Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Physics, Applied
Despite its growing importance in the energy generation and storage industry, the detection of hydrogen in trace concentrations remains challenging, as established optical absorption methods are ineffective in probing homonuclear diatomics. Besides indirect detection approaches using, e.g., chemically sensitized microdevices, Raman scattering has shown promise as an alternative direct method of unambiguous hydrogen chemical fingerprinting.
- Raman scattering
- molecular hydrogen
- detection
1. Introduction
Hydrogen (H2) is anticipated to be a major contributor to energy storage and power generation, potentially aiding decarbonization and sustainable development. Fuel cells have become increasingly sophisticated and can now power small devices such as computers, as well as larger systems such as vehicles. In principle, hydrogen can be produced from water electrolysis, preferably with surplus renewable electric power, though the majority of hydrogen production is still from fossil fuels. To help the hydrogen industry, trace sensors are necessary in monitoring inadvertent hydrogen losses. Therefore, portable and affordable H2 trace sensing devices, which can detect at or near the ambient hydrogen concentration of approximately 0.5 parts per million (ppm), are in high demand.
Despite its simple makeup, hydrogen gas remains difficult to detect at concentrations of a few ppm and below. Conventional optical methods such as cavity ring-down spectroscopy (CRDS), off-axis integrated cavity output spectroscopy (ICOS), and quantum cascade tunable infrared laser differential absorption spectroscopy (QC-TILDAS) are largely ineffective, as only quadrupole H2 transitions are infrared-active. Gas chromatography or magnetic resonance-based methods, while trace-sensitive, are bulky, expensive, and not readily portable. Thus, the most widely adopted H2 detection methods today use micro-electromechanical systems (MEMS) or metal oxide semiconductor (MOS) devices. These devices offer the advantages of high speeds, extreme miniaturization, and low costs [1]. However, poor chemical specificity and the need for recurrent calibration make accurate H2 detection at concentrations of order 100 parts per billion (ppb) a formidable challenge [2].
Recently, spontaneous Raman scattering (SRS) has become an attractive alternative due to its economical and robust nature. It is able to create a unique spectral signature for any molecular gas and, depending on the resolution, can detect dozens of analytes simultaneously, including isotopologues. Furthermore, enhancement techniques using capillaries, hollow-core photonic crystal waveguides, or optical cavities have been developed, significantly improving the prospects of SRS trace sensing applications. These techniques have demonstrated detection limits routinely in the 10 ppm range, and, in some cases, well below.
2. High-Precision Trace Hydrogen Sensing
As the simplest diatomic, hydrogen has played a unique historical role as a model for the validation of quantum mechanics molecular theory. In terms of its properties as a fuel, hydrogen has high energy content per unit of weight, making it an attractive option for transportation applications. The accurate measurement of hydrogen incorporation in solids is a matter of great interest in diverse research domains such as fuel cells, photocatalysts, and fusion reactors [3]. There has been a rapid increase in its application in the meteorological, aerospace, metallurgy, electronics, and chemical industries [4]. Outside its explosive range (4–75%), it may serve, for instance, in leak detection applications as a replacement for increasingly rare helium. At concentrations ranging from 4% to 75%, it is flammable, colorless, odorless, and thus potentially highly hazardous [5,6]. From the production, transportation, storage, and eventual utilization of hydrogen, it is essential to exercise caution and take appropriate measures to ensure the safe handling of this fuel [7,8]. Recent work has also pointed out the potential for hydrogen leaks to adversely affect the natural atmospheric removal mechanism of methane, further underlying the need for highly sensitive methods to quantify hydrogen concentrations in a variety of environments [9].
The primary requirements for a field-deployable H2 gas sensor are high sensitivity, a fast response, and stability over time. The traditional methods of gas chromatography (GC) coupled to mass spectrometry are difficult to implement for hydrogen. This is due to the fact that it is not possible to analyze a lighter gas than the carrier gas using gas chromatography coupled to mass spectrometry [10,11]. Hydrogen gas can only be analyzed by GC using a thermal conductivity detector (TCD), a helium ionization detector (HID), or an atomic emission detector (AED) [11,12]. Indirect detection of hydrogen gas by gas chromatography using electron ionization (EI) ion source mass spectrometry is possible [11,13,14]. Not only are these methods costly, but they are also time-consuming. Additionally, the equipment required for these techniques is bulky and demands skilled operators.
An alternative detection method is provided by chemically sensitized electronic transducer devices, relying on electrochemical or micro-mechanical readout [8]. Upon the deposition of hydrogen-absorbing and/or -adsorbing materials such as palladium and platinum, these sensors derive hydrogen concentrations via resistance for chemiresistors, or work functions for Schottky diodes, field-effect transistors (FETs), and metal oxide FET (MOSFET) sensors. Nanostructuring via, e.g., nanowires, can be further used to increase the surface-to-volume ratio to improve the sensing capabilities [15]. Additionally, resonator-based MEMS such as quartz crystal microbalance (QCM) and surface acoustic wave sensors have also been scrutinized extensively.
With a limit of detection (LOD) of 10 ppb, chemiresistors are one of the most effective hydrogen sensing devices. These sensors detect H2 gas by way of a change in the resistance of the semiconductor material [16] and feature a fast response (∼1 s) and recovery time (<10 s) [17]. However, a significant disadvantage is that their sensitivity is influenced by the temperature, typically optimal above 150 °C [16]. In contrast, Schottky diode sensors have a wide operating temperature range and exhibit excellent chemical stability. However, they suffer from a slow recovery time (>1 h) when exposed to certain gas atmospheres, including N22 [18]. Moreover, humidity adversely affects their performance [19]. MOSFET and FET sensors, although highly dependent on the gate morphology, are compact, highly stable, sensitive, and may have rapid response and recovery times [20]. MEMS-based surface acoustic wave sensors are efficient in mitigating the effects of temperature and pressure fluctuations but are highly affected by the surface properties of the sensing medium [21,22]. The underlying weakness of the entire family of electrochemical/electromechanical sensors is the intrinsically indirect nature of their detection mechanism, bringing about unavoidable nonspecificity and stringent calibration needs.
Spectroscopic optical techniques, by which a unique chemical fingerprint is probed, do not suffer from this drawback. However, optical infrared absorption modalities are not adequate for probing trace hydrogen, a diatomic homonuclear molecule that would only provide absorption via weak electrical quadrupole transitions [23]. Raman spectroscopy is an alternative optical technique that can be used for hydrogen detection. It is non-invasive with high selectivity, enabling hydrogen detection in complex gas mixtures [24,25,26,27]. However, due to scattering cross-sections in gases being only on the order of 10−3110−31 cm22/str, additional experimental steps are critical to make Raman scattering viable for practical sensing. For example, stimulated photo-acoustic Raman spectroscopy (PARS), in which a high-power bichromatic laser source is combined with acoustic sensing, achieved hydrogen detection at a concentration of 10 ppm and an estimated LOD of 3.4 ppm at 0.3 MPa of pressure [28]. Aside from extreme sample pressurization, which has been implemented to detect hydrogen down to concentrations of 20 ppm at 2.5 MPa [29], numerous other enhancement approaches have been explored over the years. Among waveguide-based enhancement methods, fiber-enhanced Raman scattering (FERS) has shown particular promise. In hollow-core fibers as long as 2 m, hydrogen was probed with an estimated LOD of 4.7 ppm using either rotational or vibrational transitions [30,31]. Much progress in improving FERS has also been made recently by mitigating the issue of gas throughput by utilization of larger, anti-resonant, waveguides [32]. Another means of achieving Raman enhancement is using optical resonators. Besides Purcell-enhanced Raman scattering in microresonators [33], traditional low-loss resonant optical cavities may simply be used to create circulating power many orders of magnitude greater than the pump laser’s input power. Cavity-enhanced Raman scattering (CERS) has been implemented to detect hydrogen in high concentrations [26,34,35], and, recently, also at a 100 ppm concentration at 0.1 MPa of pressure, with an estimated LOD of 0.069 ppm, and at ambient levels (≈0.5 ppm), by way of circulating power as large as 1 kW [36]. Table 1 provides a summary of selected benchmarks reported for hydrogen sensing with different techniques.
Another powerful technique for SRS enhancement uses a non-resonant multipass cavity [37,38,39,40,41,42]. Unlike Raman enhancement methods involving a resonant cavity, multipass cavity Raman scattering requires no laser frequency stabilization or interferometric cavity length control. It further does not suffer from sample gas throughput limitations. Feedback-assisted multipass cavity enhancement with a laser diode has proven to be particularly effective (Figure 1) [27,43]. With an off-the-shelf multimode blue laser diode (NUBM44), several watts of power may be recirculated to obtain effective power at the sample of near 100 W. The feedback serves the additional purpose of reducing the laser linewidth to a few wavenumbers. With feedback-assisted multipass Raman scattering, hydrogen has been detected in ambient air and in breath samples, under pressurization of up to 0.8 MPa [40]. Nevertheless, a substantial limitation in earlier measurements was the background generated by the multipass mirrors themselves, precluding accurate detection in the ppb range.
Table 1. Summary of common techniques for detection of H2, with lowest detected concentration, LOD, and measurement time, when reported.
| Method | Lowest Detected Conc. (ppm) | Estimated LOD (ppm) | Measurement Time (s) | Reference |
|---|---|---|---|---|
| Chemiresistors | 50 | 0.01 | ∼20 | [17] |
| Schottky diode | 10,000 | 0.1 | ∼10 | [44] |
| MEMS | 6 | 0.1 | ∼2 | [45] |
| IR absorption | 5000 at 0.1 MPa | 1000 | 1 | [23] |
| Raman: | ||||
| • PARS | 10 at 0.3 MPa | 3.4 | ∼10 | [28] |
| • FERS | 5 | 4.7 | 1–100 | [31] |
| • CERS | 100 at 0.1 MPa 0.5 at 0.1 MPa |
0.069 - |
500 2500 |
[36] |
| • Multipass cavity | 0.075 at 0.2 MPa | 0.06 | 600 | This work |
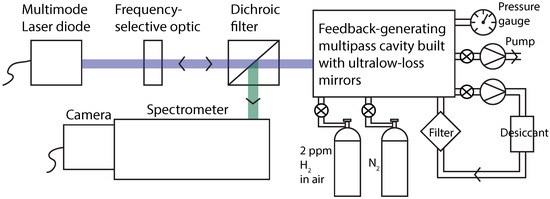
Figure 1. Diagram of experimental setup. The light from a multimode blue laser diode (Nichia NUBM44) is frequency-narrowed to 3 cm−1 by the feedback from an ultra-low-loss multipass Raman cavity using a frequency-selective optic (Optigrate volume Bragg grating). Feedback-assisted multipass SRS functions as an external cavity diode laser from which the Raman emission is dichroically coupled out to a spectrometer.
This entry is adapted from the peer-reviewed paper 10.3390/s23115171
This entry is offline, you can click here to edit this entry!
