1. Introduction
Freshwater fishes include diverse lineages collectively summing to over 18,000 species, representing ~51% of all fishes and ~25% of all vertebrates [
1,
2,
3,
4]. Freshwater fishes are important economically, culturally, aesthetically, scientifically, and educationally [
5]. They provide the basis for human nutrition, employment, and ecosystem services, including nutrient transport within aquatic ecosystems, disease vector control, and seed dispersal [
5,
6]. About one-third of all freshwater species occur in the Neotropics, with an estimated 4475 species within 71 families [
7], with new species being recognized regularly. This assemblage, however, faces a high level of anthropogenic pressure and is threatened by overexploitation, water pollution, river flow modification, degradation of habitat, and invasive exotic species [
5,
6,
8]. Studies of the ecology of many Neotropical species are relatively scarce in the peer-reviewed literature, limiting the basis for science-based actions for fisheries management and conservation.
Catfishes of the Order Siluriformes include 39 families with 498 accepted genera and 4123 species [
4]. More specifically, members of the Family Pimelodidae, the long-whiskered catfishes, and the genus
Pseudoplatystoma are distributed from South America to southernmost Mexico [
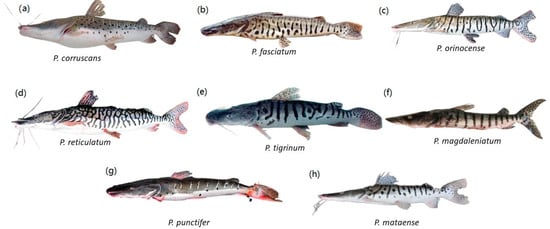
9]. They are a highly distinctive group of catfishes, with three pairs of barbels, maxillary barbels the length of their bodies, no scales, and well-developed adipose fins [
9] (
Figure 1). Many long-whiskered catfishes grow to be over 1 m in total length. The Family Pimelodidae includes 30 genera and 116 described species [
4], and many valid pimelodid species have been described only recently [
7]. Lundberg et al. [
10] suggested that there are at least 25 undescribed living species, as well as five described and many undescribed fossil species.
Figure 1. Eight Pseudoplatystoma species, members of the Family Pimelodidae; (a) P. corruscans; (b) P. fasciatum; (c) P. orinocense; (d) P. reticulatum; (e) P. tigrinum; (f) P. magdaleniatum; (g) P. punctifer; (h) P. mataense. Illustrations courtesy of Timm, C.D.; Magalhães, K.; Alvarez, F.; Sabaj Pérez; and F. Duponchelle.
Fishes of the genus
Pseudoplatystoma support important commercial and artisanal fisheries, and some species have become important to regional aquaculture [
11]. Most species of the genus are under pressure from dam construction and overfishing [
12,
13,
14,
15,
16,
17,
18]. Two species are of conservation concern:
P. corruscans is classified as Critically Endangered [
13] and
P. magdaleniatum as Endangered by IUCN [
19]. Relatively few studies have focused on species of the genus
Pseudoplatystoma, and further information on their biology, ecology, and population dynamics is needed to inform fisheries management and conservation.
2. What Species Are in the Genus, and Where Are They Distributed?
2.1. Natural History
An understanding of the natural history of the lineage provides insights into the geographic distribution of
Pseudoplatystoma species. The first and so-far only reported fossil record for the genus is a neurocranium recently discovered from late Pleistocene deposits of the Cacarana River of Argentina, which was assigned to the extant species
Pseudoplatystoma corruscans [
20]. The fossil record of freshwater fishes in South America is not as well studied as that of terrestrial mammals [
21]. Lundberg et al. [
10] present a map showing sites where fossils from the Neogene period, spanning from the end of the Paleogene Period 23 million years ago (MYA) to the beginning of the present Quaternary Period 2.6 MYA, have been found, suggesting that additional finds might be made with effort directed there.
The systematics and taxonomy of the genus
Pseudoplatystoma can be better understood in the context of the biogeological history of South America.
Pseudoplatystoma has diverse species because extensive geomorphological and physiographic processes have transformed South American drainages over time, allowing vicariance, divergence, and subsequent secondary contact of lineages [
22,
23]. Fishes of the genus
Pseudoplatystoma are currently distributed across four of the eight zoogeographic regions of South American freshwater fishes described by Géry [
24], from north to south: the Magdalenian, Orinoco, Guinea-Amazonian, and Paranean regions. Géry [
24] emphasizes that there are or have been connections between some South American river systems, some permanent (e.g., the Cassiquiera Canal joining the Rio Negro of the Amazon and the Orinoco drainages), some temporary (due to flooding in the rainy season), and some historical but no longer in existence. Current patterns of species distribution reflect these biogeographical processes, including marine incursions and the uplift of paleoarches, or ancient ridges [
25].
2.2. Systematics and Taxonomy
The isolation of freshwater refugia permitted allopatric speciation, and subsequent recolonization of the lowland allowed the mixing of the respective lineages. Hubert and Renno [
25] showed seven putative dispersal routes that gave rise to different species’ distributions. Against this background, it becomes apparent that
P. magdaleniatum,
P. orinocense, and
P. mataense persisted in northern refugia,
P. corruscans and
P. reticulatum in southern refugia, and other
Pseudoplatystoma species in western refugia. The model proposed by Hubert and Renno [
25] could explain how four species’ distributions now overlap in the Amazon basin.
Pseudoplatystoma species are not shared between the now-connected Orinoco and Amazon basins, which may be explained by the species not dispersing readily through small river systems.
The phylogeny and distributions of species across the South American landmass are linked [
10]. Until recently, three species of
Pseudoplatystoma were recognized,
P. fasciatum,
P. tigrinum, and
P. corruscans. Despite their apparent morphological homogeneity within species, the genus
Pseudoplatystoma has cryptic species [
26]. Based on morphological characteristics, Buitrago-Suárez and Burr [
26] increased the number of known species to eight:
P. punctifer and
P. tigrinum in the Amazon basin,
P. orinocense and
P. mataense in the Orinoco basin,
P. corruscans and
P. reticulatum in the Paraná basin,
P. magdaleniatum in the Magdalena basin, and
P. fasciatum in the Guyana Shield. Later, using molecular genetic analyses, Torrico et al. [
27] and Carvalho-Costa et al. [
28] invalidated the distinction between
P. fasciatum and
P. punctifer made by Buitrago-Suárez and Burr [
26]. Torrico et al. [
27] evaluated species boundaries and biological and geographic patterns within the genus
Pseudoplatystoma using a variation of mitochondrial cytochrome
b and control region sequences to reconstruct phylogenetic relationships. They confirmed aspects of the morphological classification [
26], but other elements were not supported. Morphology and molecular data supported the monophyly of the genus
Pseudoplatystoma, i.e., their descent from a common ancestor. Molecular data also showed that
P. tigrinum,
P. corruscans,
P. reticulatum, and
P. magdaleniatum were highly supported clades, validating their taxonomic status. However, there were discrepancies between morphological and molecular genetic findings regarding
P. mataense and
P. orinocoense, suggesting either mtDNA introgression between the two species or misidentification of samples. The absence of phylogenetic and clear morphological differentiation between
P. fasciatum and
P. punctifer was such that Buitrago-Suárez and Burr [
26] had erroneously separated
P. fasciatum into two distinct species, thereby invalidating the taxonomic status of
P. punctifer. On the other hand, Lundberg et al. [
29] included four species in their study of the phylogenetics of Family Pimelodidae using nuclear and mitochondrial gene sequences, omitting the species
P. punctifer. Moreover, the species
P. fasciatum,
P. tigrinum,
P. magdaleniatum, and
P. corruscans formed a well-supported, monophyletic clade embedded within the sorubimines, a lineage of ten genera. The position of
P. tigrinum differed from that in the analysis of Buitrago-Suárez and Burr [
26]. Moreover, García-Dávila et al. [
30] showed the existence of a cryptic species within
Pseudoplatystoma, with the new species resembling what Castelnau (1855) had named
Platystoma punctifer, a species with no black stripes and a distinct mouth (
Figure 1). While becoming clearer, details of the phylogeny of
Pseudoplatystoma are yet undefined and will become more firmly established only with further study of suitably large collections of lineages and the application of suitable phylogenetic markers.
2.3. Distributions
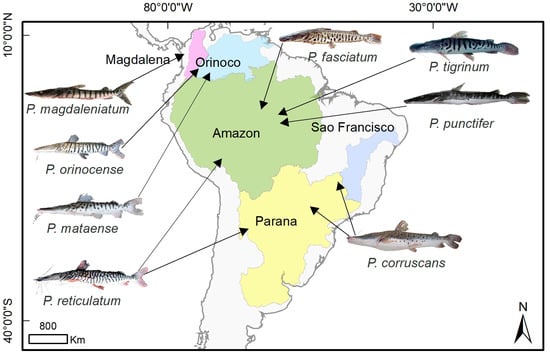
Developments in understanding the systematics of the genus
Pseudoplatystoma have led to eight species being currently recognized in the taxonomy, and their distributions (
Figure 2) are explained based on the natural history of South America. The Amazon basin contains three nominal species:
P. tigrinum,
P. fasciatum (named
P. punctifer by Buitrago-Suárez et al. [
27]), and
P. reticulatum in the Amazon River, distributed in the mainstem Amazon and its Madeira, Solimões, Xingu, Tapajós, and Jurua tributaries. Additionally, there are the cryptic species believed to be what Castelnau (1855) described as
P. punctifer:
P. cf.
punctifer [
30]. Two species occur in the Orinoco basin:
P. mataense, distributed in the Orinoco River in Venezuela and Colombia, and
P. orinocoense in the Orinoco River in Venezuela.
P. magdaleniatum is found in the Magdalena basin.
P. fasciatum is found in the Guyana Shield in the Amazon, Corantijn, Essequibo, Orinoco, (
Figure 1). Two species occur in the Paraná basin:
P. corruscans and
P. reticulatum in the central Amazon River and the Paraná River basin in Argentina, Bolivia, Brazil, Paraguay, and Uruguay. Only
P. corruscans occurs in the São Francisco basin. However, because researchers understanding of the systematics of the respective species is still not fully defined, more studies are needed to clarify the systematics, refine the taxonomy, and explain the distributions of the respective lineages in the genus.
Figure 2. Distributions of species of genus Pseudoplatystoma from the literature review data. The Magdalena basin is (represented in purple) where P. magdaleniatum is distributed. The Orinoco basin (light blue) encompasses the P. orinocense distribution. The Amazon basin (green) where P. fasciatum, P. tigrinum, P. reticulatum, and P. punctifer are distributed. P. corruscans is distributed in the São Francisco basin (blue), and P. corruscans and P. reticulatum are distributed in the Paraná basin (yellow). Illustrations courtesy of Timm, C.D.; Magalhães, K.; Alvarez, F.; Sabaj Pérez, and F. Duponchelle.
3. What Are the Life Histories and Ecologies of Pseudoplatystoma Species?
3.1. Movement Ecology
Most Pseudoplatystoma catfishes exhibit ontogenetic and feeding migrations, longitudinal migration for reproduction, and lateral migration for feeding [
31,
32]. However, the migration patterns of certain Pseudoplatystoma species need to be better understood, as there have been very few studies on the topic [
32,
33]. In the Colombian Amazon, it is believed that Pseudoplatystoma spp. migrate between 300–500 km [
34], but no movement data are available. Patterns of movement in Pseudoplatystoma have been shown to vary by sex, size, time of year, and ability of fish to cross natural barriers such as waterfalls [
18,
31,
33,
35].
While there is only a limited understanding of the movement ecology of
Pseudoplatystoma spp., especially regarding its potential importance in demographically and genetically structuring their populations, general patterns of migration of freshwater floodplains fishes suggest insights into species-level ecology of Pseudoplatystoma catfishes. The eggs and larvae of pimelodid catfishes have been found drifting in currents, in both white- and black-water rivers, and in both clear and turbid waters [
36,
37]. Catfish larvae drift mainly near the bottom of the water column, drifting distances of up to several thousand kilometers. Fast downstream movement (averaging 250 km/d) was suggested by Barthem et al. [
38] for the early life stages of some pimelodids in rain-swollen currents.
Few studies have explicitly distinguished the movement ecology of sexes and life stages of
Pseudoplatystoma catfishes. Male
P. fasciatum and
P. tigrinum usually inhabit rivers, while females inhabit riverside lakes during the low-water season [
31]. Adults of both species migrate upstream for spawning during the beginning of the high-water season in the Mamore River in the Beni basin, Bolivia. At the same time, juvenile individuals believed to be non-migratory remain in the floodplains [
31]. Adult
P. fasciatum that possessed undeveloped gonads moved upstream and passed through river rapids during the low-water season in the Madeira River [
35]; no fish were seen returning downstream. Juvenile
P. fasciatum are always caught in the floodplains; other migratory behavior is unknown. Moreover,
P. fasciatum and
P. tigrinum migrate upstream across the rapids in the Madeira River, probably seeking prey. However, despite the incomplete and seemingly inconsistent information about the timing and the direction of migration in
P. tigrinum and
P. fasciatum [
31,
33], some studies have elucidated the migration of
P. punctifer (
P. fasciatum) and
P. corruscans.
P. fasciatum performs movements both up and down river channels, and most movements occur between the dry season and the rising water period [
39].
P. fasciatum individuals were tracked using telemetry methods in the Xingu River, where they migrated up to 164 km between the dry season and the rising water period.
P. fasciatum was mainly detected moving upstream during the end of the rising-water period.
Migration and spawning patterns of
P. corruscans in the São Francisco River were observed using radio telemetry [
40]. Two migration patterns were exhibited, showing resident and migratory fish. According to the spawning stage, the migration was classified into pre-spawning staging and spawning migration. Female
P. corruscans migrated among pre-spawning staging, spawning, and non-spawning sites. Godinho et al. [
40] suggested that ripe females performed homing migration from feeding areas to the spawning sites, i.e., discrete sites where they returned multiple times. After spawning, most females migrated from the spawning site to other habitat units. The natural history of
P. corruscans in the La Plata River in the Uruguay River basin was inferred using ratios of strontium isotopes (
87Sr/
86Sr) along a transect of the otolith [
41].
P. corruscans was inferred to originate from the Paraná River, indicating a distinct nursery area. All
P. corruscans individuals showed ontogenetic shifts in their isotopic signature, with strontium ratios corresponding to catchment sub-basins, suggesting that this species presents spatially bounded reproduction in the respective areas [
41].
Given the lack of information about ontogenetic migration patterns for Pseudoplatystoma species, more studies are necessary to fully understand the life cycle of these species in the respective basins in which they are distributed. Questions that can and should be asked in future studies include, How far do the species migrate? Which habitats are used during their longitudinal and lateral migrations? Do juveniles and adults, and females and males, have the same migration patterns? Do all members of a species have the same migratory life history? Answering these questions would improve researchers understanding of the species’ life cycles and inform fisheries management and conservation for Pseudoplatystoma species.
3.2. Reproduction
The reproductive biology of
Pseudoplatystoma species is not yet well studied. The current knowledge of the reproductive ecology of
Pseudoplatystoma indicates that five of the eight species have common characteristics. Size-at-maturity varies among species between 57 cm to 82 cm, with all three species for which data are available spawning during the rising water season via total spawning, i.e., the release of all eggs in one batch (
Table 1). Larvae of
P. fasciatum were mostly observed drifting with currents during the end of the flood pulse, while larvae of
P. tigrinum were detected flowing during the start of the flood pulse in the Peruvian Amazon [
42].
Additionally, the reproductive morphology of
Pseudoplatystoma is like that of other Siluriformes.
Pseudoplatystoma species have double gonads, with elongated ovaries and testicles with digitiform projections all over their extent, features that are more evident during the reproductive period. The ovaries are covered by conjunctive and muscular tissues and blood vessels [
43]. The gonads converge at the caudal region and join a duct. The female gonads are cistovaries, meaning that the lumen is connected to the oviduct, through which the oocytes are released into the water [
44]. The male gonads are covered by conjunctive tissue that ramifies into septs filled with seminiferous tubules [
44].
The reproduction of
Pseudoplatystoma is closely related to their migratory ecology, as their reproduction is synchronized with the flood pulse and occurs during the period of rising and high waters [
32,
44,
45,
46,
47,
48] (
Table 1). The synchronization of reproduction with the upstream migration during the period of rising water enables these species to spawn when the river’s water level is favorable for the small, semi-buoyant eggs and larvae to drift with the current until they reach the inundated floodplains, which are their nursery habitats [
32,
36]. Consequently, an important life-history characteristic of the genus is that its species do not seem to provide parental care, given knowledge about their biology and considering the egg size and number. Females exhibit total spawning of numerous ova and high fecundity, which compensate for high early mortality [
31,
49,
50]; however, quantitative data about the fecundity of most of these species is lacking. Embryogenesis is rapid, lasting around 16 h at an average temperature of 23.0 ± 1.0 °C [
51]. For
P. fasciatum, the hatching takes place 14 h after fertilization at 27 °C [
52].
Table 1. Reproductive parameters of Pseudoplatystoma species.
Current knowledge of the reproductive biology of some of the species allows managers and decision-makers to take action even in the absence of more detailed studies. Based on the current information for five species in the genus, the establishment of regulations for time and minimum size-at-capture for those species lacking information could be to restrict their fisheries during the rising and high-water periods. Managers could calculate a ratio L50/Lmax for the species with available information and recommend an average ratio for the others until new studies are done.
The reproductive biology of Pseudoplatystoma species is not yet well studied, so existing knowledge gaps represent opportunities for research. One question that remains unanswered in regard to their behavioral reproductive ecology is: What is the female behavior during spawning? Do females emit any pheromonal or behavioral signal prior to or during spawning? In addition to fecundity information available for
P. fasciatum and
P. reticulatum [
54,
55], another knowledge gap relates to the fecundity of these five species: what is the range of age- or size-specific fecundities? Additionally, knowledge of the characteristics of eggs and larvae is important for understanding the reproductive ecology of
Pseudoplatystoma species. In particular, researchers must answer the questions: Do larvae perform vertical movement in the water column? What factors limit their recruitment to the juvenile stage? Are the respective species iteroparous or semelparous? If the former, how many spawning cycles occur during the lifetime? Do the species have broadcast spawning or other behaviors? Do juveniles have direct development or transformations? If the latter, how many inflection points? Answering these questions would enhance the current knowledge of reproductive biology, which is essential to managing fisheries and conserving these species.
3.3. Growth
The growth of six
Pseudoplatystoma species has been studied. As noted above, these catfishes are large-bodied compared to other Neotropical species and can grow up to about 130 cm in total length and 100 kg in weight, depending on the species [
56]. They can grow to 40 cm in their first year of life [
14]. Females grow larger and faster than males.
Most studies on the growth of
Pseudoplatystoma found a similar pattern of growth rings in calcified structures such as otoliths and vertebrae, with opaque ring formation in the hard parts of
Pseudoplatystoma, indicating fast growth occurring during the dry season [
14,
31,
50,
58,
60]. Several studies showed that one opaque ring is formed annually during the dry season and the beginning of the rainy season for
P. punctifer,
P. tigrinum, and
P. mataense in the Peruvian Amazon, Orinoco, and Mamore basins [
14,
31,
57], probably because the dry season is when prey capture is more difficult due to competition with larger catfishes and dolphins and the beginning of the reproductive stage [
14,
31,
33]. However, studies on central and eastern Amazon tributaries are necessary to determine whether the pattern of one ring per annual cycle is consistent for
P. fasciatum and
P. tigrinum. Other fish species—such as gilded catfish
Brachyplatystoma rousseauxii (Castelnau, 1855) [
58], mapara catfish
Hypophthalmus marginatus (Valenciennes, 1840) [
57], piaracatinga catfish
Calophysus macropterus (Lichtenstein, 1819) [
61], and pirarucu
Arapaima spp. [
59] in the central Amazon—form two growth rings per flood cycle [
14]. Validation of ring formation has been done for
P. punctifer,
P. tigrinum, and
P. fasciatum, but it is lacking for
P. corruscans,
P. reticulatum, and
P. magdaleniatum [
14,
31]
Generally, species that are distributed in tropical and temperate regions have faster growth rates in the warmer tropical waters because food availability is the main constraint for growth in tropical regions, while temperature constrains the growth in temperate regions [
62,
63]. For example, all species of the genus
Pseudoplatystoma that are distributed in the tropical areas of the Amazon, Orinoco, and Magdalena basins grow faster than the
Pseudoplatystoma catfishes distributed in the Paraná basin.
- Threats to Conservation of Pseudoplatystoma Species
The main threats to Pseudoplatystoma species are (1) overfishing and (2) alterations in and obstruction of river flow due to the construction of hydropower dams [64]. Other threats—such as industrial, domestic, and agricultural pollution, deforestation, introduced species, hybridization with farmed fish, and genetic erosion—may also affect populations of Pseudoplatystoma species, but in our view, they are not less pressing.
Dams interrupt streamflow and generate longitudinal and lateral hydrological changes in river ecosystems [65-67] that can be reflected subsequently in decreased fisheries yields. The most apparent effects of placing dams on rivers are the formation of a new lentic environment upstream from the dam and a tailwater environment downstream from the dam [66]. In general, six effects of dams on fisheries are (1) alteration of flood regime; (2) change in nutrient flow; (3) change in water temperature; (4) disruption of fish migration; (5) change in fish community composition; and (6) change of water chemistry [67]. For Pseudoplatystoma species, the alteration of the flood regime and disruption of migration are particularly important [32].
Alteration of the flood regime disrupts lateral connectivity, as dams cause the permanent inundation of upstream floodplains and restrict the seasonal movement of littoral zones that are part of the flood pulse [66]. Further, downstream of the dam, floodplains are not sufficiently inundated in terms of the depth, duration, and seasonality of flooding, which could result in a significant decline in Pseudoplatystoma populations and fisheries. That is, disruption of floodplain inundation is likely to affect the life cycle of Pseudoplatystoma, including disruption of migration, recruitment, and survival of cohorts. Dams blocking upstream and downstream migration can cause the decline and even extirpation of species that depend on longitudinal movements along the stream during certain phases of their life cycle [65,66]. Migratory fishes require different environments for the main stages of their life cycle: reproduction, juvenile production, growth, and sexual maturation (review in [64]). As a barrier to upstream migration, a dam prevents adults from reaching spawning grounds during the breeding season, resulting in recruitment failure and eventual extirpation of the population above the dam. Downstream migration past the dam may also be difficult or impossible for many fish species. Fish migrating into the reservoir from tributary streams may be unable to find their way to the dam and subsequently pass downstream through discharge structures. In particular, the modification of the downstream river-flow regime by an impoundment can have various adverse effects on Pseudoplatystoma fish species, including loss of stimuli for migration, loss of migration routes and spawning grounds, decreased survival of eggs and juveniles, decreased recruitment to the mature life stage and diminished fisheries production [67].
In addition to obstruction of river flow due to dams, overfishing is especially evident in parts of the São Francisco and Paraná rivers [32], particularly regarding P. corruscans stocks [32,68-70]. This species is subject to overfishing in Minas Gerais State, Brazil. P. corruscans harvest in the São Francisco River has manifested indications of collapse [70,71]. In the 1950s, the capture of thousands of P. corruscans with low fishery effort was the norm [72]. More recently, however, the yield of P. corruscans caught in a vital fishing area has declined from 10.3 kg fish/day in 1987 to 0.8 kg fish/day in 1999 [40]. In the Amazon basin, some authors have suggested that P. tigrinum and P. fasciatum are declining in mean catches and captured at small sizes [68,73–75].
With continued research, understanding of the biology and ecology of this genus would fill knowledge gaps and contribute to effective fisheries management and conservation of the eight species of the genus Pseudoplatystoma.
[64] Duponchelle, F.; Isaac, V.J.; Doria, C.; Van Damme, P.A.; Herrera-R, G.A.; Anderson, E.P.; Cruz, R.E.A.; Hauser, M.; Hermann, T.W.; Agudelo, E.; et al. Conservation of Migratory Fishes in the Amazon Basin. Aquat. Conserv. 2021, 31, 1087–1105. https://doi.org/10.1002/aqc.3550.
[65] Agostinho, A.A.; Gomes, L.C. Dams and the Fish Fauna of the Neotropical Region: Impacts and Management Related to Diversity and Fisheries. Braz. J. Biol. 2008, 68, 1119–1132.
[66] Poff, L.N.; Hart, D.D. How Dams Vary and Why It Matters for the Emerging Science of Dam Removal. Bioscience 2002, 52, 659–668.
[67] Jackson, D.; Marmulla, G.; Dams, Fish and Fisheries. Opportunities, Challenges and Conflict Resolution. FAO Fish. Tech. Paper 2001, 419, 166.
[68] Sato, Y. Reprodução de Peixes Da Bacia Do Rio São Francisco: Indução e Caracterização de Padrões; 1999. 179f. Tese (Doutorado). Centro de Ciências Biológicas e da Saúde, Universidade Federal de São Carlos, São Carlos, SP.
[69] Sato, Y.; Godinho, H.P. Migratory Fishes of the São Francisco River. In Migrotory Fish of South America; Carolsfeld, J., Harvey, B., Ross, C., Eds.; World Fisheries Trust: Victoria, BC, USA, 2003; pp. 195–232.
[70] Godinho, H.P.; Miranda, M.O.T.; Godinho, A.L.; Santos, J.E. Pesca e Biologia Do Surubim Pseudoplatystoma Coruscans No Rio São Francisco; Puc Minas: Belo Horizonte, Brazil, 1997.
[71] Godinho, A.L.; Godinho, H.P. Breve Visão Do São Francisco. Belo Horizonte: PUC Minas 468 (2003): 15-23. In Águas, Peixes e Pescadores do São Francisco das Minas Gerais; PUC Minas: Belo Horizonte, Brazil, 2003; Volume 468, pp. 15–23.
[72] Menezes, R.S. Pesca e Piscicultura No Vale Do São Francisco. Boletim da Secretaria da Agricultura Indústria e Comércio do Estado de Pernambuco 1956, 23, 43–105.
[73] Isaac, V.; Ruffino, M.; McGrath, D. In Search of a New Approach to Fisheries Management in the Middle Amazon Region. In Fishery Stock Assessment Models; Alaska Sea Grant, University of Alaska Fairbanks: Fairbanks, AK, USA, 1998; pp. 889–902.
[74] Castello, L.; McGrath, D.G.; Beck, P.S.A. Resource Sustainability in Small-Scale Fisheries in the Lower Amazon Floodplains. Fish. Res. 2011, 110, 356–364. https://doi.org/10.1016/j.fishres.2011.05.002.
[75] Agudelo, E.A.; Acosta-Santos, G.; Gómez, B.D.; Gil, R.E.; Ajiaco-Martínez; Ramírez-Gil, H. Pseudoplatystoma punctifer (Siluriformes, Pimelodidae). In: Lasso, C. A.; Agudelo Córdoba, E.; Jiménez-Segura, L. F.; H. Ramírez- Gil, H.; Morales-Betancourt, M.; Ajiaco-Martínez, R. E.; Gutiérrez,F. de P.; Usma Oviedo, J.S.; Muñoz Torres, S.E.; Sanabria Ochoa, A.I. (Eds.) I. Catálogo de los Recursos Pesqueros Continentales de Colombia. Serie Editorial Recursos Hidro- biológicos y Pesqueros continentales de Colombia. Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH). Bogotá, D. C., Colombia, 715 pp. Capitulo 7; 2011; pp. 509–512.
This entry is adapted from the peer-reviewed paper 10.3390/fishes8060306