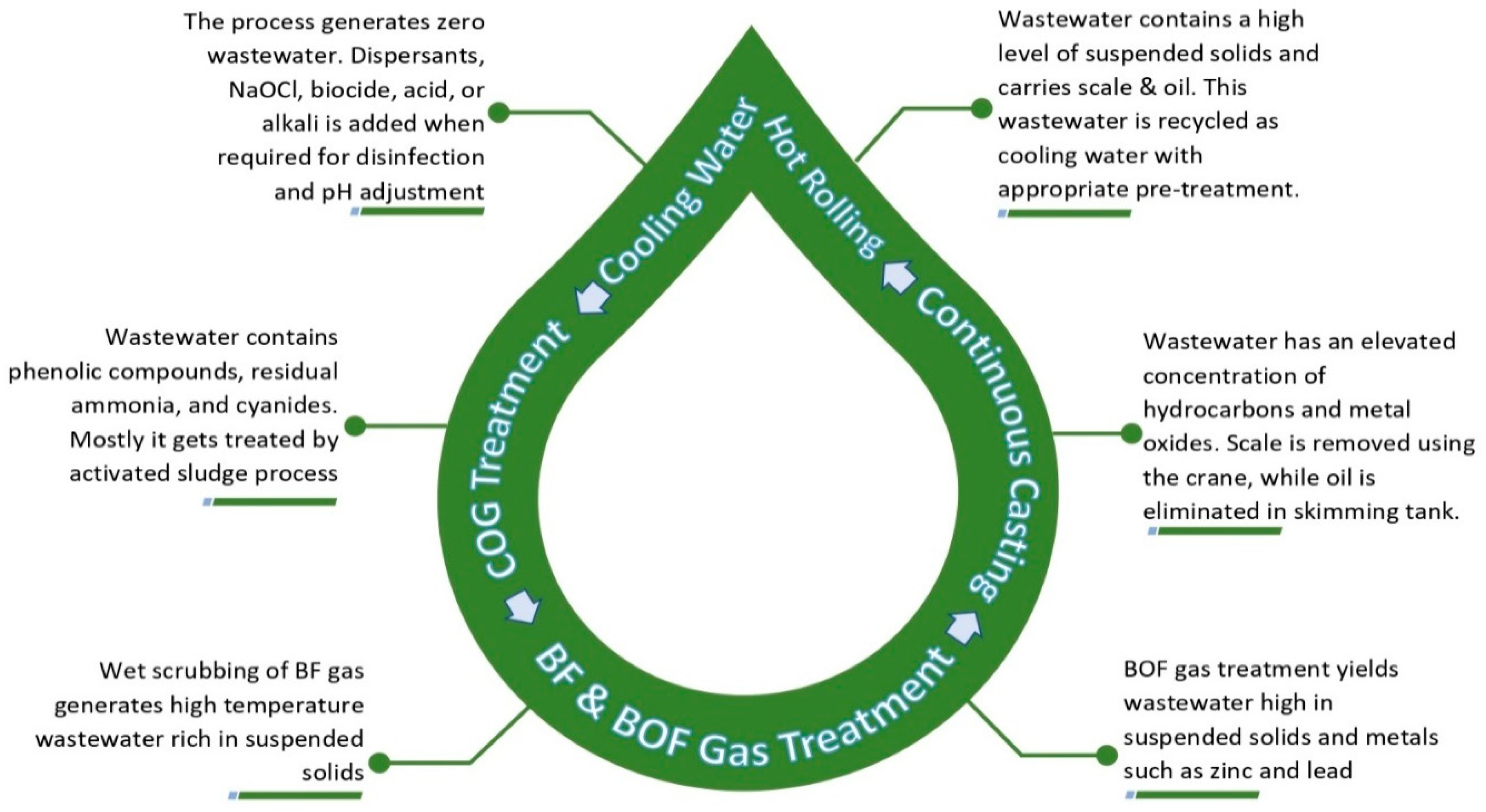
Figure 3. Steel industry wastewater characteristics cycle.
2.4. Treatment of Wastewater
The wash water from the flue gas chamber primarily contains settleable solids of higher specific gravity. Rectangular or circular settling tanks can easily eliminate the same with a retention period of around 12 h. Comparatively, fresher establishments prefer circular vortex flow clarifiers or tube settlers for rapid settling with minimal retention period (1–2 h). The effluent from the basin is separated using the overflow method, and it may contain suspended solids up to 100 mg/L. If feasible, the sludge is mechanically removed and sent to the filter press for further dewatering and recovery of essential salts (Fe2O3 and CaO) [12][13].
The scales generated from rinsing and pickling operations are of higher specific gravity and tend to settle quickly and easily. The scales are hazardous and thus should be stabilized and safely disposed of in secured landfills. The wastewater generated from the rolling operations carries free oil alongside the solids. The solids contributed from cold rolling are finer than that of hot rolling, hence the method of settling should be designed accordingly. A standard settling tank of dimensions 9 × 5.5 × 2.5 m is recommended for effluent from hot rolling, whereas a tube settler is recommended for cold rolling mill effluent. The sludge contains higher concentrations of usable nitrates that can be retracted through resource recovery. The oil issue should be addressed by a skimming tank, and its concentration should be lowered below 10 mg/L before releasing/reusing. Recently, industries have started utilizing cleansing solutions of an alkaline nature, such as NaOH, Na2CO3, phosphates, and silicates, to remove oils before the effluent enters the ETP, thereby eliminating the need for skimming. Therefore, additional FOG removal need not be performed to avoid membrane fouling in the advanced treatment systems [8][11][13].
This entry is adapted from the peer-reviewed paper 10.3390/w15122177
References
- Backman, J.; Kyllönen, V.; Helaakoski, H. Methods and Tools of Improving Steel Manufacturing Processes: Current State and Future Methods. IFAC-Pap. Online 2019, 52, 1174–1179.
- Buchmayr, B.; Degner, M.; Palkowski, H. Future Challenges in the Steel Industry and Consequences for Rolling Plant Technologies. BHM 2018, 163, 76–83.
- Silva, F.; Carvalho, A. Evaluating the financial health of the steel industry. In Directorate for Science, Technology and Innovation Steel Committee; Organization for Economic Co-operation and Development: Paris, France, 2015; Available online: http://www.oecd.org/industry/ind/Evaluating-Financial-Health-Steel-Industry.pdf (accessed on 20 June 2019).
- Lambert, P. Sustainability of metals and alloys in construction. In Sustainability of Construction Materials; Khatib, J.M., Ed.; Woodhead Publishing: Cambridge, UK, 2016.
- Labiapari, W.S.; Alcântara, C.M.; Costa, H.L.; Mello, J.D.B. Wear debris generation during cold rolling of stainless steels. J. Mater. Process. Technol. 2015, 223, 164–170.
- Hattalli, V.M.; Srivatsa, S.R. Sheet Metal Forming Processes—Recent Technological Advances. Mater. Today Proc. 2018, 5, 2564–2574.
- Colla, V.; Matino, I.; Branca, T.A.; Fornai, B.; Romaniello, L.; Rosito, F. Efficient Use of Water Resources in the Steel Industry. Water 2017, 9, 874.
- Das, P.; Mondal, G.C.; Singh, S.; Singh, A.K.; Prasad, B.; Singh, K.K. Effluent Treatment Technologies in the Iron and Steel Industry—A State of the Art Review. Water Environ. Res. 2018, 90, 395–408.
- Matino, I.; Colla, V.; Romaniello, L.; Rosito, F.; Portulano, L. Simulation techniques for an Efficient Use of Resources: An overview for the steel-making field. In Proceedings of the 2015 World Congress on Sustainable Technologies, London, UK, 14–16 December 2015; pp. 48–54.
- Arjmandi, S.; Tabesh, M.; Esfahani, S.T. Risk Analysis of Water Reuse for Industrial Cooling Water Consumptions. J. Environ. Eng. 2019, 145, 04019067.
- Sun, W.; Xu, X.; Lv, Z.; Mao, H.; Wu, J. Environmental impact assessment of wastewater discharge with multi-pollutants from iron and steel industry. J. Environ. Manag. 2019, 245, 210–215.
- Gao, C.; Gao, W.; Song, K.; Na, H.; Tian, F.; Zhang, S. Comprehensive evaluation on energy-water saving effects in iron and steel industry. Sci. Total Environ. 2019, 670, 346–360.
- Sinha, V.K. Study on the Waste Water Treatment Technology for Steel Industry Recycle and Reuse. Ph.D. Dissertation, Jharkhnad Rai University, Ranchi, India, 2016.
- Nezamoleslami, R.; Hosseinian, S.M. Data needed for assessing water footprint of steel production. Data Brief 2020, 30, 105461.
- Henriot, G. Advanced techniques for rolling mill gearing. J. Manuf. Sci. Eng. 1973, 95, 1101–1107.
- Zhang, J.; Chen, G.; Ma, Y.; Xu, M.; Qin, S.; Liu, X.; Feng, H.; Hou, L. Purification of pickling wastewater from the steel industry using membrane filters: Performance and membrane fouling. Environ. Eng. Res. 2022, 27, 200486.
This entry is offline, you can click here to edit this entry!
