Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Positron emission tomography (PET) is a non-invasive, functional imaging test utilizing ionizing radiation, the source of which is a radioactive isotope (radionuclide) administered to the patient. Myocardial perfusion imaging (MPI) with radionuclide PET is one of the non-invasive imaging methods that can provide rapid and accurate information about the extent of ischemia.
- positron emission tomography
- myocardial viability
- Infective endocarditis
- molecular imaging
- NaF
1. Myocardial Perfusion and Flow Imaging
Myocardial perfusion imaging (MPI) with radionuclide PET is one of the non-invasive imaging methods that can provide rapid and accurate information about the extent of ischemia. The gold standard for assessing perfusion is the use of O15-labeled water, a freely diffusible tracer with nearly 100% first-pass extraction from the blood [4,5]. A major limitation of this tracer is its short half-life of about 2 min, which results in the need for a cyclotron within the PET scanner neighborhood so that it can be produced and administered to the patient in a very short timeframe. Consequently, non-diffusion tracers such as N13-ammonia (13NH3) and 82-rubidium chloride (82Rb), which either have a longer half-life (13NH3) or are produced in a generator in the PET scanner room (82Rb), are more widely employed for MPI (Table 1 and Table 2) [6,7].
Table 1. Characteristics of PET radionuclides.
| Radionuclide | Half-Life | Source |
|---|---|---|
| 15O | 2 min | Cyclotron |
| 13N | 10 min | Cyclotron |
| 82Rb | 1.3 min | Generator |
| 18F | 110 min | Cyclotron |
| 68Ga | 68 min | Generator |
| 11C | 20 min | Cyclotron |
| 64Cu | 12.8 h | Cyclotron |
| 89Zr | 78 h | Cyclotron |
Table 2. PET radiotracers for myocardial perfusion imaging.
| Tracer | Isotope | Half-Life (min) |
Production | First-Pass Extraction Fraction (%) | Uptake Mechanism | Availability | Stress Modality | MBF Quantification | Injected Activity (mCi) | Effective Dose (mSv) |
|---|---|---|---|---|---|---|---|---|---|---|
| 15O-Water (H215O) | 15O | 2 | Cyclotron | ~100 | Free diffusion | + | Pharmacologic | yes | 40 | 1.7 |
| 13N-ammonia (13NH3) | 13N | 9.96 | Cyclotron | ~80 | Free diffusion and metabolic trapping (Na+/K+ ATPase) | +++ | Exercise or pharmacologic With exercise, only static images available |
with pharmacologic stress only | 20 | 1.5 |
| 82Rubidium chloride (82RbCl) | 82Rb | 1.3 | Generator | ~65 | Metabolic trapping (Na+/K+ ATPase) | ++ | Pharmacologic | yes | 60 | 1.4 |
MBF—myocardial blood flow, availability graded +/++/+++.
The obtained resting and stress perfusion images are compared, determining the presence, location and size of perfusion defects qualitatively and semi-quantitatively. By semi-quantitatively estimating the severity of the defect, one relates the degree of tracer uptake in each segment to that in which the uptake is greatest—thus obtaining relative perfusion data. When perfusion in the reference area is impaired, these data are inadequate for the actual flow rate, leading to false-negative findings—an issue that is particularly relevant in patients with multivessel coronary artery disease, as well as left main disease [8,9]. As shown in a recent study, this situation occurs in as many as 4.5% of symptomatic patients with multivessel disease [10]. Another important cohort comprises patients with normal coronary arteries in whom microvascular dysfunction is suspected: especially women, diabetic patients and patients with chronic kidney disease [6]. These obstacles have been effectively eliminated with the introduction of dynamic flow acquisitions with quantitative assessments of global and regional myocardial blood flow (MBF) and myocardial flow reserve (MFR), which is the ratio of maximal flow in a hyperemic state to myocardial blood flow at rest.
Since the first studies evaluating coronary reserve in relation to the degree of vascular stenosis were published almost 50 years ago [11], the clinical relevance of flow reserve has been widely recognized. While nowadays assessments of flow reserve are primarily performed invasively during coronary catheterization, such assessments can be conducted using PET. A great advantage of PET MBF measurements is not simply that they are noninvasive, but more importantly that they analyze not only the relative data resulting from flow through a portion of a single vessel [12], but they demonstrate the absolute values of coronary flow through the entire thickness of the tissues involved, with no additional radiation and exposures as low as 0.5 mSv [13,14,15]. The method has been shown to be highly reproducible [16], and maintains a high prognostic value independent of the body mass index (BMI) [17,18]. According to COVADIS, impaired coronary flow reserve with cutoff values ≤2.0 and impaired hyperemic MBF has been recognized as one of the diagnostic criteria for microvascular angina [19].
2. Viability
There is a complex relationship between myocardial perfusion, mechanical function and metabolism. Numerous studies have evaluated the accuracy of viability imaging for prospective identification of patients with ischemic cardiomyopathy and potentially reversible left ventricular (LV) dysfunction who can benefit from future revascularization [19,20,21,22,23]. With respect to metabolic or contractile reserve, LV impairment may have two causes: (a) irremediable necrosis equal to scarring and (b) different but overlapping reversible stunning and hibernation. The most established nuclear medicine technique to image heart metabolism utilizes 18F-fluorodeoxyglucose (18FDG)-PET, and since 1986 it continues to serve as a “gold standard” to differentiate a scar from viable hibernated myocardium [24].
Under normal conditions, myocytes as the “omnivorous” cells use free fatty acids (FFAs) as a preferable source of energy via the highly energetic, oxygen-dependent process of beta-oxidation. Prolonged ischemia is a status when the main source of energy is switched to glucose derived from anaerobic glycolysis, and in consequence leads to a substantial increase in glucose utilization [25]. This phenomenon can be depicted with PET. As a glucose analog, 18FDG is absorbed by cardiac myocytes, becoming a surrogate marker of myocardial glucose uptake. 18FDG is actively transported into the cells by GLUT-1 and four glucose transporters in the same way as a “normal” glucose molecule. As 18FDG-6-phosphate cannot be transformed back to 18FDG, it is eventually trapped inside the cell, providing an opportunity for a non-invasive assessment of glucose metabolism with PET [25]. 18FDG PET viability evaluation involves a combination of rest MPI (either PET or single photon emission computed tomography SPECT) and 18FDG metabolic imaging in order to determine one of three patterns of perfusion vs. viability: (1) normal conditions—preserved myocardial perfusion and viability. (2) Viable hibernation, a mismatch—reduced perfusion and preserved viability. (3) Non-viable scar, a match—reduced MPI and viability (Figure 1 and Figure 2) [26,27].
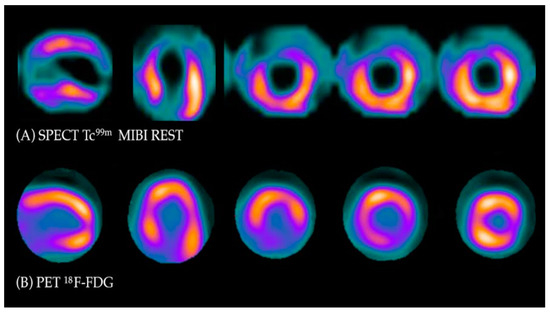
Figure 1. Myocardial viability imaging: Mismatch pattern, viable hibernated myocardium. (A) Myocardial perfusion imaging with MIBI-Tc99m SPECT: heart scans showing lack of tracer uptake in the apical region and apical segments of the anterior wall (left anterior descending coronary artery territory). (B) 18FDG viability PET. 18FDG uptake is visible in the area of the perfusion deficit, ruling out scarring and confirming viability in this region.
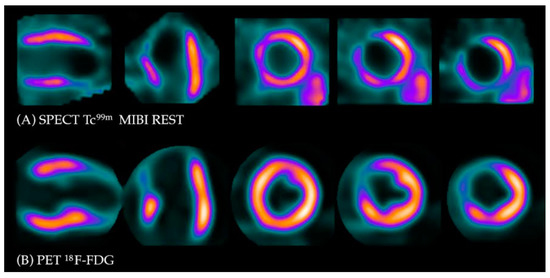
Figure 2. Myocardial viability imaging: Match pattern, non-viable scarring. (A) Myocardial perfusion imaging with MIBI-Tc99m SPECT: heart scans showing lack of tracer uptake in the apical region of the left ventricle (LAD territory). (B) 18FDG viability PET. 18FDG uptake is absent in the area of the perfusion defect, confirming myocardial scarring in this region.
The current societal guidelines by AHA/ACC/HFSA in 2022 [28] and ESC in 2021 [29] state with similar class II recommendation that non-invasive stress imaging, including PET, may be considered for the assessment of myocardial ischemia and viability in patients with CAD who are considered suitable for coronary revascularization.
These recommendations are based on landmark clinical trials. The first broad meta-analysis of 24 studies by Allman et al. in 2002, which included 3088 patients [30], explored the potential changes in the outcomes of patients with established coronary artery disease and LV dysfunction and demonstrated a strong association between myocardial viability on noninvasive testing and improved survival after revascularization [30]. No benefit was associated with revascularization without confirmed viability, irrespectively of the imaging modality used. This notion was not confirmed in the initial analysis of the STICH trial, where viability assessed with less advanced techniques in patients referred to surgical revascularization did not improve outcomes [31]; however, over longer follow-up, there was an improvement in all-cause and cardiovascular mortality with viability-guided surgical revascularization [32]. The rational for selecting PET for viability imaging was confirmed by another post hoc analysis. In the PARR-2 study, a significant reduction in cardiac events was observed in patients with 18FDG-PET-assisted management compared with patients who received optimal medical treatment in a center with easy access to 18FDG and integration with experienced clinical teams [33]. More recently, the REVIVED-BCIs2 prospective randomized trial did not support the hypothesis that percutaneous revascularization in combination with optimal medical treatment may improve event-free survival in patients with ischemic cardiomyopathy and viable myocardium compared with a strategy of medical treatment alone, even in patients with reduced ejection fraction [34,35]. Considering the aforementioned studies, the role of myocardial viability in guiding revascularization remains controversial. Balancing procedural risks and expected benefit from revascularization is still a key question in patients with ischemic heart failure—therefore, it seems that 18FDG PET myocardial viability testing is a helpful tool when it is carefully matched with the patient’s profile [36], particularly in high surgical risk patients, elderly individuals with severe LV impairment and patients with a history of prior surgical revascularization and/or complex comorbidities [36]. Additionally, patients with advanced coronary artery disease referred for high-risk revascularization of chronically occluded arteries also appear to be among those who could derive the greatest benefit from PET imaging [37,38]. Indeed, it has been previously shown that viability testing with 18FDG PET–CT or PET–MR in patients with CTO can identify those who shall show functional improvement following revascularization [39,40].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics13101791
This entry is offline, you can click here to edit this entry!
