Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Gastric cancer is an aggressive disease with low long-term survival rates. An early diagnosis is essential to offer a better prognosis and curative treatment. Upper gastrointestinal endoscopy is the main tool for the screening and diagnosis of patients with gastric pre-neoplastic conditions and early lesions. Under white-light endoscopy (WLE), early gastric cancer (EGC) should be suspected in the presence of mucosal surface irregularity and/or mucosal coloration changes.
- gastric cancer
- endoscopy
- white-light endoscopy
1. Introduction
Gastric cancer (GC) is the fourth most common cause of cancer deaths worldwide, with 768,793 cases and an estimated incidence of 10,839,103 new cases yearly, according to The International Agency for Research on Cancer. Eastern Asia has the highest incidence, accounting for 60% of the cases [1]. According to gender, both the incidence and death rate in males were more than twice that in females. Most GC cases occur in patients older than 45 years of age, with a higher incidence at 65 to 70 years.
Although advanced GC is associated with a poor prognosis and high mortality, early detection and treatment have a good prognosis, with 5-year survival rates higher than 95% [2]. Unfortunately, more than 70% of GCs in Western countries are diagnosed in advanced stages [3].
Histologically, GC is classified as diffuse (composed of non-cohesive cells) or intestinal types (gland-forming), with different epidemiological patterns [4]. Helicobacter pylori (H. pylori) is considered a risk factor for non-cardia GC, with nearly 89% of cancers associated with this pathogen infection [5], mainly intestinal-type cancer. According to Pelayo Correa’s model of carcinogenesis, a cascade of events beginning with active chronic inflammation may progress to multifocal atrophic gastritis (AG), intestinal metaplasia (IM), dysplasia, and carcinoma [6]. The eradication of H. pylori infection has been associated with a significant reduction in the incidence and mortality of GC, especially among patients younger than 50 years of age [7]. In a meta-analysis, a pooled incidence rate ratio of 0.53 (95% CI: 0.44−0.64) was observed comparing individuals who received H. pylori eradication with individuals who did not receive eradication therapy [8].
Esophagogastroduodenoscopy (EGD) is the gold-standard exam for the diagnosis of neoplastic and pre-neoplastic gastric conditions, such as AG and IM. However, in a meta-analysis of 22 studies, the authors demonstrated that nearly 10% of GCs were potentially missed during white-light endoscopy (WLE), mainly adenocarcinomas located at the gastric body. Predictive factors for diagnostic failure were a younger age (<55 years), female, advanced atrophy, adenoma, ulcer lesions, and an insufficient number of biopsies [9].
Image-Enhanced Endoscopy (IEE) has been used to overcome the diagnostic limitations of standard endoscopy. IEE refers to various methods, such as dye chromoscopy, high-resolution imaging, virtual chromoscopy, and artificial intelligence. IEE provides a better assessment of the mucosal surface, increasing the detection of subtle changes and improving the diagnosis of pre-neoplastic lesions [10][11][12].
2. Indications for Endoscopic Screening of Gastric Cancer
In Eastern countries, radiographic screening programs for GC diagnosis were implemented in the 1960s, reducing its mortality [13]. Nowadays, screening methods include radiography and EGD. A cohort study comparing the two methods showed that subjects screened by EGD had a 67% reduction in GC mortality compared with subjects screened by radiography (RR 0.327; 95% CI, 0.118–0.908) [14]. In South Korea, GC screening by EGD every 2 years was shown to be associated with a significant (≥80%) reduction in GC mortality, while for those undergoing radiographic examinations every 2 years, the reduction in mortality was only 20% [7]. In a metanalysis, EGD screening detection of GC (0.55%, 95% CI 0.39–0.75%) and early-GC (EGC) (0.48%, 95% CI 0.34–0.65%) was superior to radiography screening (GC 0.19%, 95% CI 0.10–0.31%; EGC 0.08%, 95% CI 0.04–0.13%) [15].
In Western countries, where the incidence of GC is lower than in Eastern countries, screening focuses on high-risk patients with AG or IM. The management of epithelial precancerous conditions and lesions in the stomach (MAPS) guideline recommends the use of a staging system in patients with AG and/or IM, such as the Operative Link on Gastritis Assessment (OLGA) or the Operative Link on Gastritis Assessment based on Intestinal Metaplasia (OLGIM) systems [16][17]. Patients with extensive atrophy and/or IM, i.e., affecting both antral and corpus mucosa, should be identified and sampled as they are considered to be at higher risk for GC [17]. These patients should be followed-up with a high-quality EGD every 3 years. In patients with a family history of GC, close follow-up is suggested (e.g., every 1–2 years after diagnosis). The association of high-risk OLGA stages (III/IV) with GC was demonstrated in a meta-analysis including 6 case–control studies and 2 cohort studies (2.64 and 27.7 times higher chance of GC than lower OLGA stages, respectively). Among patients with OLGIM III/IV, the risk of GC was 3.99 times higher (95% CI 3.05–5.21) [18].
The American Gastroenterological Association (AGA) suggested EGD surveillance every 3–5 years in patients at high risk for GC, including those with advanced AG, extensive and incomplete IM, a family history of GC, and new immigrants from areas of high risk, such as East Asia or South America. The AGA also recommended testing and the eradication of H. pylori infection among individuals with IM [19][20]. Similarly to the AGA, the American Society of Gastrointestinal Endoscopy (ASGE) recommended surveillance for high-risk individuals with IM, as well as testing and treating H. pylori infection [21].
The British Society of Gastroenterology emphasized the importance of IEE for the diagnosis of pre-neoplastic lesions. The society advised a baseline endoscopy among individuals with laboratory evidence of pernicious anemia, such as vitamin B12 deficiency, and positive gastric parietal cell or intrinsic factor antibodies [22].
3. White-Light Endoscopy (WLE)
EGD has the possibility of identifying pre-neoplastic mucosal changes, detecting early-stage GC, and reducing cancer-related mortality by diagnosing and treating gastric mucosal alterations and/or EGC. Under WLE, EGC should be suspected in the presence of mucosal surface irregularity and/or mucosal coloration changes. Spontaneous bleeding, pallor coloration, and alterations in the mucosa surface and in light reflection should raise a concern about neoplastic lesions, as shown in Figure 1.
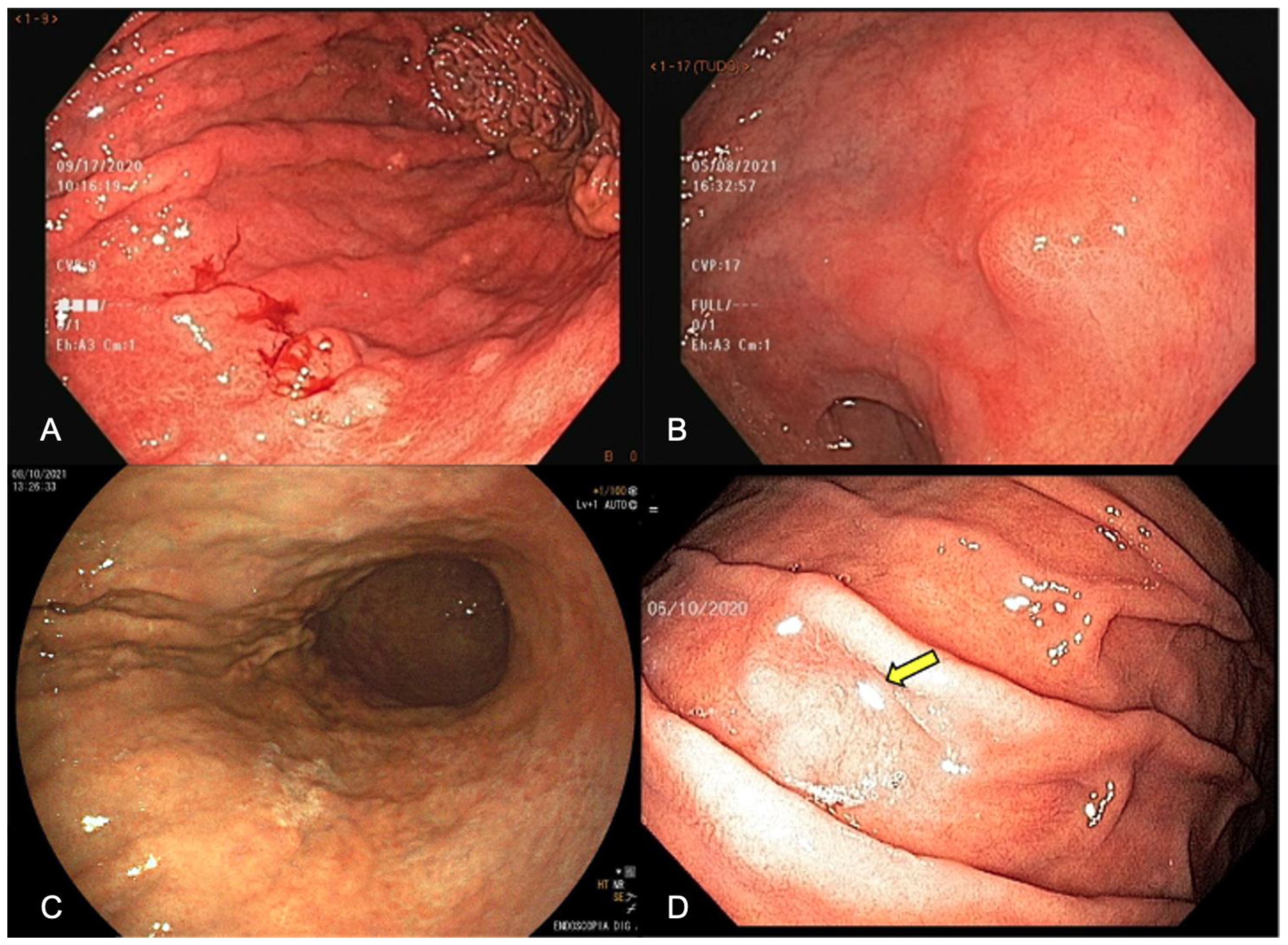
Figure 1. Endoscopic appearance of early gastric cancer (A) white-light endoscopy (WLE) showing spontaneous bleeding, (B) WLE showing irregular surface, showing a slightly elevated lesion with a central depression, (C) pallor color change in the posterior wall of the stomach enhanced by FICE (Flexible spectral imaging color enhancement), (D) change in light reflection (arrow) under WLE.
Early gastric cancer is defined as a lesion confined to the mucosa and submucosa (T1), regardless of lymph node involvement. These lesions usually manifest as superficial lesions (type 0), which can be subclassified into polypoid (Type 0-I), flat (Type 0-II), and excavated (Type 0-III). Flat lesions are categorized as slightly elevated (0-IIa), completely flat (0-IIb), or slightly depressed (0-IIc). Superficial tumors with two or more components should have all the components described (e.g., 0-IIa + IIc) [23] (Figure 2).
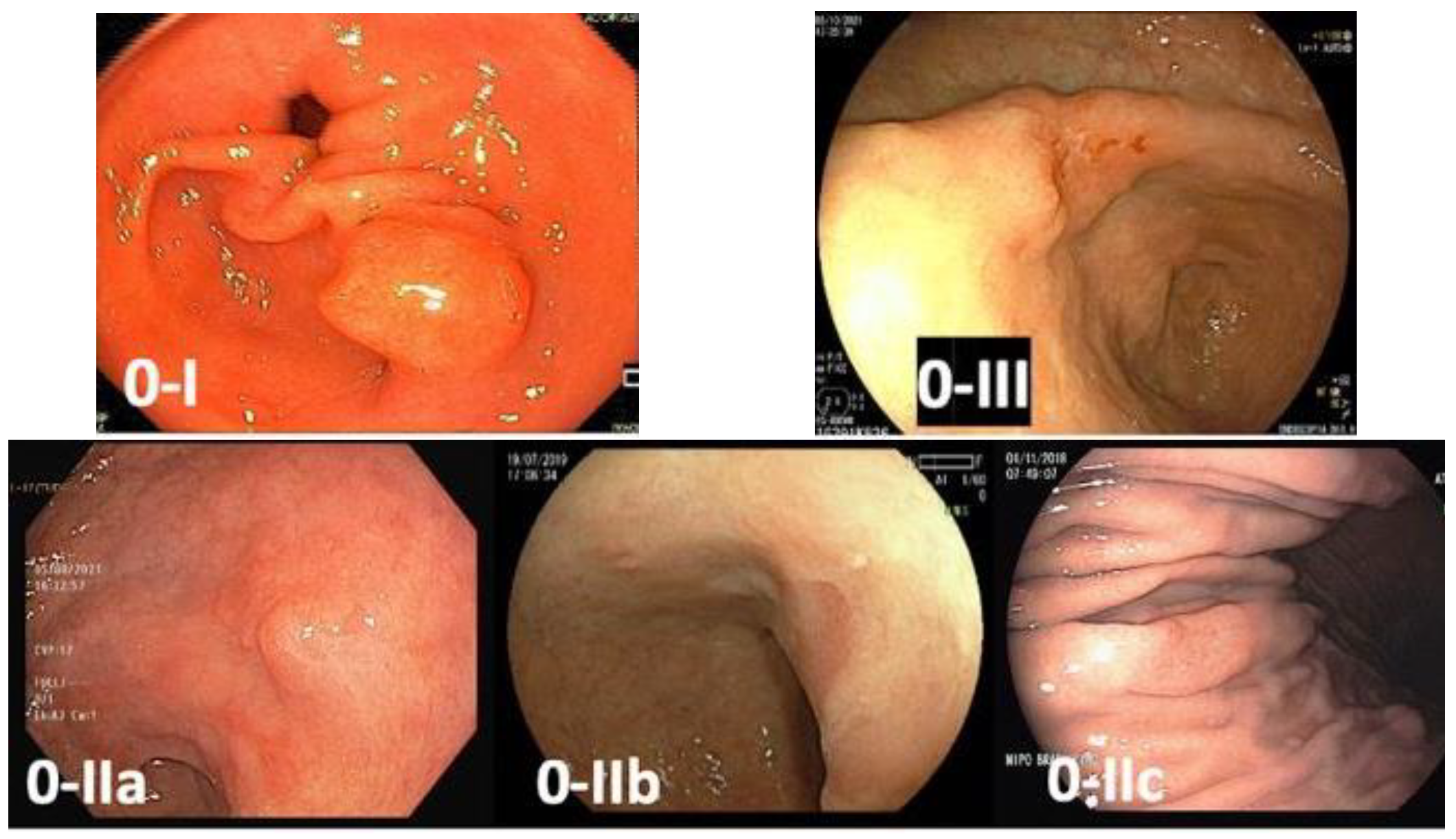
Figure 2. Paris classification of gastric superficial lesions—0-I: polypoid lesion; 0-IIa: flat and slightly elevated lesion; 0-IIb: completely flat lesion; 0-IIc: flat and slightly depressed lesion; 0-III: excavated lesion.
A high-quality level of endoscopic examination is imperative to make a proper diagnosis of early neoplastic lesions. The use of pre-endoscopy medications (mucolytic and defoaming agents), high-definition endoscopes, adequate inspection time, obtaining index images, and the application of the MAPS biopsy protocol when AG and chronic inflammation are suspected have been recommended by the European Society of Gastrointestinal Endoscopy (ESGE) guidelines [24][25].
3.1. Mucolytic and Defoaming Agents
To achieve a proper mucosal evaluation during EGD, the cleansing of mucus, bubbles, and foam is important. The most common mucolytic agents used worldwide are Pronase® and n-acetylcysteine, and both are used to eliminate gastric mucus using non-osmotic solutions. Simethicone (activated dimethicone) is a commonly used defoaming agent that decreases the surface tension of gas bubbles without significant adverse interactions. It can improve the mucosal observation when used 20 min before the procedure [26].
3.2. Antispasmodic Agents
Antispasmodic agents, such as cimetropium bromide, scopolamine, and hyoscine N-butyl bromide, can reduce peristalsis and may be used during EGD [27]. However, there is no scientific evidence supporting the benefits of antispasmodic agents in the detection rate of upper gastrointestinal (GI) neoplasia. In addition, patients may have adverse reactions to these drugs, such as arrhythmia, benign prostate hypertrophy, and glaucoma. Thus, its use should be selective and at the discretion of the endoscopist.
3.3. Inspection Time
Limited consensus exists about the optimum inspection time for EGD compared to the colonoscopy quality protocol recommendation (withdrawal time of 6 min or more). In a retrospective Korean study [28], endoscopists who dedicated at least 3 min to evaluate the gastric mucosa detected more gastric adenomas or cancers than faster endoscopists (0.28% versus 0.20%, respectively; p < 0.01). A Japanese study showed that faster endoscopists, with a mean inspection time below 5 min, may overlook neoplastic lesions in the upper gastrointestinal tract. In this study, endoscopists were classified into fast (<5 min examination), moderate (between 5–7 min), and slow (>7 min) groups. The odds ratio of diagnosing neoplastic lesions was 1.90 for the moderate group and 1.89 for the slow group compared to the fast group (p = 0.03 and p = 0.06, respectively) [29].
3.4. High-Resolution Endoscopes
The resolution of an image depends on the pixel density, which is directly associated with the capacity to distinguish two adjacent points. Higher pixel density endoscopes provide higher imaging resolution. Standard endoscopes produce a signal image with a resolution of 100,000 to 400,000 pixels. High-resolution or high-definition endoscopes generate images with up to 1,000,000 pixels [30]. The development of high-resolution endoscopes enabled to distinguish subtle mucosal surface details compared to standard endoscopes, allowing more accurate suspicious diagnoses and targeted biopsies [31].
3.5. Obtain Index Images
The requirements of minimum photo documentation vary from each endoscopy society recommendation. While USA guidelines do not specify the minimum number of EGD images to report, the ESGE suggested at least ten images indicating the anatomical index [32]. In Japan, a systematic screening protocol for the stomach included 22 images, with the rationale to study the full gastric mucosa and avoid blind spots [33]. The World Endoscopy Organization proposed a total of 28 image areas, including the hypopharynx [34]. Adherence to a standardized photo protocol may increase the EGD neoplastic detection rate because the endoscopist must examine the entire stomach, including potential blind spots.
3.6. Target Biopsies of Suspicious Lesions
Target biopsies improve the diagnosis of GC. The ESGE recommends at least six biopsies of suspected advanced GC [35]. For EGC, only one to two targeted biopsies are recommended to avoid scars or submucosal fibrosis, as it is best staged and treated by endoscopic resection [36].
Moreover, some conditions, including chronic AG or IM, carry a higher risk for GC. The updated Sydney system recommended at least five biopsies: two from the antrum (greater and lesser curvature, 3 cm from the pylorus), one from the incisura, and two from the gastric body (from lesser curvature and greater curvature) [37]. The MAPS I and II guidelines question the necessity of sampling the incisura, as it yielded minimal additional diagnostic information, with more costs [17]. The inclusion of an incisura biopsy, however, increased the proportion of patients classified as high-risk stages (OLGA III/IV or OLGIM III/IV) [38][39]. It is also important to label the biopsies from different sites, and to apply a validated staging system, such as OLGA or OLGIM.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15092445
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249.
- Crew, K.D.; Neugut, A.I. Epidemiology of Gastric Cancer. World J. Gastroenterol. 2006, 12, 354–362.
- Minicozzi, P.; Innos, K.; Sánchez, M.-J.; Trama, A.; Walsh, P.M.; Marcos-Gragera, R.; Dimitrova, N.; Botta, L.; Visser, O.; Rossi, S.; et al. Quality Analysis of Population-Based Information on Cancer Stage at Diagnosis across Europe, with Presentation of Stage-Specific Cancer Survival Estimates: A EUROCARE-5 Study. Eur. J. Cancer 2017, 84, 335–353.
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49.
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global Burden of Cancers Attributable to Infections in 2012: A Synthetic Analysis. Lancet Glob. Health 2016, 4, e609–e616.
- Correa, P.; Houghton, J. Carcinogenesis of Helicobacter Pylori. Gastroenterology 2007, 133, 659–672.
- Mabe, K.; Inoue, K.; Kamada, T.; Kato, K.; Kato, M.; Haruma, K. Endoscopic Screening for Gastric Cancer in Japan: Current Status and Future Perspectives. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2022, 34, 412–419.
- Lee, Y.-C.; Chiang, T.-H.; Chou, C.-K.; Tu, Y.-K.; Liao, W.-C.; Wu, M.-S.; Graham, D.Y. Association Between Helicobacter Pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-Analysis. Gastroenterology 2016, 150, 1113–1124.e5.
- Pimenta-Melo, A.R.; Monteiro-Soares, M.; Libânio, D.; Dinis-Ribeiro, M. Missing Rate for Gastric Cancer during Upper Gastrointestinal Endoscopy: A Systematic Review and Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1041–1049.
- Zhao, Z.; Yin, Z.; Wang, S.; Wang, J.; Bai, B.; Qiu, Z.; Zhao, Q. Meta-Analysis: The Diagnostic Efficacy of Chromoendoscopy for Early Gastric Cancer and Premalignant Gastric Lesions. J. Gastroenterol. Hepatol. 2016, 31, 1539–1545.
- Fiuza, F.; Maluf-Filho, F.; Ide, E.; Furuya, C.K.; Fylyk, S.N.; Ruas, J.N.; Stabach, L.; Araujo, G.A.; Matuguma, S.E.; Uemura, R.S.; et al. Association between Mucosal Surface Pattern under near Focus Technology and Helicobacter Pylori Infection. World J. Gastrointest. Endosc. 2021, 13, 518–528.
- Zhang, Q.; Wang, F.; Chen, Z.-Y.; Wang, Z.; Zhi, F.-C.; Liu, S.-D.; Bai, Y. Comparison of the Diagnostic Efficacy of White Light Endoscopy and Magnifying Endoscopy with Narrow Band Imaging for Early Gastric Cancer: A Meta-Analysis. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2016, 19, 543–552.
- Oshima, A.; Hirata, N.; Ubukata, T.; Umeda, K.; Fujimoto, I. Evaluation of a Mass Screening Program for Stomach Cancer with a Case-Control Study Design. Int. J. Cancer 1986, 38, 829–833.
- Hamashima, C.; Shabana, M.; Okada, K.; Okamoto, M.; Osaki, Y. Mortality Reduction from Gastric Cancer by Endoscopic and Radiographic Screening. Cancer Sci. 2015, 106, 1744–1749.
- Faria, L.; Silva, J.C.; Rodríguez-Carrasco, M.; Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Libânio, D. Gastric Cancer Screening: A Systematic Review and Meta-Analysis. Scand. J. Gastroenterol. 2022, 57, 1178–1188.
- Dinis-Ribeiro, M.; Areia, M.; de Vries, A.C.; Marcos-Pinto, R.; Monteiro-Soares, M.; O’Connor, A.; Pereira, C.; Pimentel-Nunes, P.; Correia, R.; Ensari, A.; et al. Management of Precancerous Conditions and Lesions in the Stomach (MAPS): Guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012, 44, 74–94.
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of Epithelial Precancerous Conditions and Lesions in the Stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) Guideline Update 2019. Endoscopy 2019, 51, 365–388.
- Yue, H.; Shan, L.; Bin, L. The Significance of OLGA and OLGIM Staging Systems in the Risk Assessment of Gastric Cancer: A Systematic Review and Meta-Analysis. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2018, 21, 579–587.
- Gupta, S.; Li, D.; El Serag, H.B.; Davitkov, P.; Altayar, O.; Sultan, S.; Falck-Ytter, Y.; Mustafa, R.A. AGA Clinical Practice Guidelines on Management of Gastric Intestinal Metaplasia. Gastroenterology 2020, 158, 693–702.
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021, 161, 1325–1332.e7.
- ASGE Standards of Practice Committee; Evans, J.A.; Chandrasekhara, V.; Chathadi, K.V.; Decker, G.A.; Early, D.S.; Fisher, D.A.; Foley, K.; Hwang, J.H.; Jue, T.L.; et al. The Role of Endoscopy in the Management of Premalignant and Malignant Conditions of the Stomach. Gastrointest. Endosc. 2015, 82, 1–8.
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology Guidelines on the Diagnosis and Management of Patients at Risk of Gastric Adenocarcinoma. Gut 2019, 68, 1545–1575.
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 3rd English Edition. Gastric Cancer 2011, 14, 101–112.
- Kim, S.Y.; Park, J.M. Quality Indicators in Esophagogastroduodenoscopy. Clin. Endosc. 2022, 55, 319–331.
- Bisschops, R.; Areia, M.; Coron, E.; Dobru, D.; Kaskas, B.; Kuvaev, R.; Pech, O.; Ragunath, K.; Weusten, B.; Familiari, P.; et al. Performance Measures for Upper Gastrointestinal Endoscopy: A European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy 2016, 48, 843–864.
- Chang, W.-K.; Yeh, M.-K.; Hsu, H.-C.; Chen, H.-W.; Hu, M.-K. Efficacy of Simethicone and N-Acetylcysteine as Premedication in Improving Visibility during Upper Endoscopy. J. Gastroenterol. Hepatol. 2014, 29, 769–774.
- Kim, S.Y.; Park, J.M.; Cho, H.S.; Cho, Y.K.; Choi, M.-G. Assessment of Cimetropium Bromide Use for the Detection of Gastric Neoplasms During Esophagogastroduodenoscopy. JAMA Netw. Open 2022, 5, e223827.
- Park, J.M.; Huo, S.M.; Lee, H.H.; Lee, B.-I.; Song, H.J.; Choi, M.-G. Longer Observation Time Increases Proportion of Neoplasms Detected by Esophagogastroduodenoscopy. Gastroenterology 2017, 153, 460–469.e1.
- Kawamura, T.; Wada, H.; Sakiyama, N.; Ueda, Y.; Shirakawa, A.; Okada, Y.; Sanada, K.; Nakase, K.; Mandai, K.; Suzuki, A.; et al. Examination Time as a Quality Indicator of Screening Upper Gastrointestinal Endoscopy for Asymptomatic Examinees. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2017, 29, 569–575.
- Jang, J.-Y. The Past, Present, and Future of Image-Enhanced Endoscopy. Clin. Endosc. 2015, 48, 466–475.
- Bhat, Y.M.; Abu Dayyeh, B.K.; Chauhan, S.S.; Gottlieb, K.T.; Hwang, J.H.; Komanduri, S.; Konda, V.; Lo, S.K.; Manfredi, M.A.; Maple, J.T.; et al. High-Definition and High-Magnification Endoscopes. Gastrointest. Endosc. 2014, 80, 919–927.
- Rey, J.F.; Lambert, R. ESGE Quality Assurance Committee ESGE Recommendations for Quality Control in Gastrointestinal Endoscopy: Guidelines for Image Documentation in Upper and Lower GI Endoscopy. Endoscopy 2001, 33, 901–903.
- Yao, K. The Endoscopic Diagnosis of Early Gastric Cancer. Ann. Gastroenterol. 2013, 26, 11–22.
- Emura, F.; Sharma, P.; Arantes, V.; Cerisoli, C.; Parra-Blanco, A.; Sumiyama, K.; Araya, R.; Sobrino, S.; Chiu, P.; Matsuda, K.; et al. Principles and Practice to Facilitate Complete Photodocumentation of the Upper Gastrointestinal Tract: World Endoscopy Organization Position Statement. Dig. Endosc. 2020, 32, 168–179.
- Pouw, R.E.; Barret, M.; Biermann, K.; Bisschops, R.; Czakó, L.; Gecse, K.B.; de Hertogh, G.; Hucl, T.; Iacucci, M.; Jansen, M.; et al. Endoscopic Tissue Sampling—Part 1: Upper Gastrointestinal and Hepatopancreatobiliary Tracts. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2021, 53, 1174–1188.
- Chiu, P.W.Y.; Uedo, N.; Singh, R.; Gotoda, T.; Ng, E.K.W.; Yao, K.; Ang, T.L.; Ho, S.H.; Kikuchi, D.; Yao, F.; et al. An Asian Consensus on Standards of Diagnostic Upper Endoscopy for Neoplasia. Gut 2019, 68, 186–197.
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and Grading of Gastritis. The Updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181.
- Isajevs, S.; Liepniece-Karele, I.; Janciauskas, D.; Moisejevs, G.; Funka, K.; Kikuste, I.; Vanags, A.; Tolmanis, I.; Leja, M. The Effect of Incisura Angularis Biopsy Sampling on the Assessment of Gastritis Stage. Eur. J. Gastroenterol. Hepatol. 2014, 26, 510–513.
- Varbanova, M.; Wex, T.; Jechorek, D.; Röhl, F.W.; Langner, C.; Selgrad, M.; Malfertheiner, P. Impact of the Angulus Biopsy for the Detection of Gastric Preneoplastic Conditions and Gastric Cancer Risk Assessment. J. Clin. Pathol. 2016, 69, 19–25.
This entry is offline, you can click here to edit this entry!
