Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Salivary gland carcinomas (SGCs) are a diverse collection of malignant tumors with marked differences in biological activity, clinical presentation and microscopic appearance. Although the etiology is varied, secondary radiation, oncogenic viruses as well as chromosomal rearrangements have all been linked to the formation of SGCs. Epigenetic modifications are any heritable changes in gene expression that are not caused by changes in DNA sequence. It is widely accepted that epigenetics plays an important role in SGC development.
- Salivary gland cancers
- epigenetic modifications
- DNA methylation
- noncoding RNAs
- histone modifications
1. Introduction
The embryonic development of the tubulo-acinar exocrine organ known as the salivary gland begins between week 6 and week 8 of intrauterine life. Submandibular and sublingual glands originate in the embryonic endoderm, while the parotid gland is thought to develop from the oral ectoderm [1]. Salivary glands have a two-tiered structure with luminal (acinar and ductal) and abluminal (myoepithelial and basal) cell layers. Rapid entry into the cell cycle makes these cells vulnerable to neoplastic transformation [2]. Salivary gland carcinomas (SGCs) are uncommon compared to the other carcinoma types but are common in the context of head and neck tumours [3,4]. Salivary gland carcinomas (SGCs) account for between 3–6% of all head and neck malignancies. The parotid gland is the most commonly involved, especially by benign type followed by the submandibular gland and the minor salivary glands. Among the malignant histological subtypes are mucoepidermoid carcinoma (MEC), carcinoma ex pleomorphic adenoma, intraductal carcinoma, acinic cell carcinoma, adenoid cystic carcinoma (ACC), and carcinosarcoma [5,6,7]. Mucoepidermoid carcinoma is further classified into a low-grade and high-grade tumor where the treatment approaches are significantly differed. It is challenging to get earlier diagnosis of these SGCs and deliver adequate treatment due to existing high histological heterogeneity.
Salivary gland carcinomas (SGCs) are exceptionally rare, hence very little is known about their etiology. A few studies have reported that alcohol consumption, tobacco use, diet high in animal fat and low in vegetables, and heavy cell phone use are associated with an increased risk of SGCs [8,9]. Radiation exposure (such as radiotherapy to the head and neck) and certain occupational exposures (such as silica dust, nickel alloy dust, asbestos, and rubber products manufacturing and mining) have also been implicated [9]. A history of cancer [10] and perhaps exposure to the human papillomavirus [11], Epstein Barr virus [9], and HIV [12] have also been identified (Figure 1).
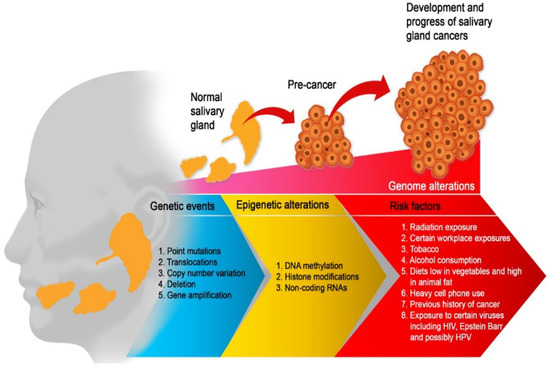
Figure 1. Etiological factors of salivary gland carcinoma. Multiple factors are working together to drive SGC from a few aberrant cells to a tumour phenotype with the capacity to metastasis. Therefore, the optimum environment for malignant development is maintained by a complex interplay of genetic events, risk factors, and epigenetic mechanisms. All of these factors work together to promote an unstable genome and hence, promote cancer progression.
Epigenetic and genetic changes have been proposed as etiological variables, but there are yet few research investigating its function in SGT (Figure 2) [1,3,13]. Epigenetic events can take the form of DNA methylation, alterations in the expression of non-coding RNAs such as microRNAs (miRNAs), or abnormalities in the structural modification of histones [14,15,16]. Several cancers, including SGCs, develop and progress due to epigenetic alterations that cause considerable changes in gene expression [3]. In addition, significant genetic alterations have been documented in all SGCs, and these alterations can be grouped according to their role in prediction, diagnosis and prognosis [13].
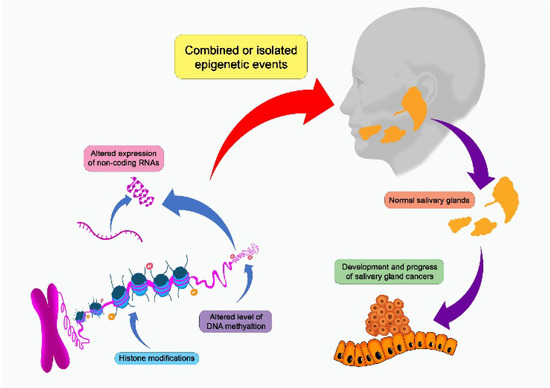
Figure 2. Salivary gland carcinomas can be affected by a number of epigenetic events that can alter the development and progression of the cancer.
2. Epigenetics Mechanisms
Epigenetics is a broad word that refers to molecular mechanisms that affect gene expression without altering the DNA base sequence. Transcription regulators, epigenetic writers, gene imprinting, histone modification as well as DNA methylation are important epigenetic processes implicated in gene expression alterations (Figure 2) [17]. DNA methylation involves transformation of methylated cytosine by treatment with sodium bisulfite, into thymine and two distinct probes which used to target each site of CpG [18]. Mechanism of histone modifications involves chemical post-translational modifications (PTMs) such as sumoylation, ubiquitylation, acetylation, phosphorylation as well as methylation, to the histone proteins, that causes chromatin structure to change or attract histone modifiers [19]. Another epigenetic mechanism is genomic imprinting that impacts a small group of genes, resulting in monoallelic expression of genes which is parental specific origin in manner. Gene expression as well as genomic region compaction are controlled by epigenetic alterations, which are produced by specific enzymes called as “writers” and eventually identified by the effector proteins called as “readers” and removed by erasers, all of which together contribute to the regulation of gene transcription, and abnormalities can result in tumor formation as well as development [20]. Additionally, the creation of a different research known as nutrigenomic results from epigenetic regulation via diverse nutritional substances [21,22].
3. Epigenetic Alterations in Salivary Gland Tumors
In scope of head and neck neoplasms, Salivary gland carcinomas (SGCs) are particularly prevalent, considering their uncommonness in comparison to other malignancies [73]. These lesions possess assorted or diversified collection of benign as well as malignant tumors exhibiting great variation in their clinical demonstration as well as microscopic presentation, and their biological activity varies in accordance with lesion [74]. On account of their unexpected biological as well as clinical activity, these tumors offer great difficulty in management, which can result in therapeutic failure [44]. SGCs account for 5% of all the head and neck malignancies, including a worldwide yearly incidence ranging from 0.4–13.5 cases per 100 individuals [75]. More than thirty tumors have been identified by world health organization (WHO) stratification of head and neck cancers (HNCs). The mucoepidermoid carcinoma (MEC) have been considered as the most prevalent malignant carcinomas. Multiple investigations on this issue have been conducted; nevertheless, causative elements are still unclear, while chemotherapy, chromosomal rearrangement as well as secondary radiation, can be linked to progression of SGCs.
The epigenetic changes have been proposed as causative variables, however, there have been an infrequent investigation examining their relevance in SGCs [3]. Variations in DNA sequence altering the protein expression, but do not change the order of nucleotide bases are characterized as epigenetic. Epigenetic alterations are important in physiological activities such as replication, transcription as well as DNA repair. As a result, changes in these pathways might result in formation as well as development of several neoplasms [14]. Epigenetic variations have been classified into three types such as: methylation of DNA (Table 1), histone’s structural modifications as well as variable expression of noncoding RNAs such as microRNAs (miRNAs) [14,15,16]. These epigenetic modifications cause a widespread downregulation of gene expression patterns, which leads to progression as well as development of a variety of cancers such as SGCs [15,22,23,24]. Although, these processes are changeable hence, a complete knowledge of these alterations may lead to the identification of novel therapeutic targets for a variety of disorders, such as cancer [14,15,16]. Recent investigations have suggested that these three epigenetic changes might be implicated in formation as well as progression of SGCs [3]. However, the majority of studies have focused on malignant SGCs. It has been established that epigenetic changes can also contribute to benign SGCs [23]. Various approaches as well as models such as (human biopsy tissue and cancer stem cells) are being used to explore these modifications, with genomic research revealing the epigenetic landscape of SGCs [15,23,24,28]. However, several investigations have been conducted, elucidating comprehensive and detailed function of epigenetic modifications in SGCs. A limited review is available that summarizes recent information on this genetic pathway.
Table 1. Description of methylated genes contributing to the pathogenesis of SGCs.
| Carcinoma Type | Methylated Genes | References |
|---|---|---|
| Mucoepidermoid Carcinoma | P14, CLIC3, CDH1, APC, Mint1, PGP9.5, Timp3, p16(INK4A), RUNX3, DAPK, MGMT, RARβ2 and RASSF1 | [3,11,23] |
| Adenoid cystic carcinoma | P15, p18, p19, p21, APC, Mint1, PGP9.5, Timp3 Cyclin-dependent kinase inhibitors (p27), HCN2, AQP1, SBSN, RUNX3, DAPK, MGMT, RARβ2 and RASSF1 | [3,26,28,31] |
| CA-Ex-PA | RASSF1, p53, p16(INK4A), promoter methylation in CDH1, P14ARF | [29,32,41] |
| Acinic cell carcinoma | RASSF1 (Ras association domain family protein1 isoform A) and RARβ2, DAPK, and MGMT | [3] |
3.1. DNA Methylation in Mucoepidermoid Carcinomas (MECs)
DNA methylation, a crucial pathway of regulating gene expression, has been linked to neoplasm formation as well as metastasis. In fact, hypermethylation has been proposed as one of the primary pathways for inactivation of tumor suppressor genes (TSGs) [76]. A study conducted by Nikolic et al. in 2018 [23], reported methylation of p53 (TSG) in thirty-five cancer samples as well as repressing of p14ARF (an important regulator of p53 (TSG) function). These events can contribute to the major processes implicated in pathogenesis of mucoepidermoid carcinoma (MECs). However, this is only a qualitative methylation study, and hundred percent of cases indicated epimutations. Even with normal (wild type-unmethylated) p53, a cell would be unable to repair damage if p14 is hypermethylated or otherwise inactivated. Study conducted on aetiology of MECs, by Nikolic et al. [23], confirms significance of epigenetic p14 inactivation, is in line with earlier investigations on carcinoma ex-pleomorphic adenoma (CA-Ex-PA) [77]. Nishimine et al. examined seven MEC specimens for detection of p14 variations and reported no methylation in any specimen, with one deletion. p14 promoter methylation was found to be 19.4 percent while considering intact salivary gland carcinoma (SGC) specimen with dominant adenoid cystic cell carcinoma (AdCC) [78]. Hypermethylation of p14 promotor had been discovered by Ishida and colleagues, in twenty percent specimens of oral squamous cell carcinomas (SCCs). This observation showed a substantial correlation with subsequent clinical presentations, indicating that it might be a critical molecular process in cancer development [79].
Cyclin dependent kinase inhibitors (CKIs) are encoded by p16INK4a TSG, that plays a crucial role in controlling cell-cycle at G1/S phase inspection-point. Lack of a functioning p16 protein result in abnormal regulation of cell-cycle, promoting cancer cell growth. Nikolic et al. [77] observed 60% MEC specimens with hypermethylation of p16, this investigation is consistent with prior studies revealing significance of epigenetic process in SGCs, with rates ranging from 29% to 47% [31,42,59]. Guo et al. [42] investigated thirty-four percent p16 methylation and found no p16 methylation in specimens of MECs. Weber et al., on the other hand, determined that aberrant INK4a-ARF/p53 pathway by various processes found to be a highly common occurrence (84%) in HNC squamous cell carcinomas (SCCs) [59]. Changed levels of TP53 methylation are linked to a variety of cancers such as oral malignancies [80]. Nikolic et al. reported TP53 hypermethylation as an infrequent occurrence in SGCs, but it has increased occurrence of p14 methylation. Specifically, rare TP53 hypermethylation prevents p53 preclusion from pathogenic event, however, instead reinforce the concept that silencing of p14 leads to an inactivation of p53. Hypermethylation of TP53 has been reported by various researchers, in malignant tissues [81], although TP53 hypermethylation as a typical occurrence in healthy cells reported by others [80], which is consistent with ramifications of Nikolic et al.
Function of hTERT gene as well as of telomerase is controlled by significant monitoring systems which include its promotor methylation, but this is occasionally contradicted by generic pattern of DNA methylation as pathways for gene repression [82]. According to Renaud et al., enabled transcription as well as hTERT inhibitors binding arrest are the results of methylated CpG island in hTERT promoter [83]. Nikolic et al. [23], reported malignancies with a significantly greater frequency of hypermethylated hTERT than controls, indicating that formation of MECs may involve this molecular process. Methylation of hTERT is linked with reduced histological grading as well as clinical phases, suggesting that it plays a function in early stages of carcinogenesis. However, methylation of p16 is linked with worse survival rate in HNCs [84], ramifications identical to Nikolic et al. [23], reported that p14 and/or p16 had no effect on survival rate in HNSCCs [85], oral as well as oropharyngeal tumors [86].
The methylation patterns of genes implicated in angiogenesis, cell-cycle regulation and/or DNA repair are frequently abnormal in neoplastic cells [76]. Toyota et al. suggested CpG island methylator phenotype (CIMP). All malignancies were divided into two groups, group 1 is methylation of complete genome and group 2 is the infrequent methylation events, according to the investigators. The first group is more susceptible to promoter methylation-mediated transcriptional suppression of many TSGs [87].
Sasahira et al., discovered that dysregulation of RUNX3 due to DNA hypermethylation as well as protein mislocalization, was substantially linked to metastasis of MEC as well as AdCC, cancer development and SGCs. The promoter region of RUNX3 gets methylated which leads to an inactivation of RUNX3, reported in several carcinomas [43]. SGCs reported to have frequent inactivation of RUNX3 as compared to intact salivary gland tissues, as well as dysregulation of RUNX3 is linked to low survival rate in MEC and AdCC [43].
3.2. DNA Methylation in Adenoid Cystic Carcinoma (AdCC)
While we are learning more about genetic changes in AdCC, epigenetic environment is still mostly unclear. An effort has been made by Ling et al. to discover significantly methylated genes to better understand the pathogenesis of AdCC [39]. The modulation of oncogene as well as TSG expression by methylation of DNA promoter is critical in tumorigenesis of AdCC and can contribute to several types of human malignancies [88]. Indeed, AdCC methylome has been analyzed and four genes have been verified [89]. Ling et al., adopted a xenograft-based process involving methylation patterns of complete genome as well as pharmacological demethylation, since viable cell lines were unavailable for the research [90]. Ling et al., reported hypomethylation of HCN2 (potential oncogene) promoter in AdCC cases by using an indifferent inspection for methylated gene promoters [39]. Oncogenic HCN2 may contribute to the AdCC pathogenesis, because of hypomethylation of its promoter. Moreover, HCN2 promoter’s hypomethylation is associated with distant metastasis, recurrence as well as local recurrence of AdCC primary cancers. However, k+ and Na+ are usually conducted by HCN2 [91,92] and HCN2 is permeable to Ca2+. It has been proposed that overexpression of HCN2 may engage in pathogenic Ca2+ signaling [93].
Daa et al., investigated methylation patterns of several CKI genes, focusing on p27 expression [26]. A low occurrence of p27 (26.5%) methylation was reported but, p21, p19, p18 as well as p15 showed high occurrence of methylation ranging from 68.8% to 92.3% [23]. These findings complemented as well as broadened the investigations of Li et al. [31] and Maruya et al. [94], reporting that CKI genes are associated with frequent methylation in AdCC. Promoter methylation suppresses gene expression, considered as a general assumption. As a result, findings of Daa et al., suggested that multiple CKI gene expression may be reduced in AdCC [26]. Furthermore, significant prevalence of CKI gene promoter’s methylation indicates that abnormal methylation occurs early in the course of ACC. Daa et al., reported, nuclear p27 expression in all AdCC specimens including healthy salivary gland cells surrounding AdCC cells [26], validating investigations of Takata et a [95]. Accumulation of p27 is widely recognized in nucleus of tumor cells in the G0 phase, identical to healthy salivary gland cells, as well as acts as a cell cycle inhibitor [96]. Investigations of Daa et al., point to p27 downregulation as a potential contribution to AdCC carcinogenesis [26], with methylation as the likely pathway behind this dysregulation, as has been documented in other malignancies [95,96,97,98,99]. There are various probable explanations for dysregulation of p27 in cancer that is linked to DNA methylation level. Another explanation is that the cancer cells differ in their p27 promoter’s methylation level. In mammalian DNA, methylation occurs at cytosine residues, following cell division by DNA methyltransferase, only certain cancer cells have methylated genes at any one moment. Another possibility is that methylation occurs exclusively in one allele of a gene. p27 would be expressed by various cancer cells in this case, and the PCR product produced in MSP would only use M-primers [26].
In several forms of HNCs, including oral squamous cell, laryngeal, thyroid as well as nasopharyngeal, methylation of a promoter is a frequent process underpinning the inactivation of a variety TSGs, including MGMT, hMLH1, DAPK, RASSF1A, p16INK4a as well as E-cad [100]. Methylation of promoters of DAPK, RASSF1A and 16INK4a was found to be frequent in AdCC of salivary gland [31]. Zhang et al., reported methylation of E-cad promoter in 57 percent of individuals having AdCC of salivary glands, with greater incidence of any of the five gene promoters studied in same cancer [28]. Findings of Zhang et al. [28], were similar with previous evidence in which methylation of E-cad promoter was found in seventy percent of individuals with AdCC of salivary gland [35]. Zhang et al., reported E-cad promoter methylation was linked to a decrease in expression of E-cad protein, in AdCC patients, indicating methylation of E-cad causes a dysregulation of E-cad protein [28].
3.3. DNA Methylation in Carcinoma Ex-Pleomorphic Adenoma (Ca Ex-PA)
DNA methylation is identified to have a role in tumor progression and growth, as well as determining the methylation level of TSGs constitutes a promising method for initial cancer identification [76]. Cancerous cells frequently have abnormal methylation of several genes, including those that control angiogenesis, DNA repair and cell cycle [101]. There is a scarcity of data on the epigenetics of SGCs, especially the methylation levels of p14 as well as p16. Nikolic et al., investigated p14 as well as p16 TSG promoter’s hypermethylation as a frequent occurrence in CXPA based on its significant incidence, despite its lack of diagnostic significance [77]. DNA methylation level is influenced by ethnicity as well as race, which may elucidate greater prevalence of p14 as well as p16 promoter hypermethylation identified in a study conducted by Nikolic et al. [77]., when compared to earlier publications [102,103,104].
Nikolic et al., suggest that an increase in telomere length or telomeric instability, can contribute to the etiology of CXPA. Importantly, the CXPA showed significant telomere length variability, which is commonly associated with (alternative lengthening of telomeres) ALT phenotype. Findings of Nikolic et al., showed that hypermethylation of p14ARF results in extended telomeres (p = 0.013), as well as while identical findings have not been discovered in literature, they support the idea that p14 silencing impacts p53-associated cancer suppression [77].
Gene expression changes are primarily conducted by epigenetic as well as genetic methods. Whereas epigenetic variations cause transcriptional changes, genetic variations generally modify the structure or number of specific gene [105]. Methylation of CpG island in promoter area is a typical epigenetic technique for altering gene expression. This regulation is mostly accomplished by the inactivation of TSGs RASSF1A, DAPK, MGMT as well as p16. In several cancers, the silencing of E-cadherin is primarily determined by modified methylation level of CDH1 promoter [105,106,107]. Silencing of CDH1 is linked to advanced tumor stage and an aggressive character [105]. This is the first study to look at the methylation status of the CDH1 promoter’s methylation level in carcinoma Ex-pleomorphic adenoma (CXPA), first time investigated by Xia et al. [29]. Investigations of Xia et al., found a link between methylation of CDH1 promoter and E-cadherin expression [29]. They identified lack of E-cadherin expression in 35.14% (13/37) of patients with CXPA. These investigations are comparable to the findings of Zhang et al. [49] reporting, negative identification rate of 38.33% in sixty AdCC patients. Although, no expression of E-cadherin was identified in 87.26% (18/23) of oral squamous cell carcinoma (SCC) patients as well as in 68.42% (26/38) of eyelid SCC patients. According to Xia et al., Bisulfite-assisted genomic sequencing PCR (BSP) is considered as the gold standard method which not only identifies the DNA methylation at each CpG location separately but also identifies methylation of CDH1 promoter [29]. Xia et al., investigated 67.57% (25/37) methylation rate of CDH1 in CXPA [29]. This percentage is identical to multiple other carcinomas, such as: colorectal cancer (52%) [108] breast cancer (65–95%) [108,109,110,111] as well as primary lung carcinoma (88%) [112]. Selective DNA methylation has been reported by Xia et al., in the first four CpG regions compared to the remaining CpG regions [29].
The relationship between E-cadherin expression as well as CDH1 methylation was determined by Xia et al., in cases with CXPA [29]. In clinical cases, CDH1 methylation was shown to be strongly linked with lower expression of E-cadherin (p < 0.001). Furthermore, Xia et al., examined methylation level of CDH1, associated CDH1 mRNA as well as protein levels in SM-AP1 (stromal membrane-associated protein 1) and SM-AP4 cell lines. Cells with increased CDH1 methylation levels expressed less E-cadherin, which was consistent with the previous findings. Although, according to investigations of Xia et al., in each case, decreased levels of E-cadherin expression were not related with promoter methylation of CDH1 [29]. One sample from a low-methylation group showed no expression of E-cadherin. Apart from promoter methylation, there are other pathways which result in suppression of CDH1 production including translational as well as post-translational control, particular transcriptional factors, inactivating gene mutations, loss of heterozygosity (LOH) at 16q22.1 and changes in chromatin structure [113,114,115].
Hence, it can be proposed that DNA methylation in patients with CXPA, predominantly but not completely control levels of E-cadherin expression, both in vitro as well as in vivo. Further research into other regulatory pathways controlling CDH1 in CXPA may be conducted. Reduced expressions of E-cadherin have been linked to tumor recurrence, metastasis and invasion in individuals with breast [111], bladder [116], and oral squamous cell carcinomas [117].
p16INK4a reported no specific variations or microdeletions in any exon in forty-two patients of PA, although twenty eight percent patients reported p16INK4a promoter methylation, which associated with lack of mRNA production. Augello reported fourteen percent hypermethylation of p16INK4a TSG promoter. This disparity between research of Augello et al., and the other data is most likely attributable to dietary influence. As previously documented, TP53 variations as well as p16INK4A methylation arise only in epithelial elements of PAs, indicating that these portions of adenoma may possibly progress into tumor [118].
3.4. DNA Methylation in Acinic Cell Carcinoma
Other benign as well as malignant neoplasms were investigated for variations in epigenome of SGCs (Table 1). RASSF1 and retinoic acid receptor beta2 (RARβ2) genes were found to be frequently methylated in salivary duct carcinoma as well as acinic cell carcinoma. The nuclear receptor superfamily includes human RARβ2 as a member of this family, which performs an important function in controlling the effects of retinoic acid on cell proliferation as well as differentiation. Lack of RARβ2 expression has been linked to the development of mammary ductal carcinoma.
The Histone hypoacetylation H3 (lys9) was shown to be notably prominent in malignant SGCs in contrast to benign cancer in intriguing research incorporating 84 samples of SGCs (42 malignant and 42 benign) [44]. Researchers also found that acetylated tumour cells were less likely to multiply [44]. Thus, unlike in other malignancies like breast cancer as well as pancreatic adenocarcinoma, H3 acetylation has a negative effect on proliferation in SGCs [44]. This is likely because various tissues and organs have unique processes that contribute to tumor formation and can function in opposite ways.
Salivary ACC is an aggressive kind of SGC, and research into histone changes is progressively advancing towards the quest for novel target therapy [45]. Long-term survival is less encouraging because recurrent tumors and metastasis to distant organs such as liver, bones and lungs are common side effects of therapy for this malignancy [45]. Additional research found that multiple genes involved in chromatin remodeling (such as AT-rich interaction domain containing 5B [ARID5B], lysine-specific demethylase 5A [KDM5A], SW/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily A, member 2 [SMARCA2], and chromodomain helicase DNA-binding protein 2 [CHD2]) were aberrant in 50% of AdCC [119,120]. Mutations in chromatin regulator genes were found in 35% of ACC tumors in another investigation [102].
Out of the 22 malignant SGT patients, Pouloudi et al., reported that 14% were HDAC-1 positive (3 cases), 82% were HDAC-2 positive (18 cases), 36% were HDAC-4 positive (8 cases), and 18% were HDAC-6 positive 4 cases [121]. However, Pouloudi et al., did not find any significant relationships between gender or age of the patients and HDAC expression, previous research has revealed similar links across a variety of carcinoma types. Specifically, research has linked male genders and younger age group of patients to elevated HDAC-1 expression in mobile tongue SCC [122], whereas in gastric carcinoma [123] it has been linked to older age group of patients. By contrast, instances of invasive ductal breast cancer where HDAC-6 expression was high had younger individuals [124].
3.5. Non-Coding RNAs Contributing to SGCs
Expression of many different genes may be influenced by a class of RNA (transcribed) molecules called noncoding RNAs (ncRNAs) [15,16,125,126]. SGCs may be more aggressive and develop at a faster rate if miRNAs play a role in their development [46]. One of the most upregulated miRNAs in MEC tissues was miR-302a, and in-vitro, its overexpression in SGT cell lines led to cancer cell invasion [46]. The t (11;19) translocation that causes CRTC1-MAML2 gene fusion is a significant oncogenic driver for the development of MECs that cause various disorders [127]. Recent research has identified a particular lncRNA (LINC00473) as a key regulator of the oncoprotein CRTC1-MAML2 [47]. Furthermore, bioinformatic research found that over 3091 lncRNAs were changed throughout the pathogenesis of MEC; however, the clinicopathologic relevance of these results required additional study [52]. Additionally, miR-34a and miR-21 were elevated, while miR-20a was downregulated, and MEC lacked the majority of related-apoptosis transcripts, when studying the expression of transcripts and miRNAs implicated in the regulation of apoptosis in MECs [51]. Furthermore, interaction of other genes involved in this malignancy with lncRNAs has also been reported, however the mechanism associated with this connection is not entirely known [50].
3.6. Tumour to Tumour Interactions
Tumour to tumour interaction plays critical role in the carcinogenesis. Imperatively, there are multiple factors and proteins produced by the tumour and these tumoural markers create a complex interaction in the tumour ecosystem. The interaction of some of these markers in the tumour microenvironment has significant impact on the tumour biology and characteristics. For instance, the intratumoural lymphocytes has been associated with high risk of neck nodes metastasis and high-grade tumours in acinic cell carcinoma [128]. This is likely due to the presence of PDL1 expression in higher level in association with elevated immune cell infiltration of T and B cells. This underlies why the acinic cell carcinoma has been shown to have unfavourable prognosis and has higher risk of lymph node metastases.
Additionally, the process of cancer metastases is important as it influence the prognosis of patient with SGCs. The cancer cells infiltrate the lymphatic and blood vessels through the migration of extracellular matrix, where the main enzyme systems of MMPS is required, and this is located in the invadopodia of cancer cells. In adenoid cystic carcinoma, Lissencephaly 1 (L1S1) regulates the invadopodia formation and has been shown to associate with matrix metalloproteinases (MMPs) expression. Lissencephaly L1S1 is a microtubule associated protein which regulates the microtubules stability, and it can mitigate the metastatic potential of ACC through the invadopodia formation and ECM degradation [129]. Also, integrin linked kinase (ILK), play important role in ECM interactions, with presence of other cofactors such as growth factors and integrin, which regulates cells differentiation, migration and apoptosis. This has positive roles in tumour progression and transformation [130].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15072111
This entry is offline, you can click here to edit this entry!
