The gut microbiome is made up of bacteria, fungi, viruses and archaea, all of which are closely related with human health. As the main component of enterovirus, the role of bacteriophages (phages) in chronic liver disease has been gradually recognized. Chronic liver diseases, including alcohol-related liver disease and nonalcoholic fatty liver disease, exhibited alterations of the enteric phages. Phages shape intestinal bacterial colonization and regulate bacterial metabolism. Phages adjoining to intestinal epithelial cells prevent bacteria from invading the intestinal barrier, and mediate intestinal inflammatory response. Phages are also observed increasing intestinal permeability and migrating to peripheral blood and organs, likely contributing to inflammatory injury in chronic liver diseases. By preying on harmful bacteria, phages can improve the gut microbiome of patients with chronic liver disease and thus act as an effective treatment method.
1. Introduction
The phage is small and has a tadpole shape, microsphere shape and fine rod shape. Phages are composed of genomic nucleic acids and capsid protein, and nucleic acids can be linear double-stranded DNA, circular single-stranded DNA, or linear single and double-stranded RNA [
1]. Some phages without tails are being recognized; some phages present an outer lipid membrane in addition to their protein capsid. Most phages in human intestinal flora belong to the order
Caudovirales, with double-stranded DNA smaller than 200 KB in size. Megaphage with a very large genome (540 KB in length) has been found in animal and human gut [
2].
Phages can dissolve bacteria to complete their life cycle [
3]. Gentle survival strategies are also used, some of which are even beneficial to bacteria. For example, temperate phages steadily integrate into bacterial genomes and confer antibiotic resistance to the host. Phages can be classified as either lytic or lysogenic depending on the types of interaction with their host [
4] (
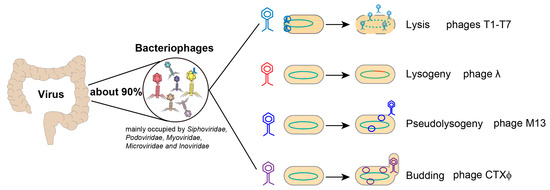
Figure 1).
Figure 1. Characteristics of intestinal virus. Bacteriophage is the most abundant component of human intestinal virome (accounting for about 90%). For lytic growth, phages infect bacteria and synthesize their own components, then release new virions by lysing host bacteria. During lysogenic growth, phages inject their genomes into the bacteria and integrate them into the bacterial chromosome. When the phages enter the bacteria body but remain in a static state it is called pseudolysogeny. Finally, some phages infect bacteria and then bud to produce new phages.
In recent years, a growing number of works have revealed the effects of enteric colonizing viruses on human health and disease, including chronic liver disease [
9]. The role of enteroviruses in the progression and prognosis of chronic liver disease has been further understood, and some phages have been used as treatment for chronic liver disease [
4]. By preying on bacteria, phages participate in the microecological balance of the gut and affect human health. Phages have been reported regulating the immune microenvironment of the intestine, and affecting blood glucose homeostasis [
10,
11]. The ecological balance between phages and bacteria is key to maintaining health.
2. Alterations in the Enteric Phages of Chronic Liver Diseases
2.1. Alcoholic Liver Diseases
Patients with alcohol-use disorder were reported with decreased abundance of
Propionibacterium,
Lactobacillus and
Leuconostoc phages, and increased abundance of
Streptococcus and
Lactococcus phages compared with controls, whereas after 2 weeks of alcohol abstinence, all phages were increased [
12]. Alcohol-use disorder patients with progressive liver disease were more abundant in
Enterobacteria and
Lactococcus phages than nonprogressive individuals. Patients with alcoholic hepatitis had the highest viral diversity and richness, compared with controls and patients with alcohol dependence [
13].
Alcoholic hepatitis patients were dominated by
Lactobacillus,
Streptococcus and
Escherichia phages, and alcohol-use disorders were mainly characterized by
Lactococcus phages. When compared with controls, alcoholic hepatitis patients had decreased abundances of
Lactococcus and
Parabacteroides phages, and increased abundance of
Lactobacillus,
Escherichia,
Enterobacteria and
Enterococcus phages. The
herpesviridae was exclusively detected in stool samples from patients with alcoholic hepatitis, and not found in patients with alcohol-use disorders and controls [
13,
14].
2.2. Nonalcoholic Fatty Liver Disease
Experimental nonalcoholic liver disease animals were found with changes of intestinal virus. The short-term high-fat diet (16 weeks) increased the alpha diversity of viromes in mice, but the long-term high-fat diet (28 weeks) restored the alpha diversity of viromes identically to the control group [
16]. What is more, Eukaryotic viruses
Phycodnaviridae and
Mimivirdae gradually replaced
Siphoviridae in the dominant position of mice intestines during feeding with a high-fat diet. Viral communities of mice with a high-milk-fat diet were sensitive to dietary disturbances, in contrast to mice with baseline low-fat diet [
17].
2.3. Liver Cirrhosis
The similarity of disease progression of liver cirrhosis and gut virus were sparse. Jasmohan S Bajaj et al. reported compensated liver cirrhosis had similar alpha diversity of phage genera with controls [
20]. Beta diversity analysis at the levels of phage families and phage genera showed liver cirrhosis patients were clustered. Furthermore, lactulose and rifaximin treatment of cirrhosis with hepatic encephalopathy had no effects on the intestinal phage genera. Correlation networks between phages and bacterial species were significantly reduced in compensated cirrhosis compared with controls.
Faecalibacterium,
Streptococcus,
Lactobacillus,
Microbacterium and
Lactococcus phages were obviously increased in patients with cirrhosis, and
Kagunavirus phage was decreased, compared with healthy controls.
Bullavirinae,
Felixounavirus,
Streptococcus,
Escherichia and
Pseudomomas phages were positively related while
Faecalibacterium phages were negatively related with the model for end-stage liver disease (MELD) scores.
3. Underlying Mechanisms of Phages in Chronic Liver Diseases
3.1. Phages Shape Intestinal Bacterial Colonization
Bacteriophages are viewed as “commensal” gut-resident viruses, regulate bacterial composition and help maintain a “healthy” miccobiota status [
21,
22]. How do bacteria live with deadly phages? The mutual cycle of resistance evolution and infection resistance evolution provides the theoretical basis for the coexistence of bacteria and phages [
23,
24]. Recent studies have shown that the physical environment in which bacteria and phages reside plays an important role in promoting bacteria-phage coexistence [
23].
The influence of phages on bacterial evolution is universal; about 2 × 10
16 phage-mediated gene transfer events occur every second [
26]. Through transformation, generalized and specialized transduction and chromosomal rearrangements, phages transfer DNA into bacterial genome, and provide virulence genes to bacteria. The Vibrio cholerae toxin gene was derived from the filamentous phage CTXΦ(25) [
27]. Phages encoding virulence factors are involved in the emergence of new epidemic strains of Salmonella [
28]. By trapping iron, phages can affect the virulence of intestinal bacterial pathogens such as Vibrio vulnificus, Salmonella typhimurium, and Yersinia [
29]. Phage-mediated exchange of resistance gene islands among enterococci provides a survival advantage for enterococci in the face of complex environmental factors [
30]. Phage has been found hitchhiking on carrier bacteria to facilitate its infection of host bacteria, and promote carrier bacteria colonization [
31].
3.2. Phages Regulate Bacterial Metabolism
With advances in genome sequencing, phages are found to be able to alter the metabolism of their hosts by carrying auxiliary metabolic genes (AMGs) [
46]. Phages were suggested help digest plant-derived polymers by encoding the CAZyme gene which frees beta-1, 4-linked mannoses from galactomannan and glucomannan [
47]. Phages prey on harmful bacteria and can eliminate their harmful metabolites [
48]. Tryptophan is decarboxylated by gut bacteria to produce tryptamine, an important neurotransmitter. Phage (T4 and F1) intervention resulted in a significant decrease in the abundance of
C. sporogenes in the intestinal tract of mice, along with a decrease in the content of tryptamine [
32].
3.3. Phages and Intestinal Barrier
Numerous studies have shown that phages can translocate directly through the mucosal barrier [
49] (
Figure 2). Phages administered orally, intranasally and intraperitoneally can appear rapidly in the blood and accumulate in the kidneys, spleen, liver and thymus [
50]. A rat treated with a bacteriophage cocktail for 10 days showed increased intestinal permeability [
51]. The mechanism of phage penetration of the mucosal barrier is still not completely clear. There are several hypotheses: phage uptake by epithelial cells through transcytosis; phages hidden in bacteria cross the epithelial barrier through the Trojan horse mechanism; phages enclosed in intestinal contents and ingested by enteric dendritic cells; or phages pass through damaged epithelial barriers [
52]. High levels of phages were found in the mucous layer of the gut. Phages use their Ig-like domain proteins to bind to the variable glycan residues that coat the mucin glycoprotein, and colonize the intestinal mucous layer [
53]. Phages in close proximity to intestinal epithelial cells may be a previously unrecognized antimicrobial defense mechanism that prevents bacteria from invading the intestinal barrier, and actively protects mucosal surfaces [
54]. However, bacteriophages can also induce increased intestinal permeability [
44,
51]. Disruption of intestinal barrier integrity was found in rats ten days after administration of a bacteriophage cocktail active against
Staphylococcus spp.,
Streptococcus spp.,
Proteus spp.,
Pseudomonas spp.,
E. coli,
K. pneumonia, and
Salmonella spp. Phages can also penetrate epithelial cell layers and migrate to peripheral blood and organs, likely contributing to inflammatory injury in chronic liver disease [
55]. The relationship between phage and the intestinal barrier deserves further study.
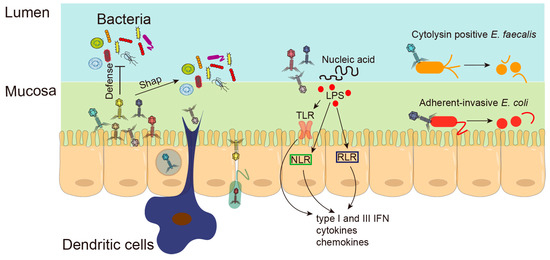
Figure 2. Underlying mechanisms of phages in chronic liver diseases. Phages not only shape the intestinal bacterial colonization, but also prevent bacteria from invading the intestinal barrier. Phages can directly pass through damaged epithelial barriers or be taken up by epithelial cells through transcytosis. Phages hidden in bacteria can cross the epithelial barrier through the Trojan horse mechanism; Phages can also be ingested by enteric dendritic cells. Bacterial components and phage DNA, released from the lysed bacteria, trigger intestinal immunity through Toll-like receptors (TLRs), NOD-like receptors (NLRs) and RIG-I-like receptors (RLRs) in epithelial cells or immune cells. Phages against adherent-invasive
E. coli were found to be protected from DSS-induced colitis. Phages isolated from sewage were able to lyse the intestinal cytolytic
E. faecalis strain and alleviated alcohol-induced liver damage.
3.4. Phages and Intestinal Inflammation
Bacterial components and phage DNA, released from the lysed bacteria, are the main ligands that trigger intestinal immunity through pattern recognition receptors (PRRs) in epithelial cells or immune cells (
Figure 2). In addition, bacteriophages can directly motivate inflammatory responses in epithelial cells and immune cells in the intestine [
56]. Phages have immunomodulatory activity, affecting the function of major populations of immune cells involved in innate and adaptive immune responses including phagocytosis and respiratory burst of phagocytes, cytokine production and production of antibodies against non-phage antigens [
57]. Increased phage load causes expansion of immune cells in the gut and stimulates IFN-γ secretion through the nucleotide-sensing receptor TLR9, then exacerbates the colon inflammatory response [
58]. Phage-caused intestinal inflammation exacerbates the hyperpermeability of the gut.
Anti-T4-like phage antibodies are common in humans, and the capsid proteins Hoc and gp23* contribute significantly to the immune memory of phage T4 [
59]. There was evidence that the
B. thetaiotaomicron,
L. plantarum and
E. coli phages induce IFN-γ production in a microbia-independent manner through toll-like receptor 9 (TLR) in the gut [
58]. Higher levels of plasma LPS and serum levels of tumor necrosis factor-alpha (TNF-α), IL-1β, and IL-6 were found in rats challenged with the commercial bacteriophage cocktail [
44]. Several Toll-like receptors (TLRs), NOD-like receptors (NLRs) and RIG-I-like receptors (RLRs) have been reported as virus PRRs sensors [
56].
4. Phage Therapy for Chronic Liver Diseases
Phage has been reported since 1917 as a treatment for dysentery [
69]. This sparked enthusiasm for phages’ treatment of diseases related with specific bacterial pathogens. Over the past two decades, phages have proved effective in fighting antibiotic-resistant bacterial pathogens and the infections they cause [
70,
71,
72,
73]. These phages have been successfully used in mice or patients for pathogenic infections such as
Pseudomonas aeruginosa,
Acinetobacter baumannii,
Vibrio parahaemolyticus,
Clostridium difficile,
Staphylococcus aureus,
Mycobacterium abscessus and
Vibrio cholerae [
54,
74,
75]. Researchers have found that a higher abundance of
Caudovirales or a higher alpha diversity of bacteriophage in the donor proved more effective in treating
C. difficile infection with fecal bacteria transplantation [
76,
77].
There have been many clinical trials and case reports of the use of phages in the treatment of gastrointestinal and chronic liver disease [
78,
79]. Mice receiving phage against adherent-invasive
E. coli were found to be protected from DSS-induced colitis [
80]. A phase I/IIa randomized, double-blind, placebo-controlled clinical trial is underway to assess the safety and efficacy of oral administration of phages that target intestinal adherent-invasive
E. coli in patients with Crohn’s disease in remission (NCT03808103) [
78]. Phages can successfully rescue mice from severe infections caused by multidrug-resistant Klebsiella pneumoniae (ST258) [
81]. The use of phage in the treatment of ALD has achieved promising results [
15]. Four phages isolated from sewage were able to lyse the intestinal cytolytic E. faecalis strain.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms11051181