Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Tissue engineering and regenerative medicine (TERM) holds great promise for addressing the growing need for innovative therapies to treat disease conditions. To achieve this, TERM relies on various strategies and techniques. The most prominent strategy is the development of a scaffold. Polyvinyl alcohol-chitosan (PVA-CS) scaffold emerged as a promising material in this field due to its biocompatibility, versatility, and ability to support cell growth and tissue regeneration.
- polyvinyl alcohol
- chitosan
- scaffold
- tissue engineering
1. Tissue Engineering
Tissue engineering (TE) is a vital area of biomaterials application as the various approaches can be used to treat abnormalities in tissues and organs [1][2]. TE aims to create a 3D cell-containing scaffold implanted into the body to treat a disease or repair damage [3]. The standard in vitro culture system cannot mimic the intricacies of the cellular microenvironment and seldom facilitates the integration of cells into fully functional tissue. Therefore, providing an appropriate scaffold of a diverse range of natural and synthetic materials will lead to the development of functional tissue.
In recent years, PVA-CS was widely used as a TE scaffold. PVA-CS scaffold could be a good platform for tissue regeneration alone or in combination with other polymers and cellular components [4]. The scaffold provides a 3D architecture that imitates the original tissue’s extracellular matrix (ECM), providing a favorable environment for stem cell growth and differentiation [5]. Stem cells are undifferentiated cells that can develop into distinct types of specialized cells. Depending on the application and regenerated tissue, different types of stem cells can be integrated with biomaterial scaffolds. The combination of stem cells and biomaterial scaffolds presents a promising approach for both in vitro and in vivo TE applications [5].
PVA-CS scaffold is a promising biomaterial for cartilage repair, while mesenchymal stem cells (MSCs) are emerging as a better option due to their unique properties and potential to promote tissue regeneration and repair [6]. MSCs are a type of adult stem cell that can differentiate into multiple cell types, including chondrocytes, adipocytes, osteocytes, etc. When MSCs are introduced into damaged cartilage tissue, they can help stimulating the repair process and promote the growth of new cartilage cells. One of the advantages of using MSCs for cartilage repair is that they can be obtained from a variety of tissues, including bone marrow (BM), adipose tissue (AT), and umbilical cord tissue (UCT). This means that MSCs can be easily isolated and propagated in vitro, making them a readily available option for cartilage repair and cell-based therapy [7]. In addition, MSCs were shown to secrete various growth factors and cytokines that can promote tissue regeneration and repair. These factors can help stimulate new blood vessel growth, reduce inflammation, and promote the growth of new cartilage cells [6]. This could be the reason why MSCs are often combined with natural or synthetic hydrogels to enhance biocompatibility, biodegradability, and cellular response.
In 2015, Dashtdar et al. investigated whether MSCs seeded in PVA-CS hydrogel could result in comparable or even better cartilage healing than that of previously established alginate-transplanted model. This study confirmed that the PVA-CS-MSCs construct leads to comparable treatment outcomes in the rabbit cartilage defect model, thus suggested for for clinical applications in cartilage regeneration [8]. In extension to this study, the group implanted the PVA-CS in a cadaveric knee cartilage defect using a minimally invasive arthroscopic technique as part of the technical validity prior to the clinical trial study (Figure 1). In 2019, Peng et al. demonstrated that the hydrogel PVA-CS provided an excellent surface for rabbit bone marrow mesenchymal stem cells (rBM-MSCs) adhesion and proliferation. In addition, this group demonstrated that PVA-CS caused no cytotoxicity and achieved the best cartilage repair compared to scaffold alone in an in vivo rabbit model [9].
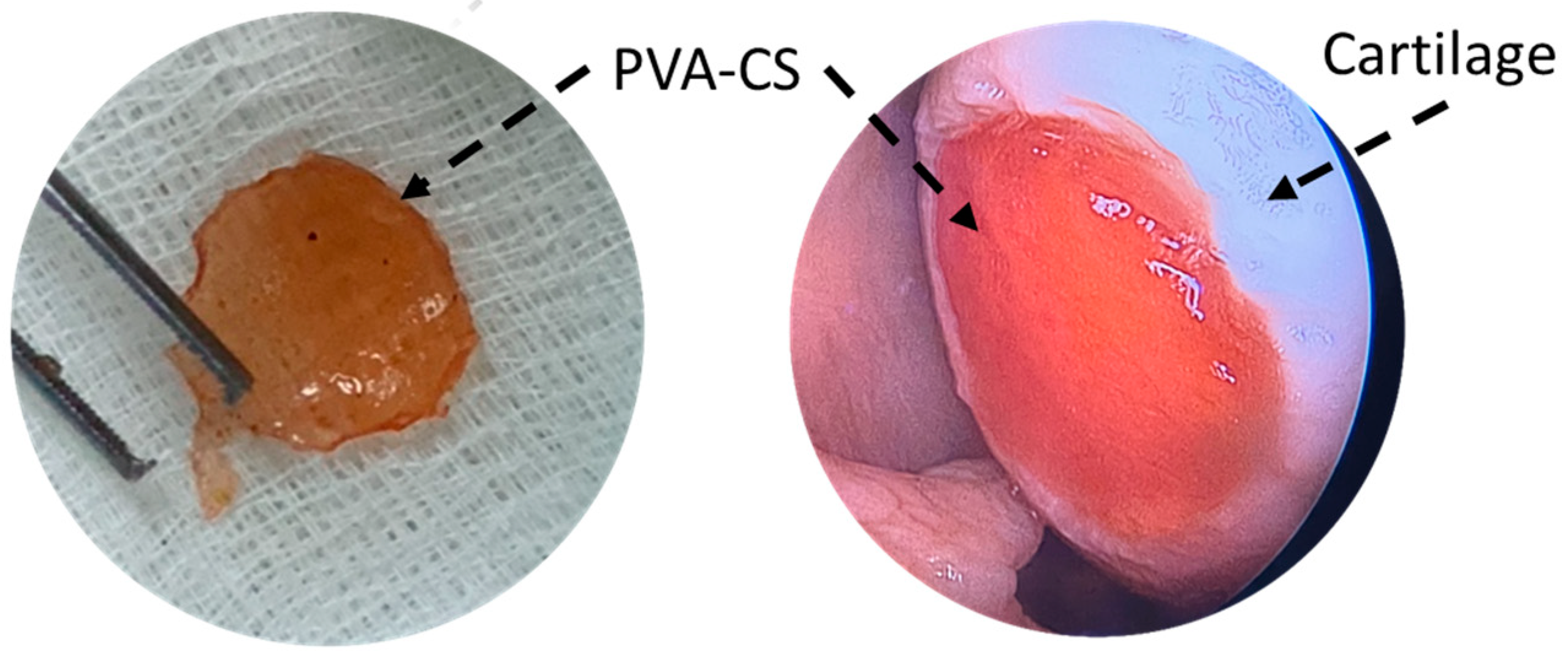
Figure 1. PVA-CS was implanted at the cadaveric knee cartilage defect using a minimally invasive arthroscopic technique.
Nour-Eldeen et al. established a scaffold that allowed adipose-derived mesenchymal stem cells (ADSCs) to proliferate and differentiate into chondrocyte-like cells using PVA-CS nanofiber scaffolds [10]. Characterization of seeded cells, including cell morphology, analysis of surface markers, and chondrogenic differentiation, were studied in vitro. This study suggests that using PVA-CS nanofiber scaffolds had a promoting effect on chondrogenic differentiation of ADSCs, as demonstrated by significant upregulation of aggrecan and collagen type II alpha 1 Chain (COL2A1), suggesting PVA-CS-ADSCs nanofiber scaffolds can potentially be used to improve the pathophysiology of osteoarthritis (OA). Various types of PVA-CS nanofibers were investigated for biomedical applications. PVA-CS nanofibers can be synthesized through different electrospinning techniques, resulting in different fiber morphologies and properties [11]. Many studies incorporated various biological and polymeric materials into PVA-CS nanofibers to improve their properties.
Moreover, the osteoconductive and tissue regeneration performance of the fabricated scaffold was demonstrated with and without AT-MSCs in vivo rat model [12]. Abazari et al. incorporated hydroxyapatite (HA) and platelet-rich plasma (PRP) into PVA-CS to study MSCs survival and osteogenic differentiation potential in vitro. The in vivo study showed that PVA-chitosan-HA(PRP) successfully repaired bone defects to a considerable extent. However, when MSCs were seeded onto PVA-chitosan-HA(PRP), the defects were almost filled. Therefore, it can be inferred that PVA-chitosan-HA(PRP) alone or with cultured stem cells has a promising option as an efficient bone implant.
Recently, Wee et al. investigated the impact of transforming growth factor-beta 1 (TGF-β1) and -β3 on the chondrogenic differentiation of rBM-MSCs, grown on the PVA-CS-PEG (polyethylene glycol) scaffold in comparison to pellet cultures [13]. The study reported that utilization of the PVA-CS-PEG scaffold improved both the proliferation and chondrogenic differentiation of rBM-MSCs. However, no significant differences were observed between the cultures supplemented with or without TGF-β, suggesting no effect of TGF-β1 and TGF-β3 in chondrogenic differentiation. Enhanced cell proliferation observed in PVA-CS-PEG scaffolds may be attributed to the positive charge of chitosan, which facilitates the adhesion and proliferation of BM-MSCs on the scaffold [14]. Moreover, the PVA-CS-PEG scaffold offers a beneficial 3D porous structure that enables high-density cell proliferation of BM-MSCs within the scaffold due to its large surface area-to-volume ratio [13].
Mohammadi et al. developed a novel 3D nanofiber hybrid scaffold of poly(ε-caprolactone), PVA, and CS for bone tissue engineering using MSCs via a multi-jet electrospinning method [15]. The scaffolds’ chemical, physical, and structural properties were investigated to determine their impact on the differentiation of MSCs into osteoblasts and the proliferation of the differentiated cells. SEM microscopic images of MSCs seeded and differentiated on the scaffold showed that the cells attached, permeated, and uniformly distributed within the construct. Additionally, the expression of osteoblastic differentiation markers, including osteocalcin (OCN), osteopontin (OPN), alkaline phosphatase (ALP), and bone sialoprotein (BSP) exhibited an upregulation in constructs cultured in osteogenic media suggested that nanofibrous scaffolds may be favorable for TE [15]. The mechanisms by which PVA-CS nanofibers scaffold promotes osteoblasts differentiation and proliferation of MSCs are multifactorial and involve both physical and chemical cues. The 3D structure of the nanofibers scaffolds that mimic the natural ECM of bone tissue allows for the adhesion and proliferation of MSCs, providing a suitable microenvironment for osteoblast differentiation. Additionally, the high surface area-to-volume ratio allows for the efficient exchange of nutrients and waste products between the cells and the culture medium [16][17].
Interestingly, CS was shown to stimulate osteoblast differentiation and mineralization. Mathews et al. demonstrated the osteogenic potential of CS in a 2D culture system. This study presented novel findings indicating that CS enhanced mineralization by upregulating the genes involved in mineralization as well as calcium-binding proteins such as OPN, Integrin binding sialoprotein (IBSP), Collagen type I alpha 1 chain (COL1A1), ALP, and OCN [18]. Chen et al. developed chitosan nanofibers to investigate their impact on osteoblast maturation and the underlying mechanisms of action in vitro [19]. This study reported that chitosan nanofibers could promote the growth and development of osteoblasts by regulating the expression of genes associated with osteoblasts function, including OPN, OCN, and ALP through the Runt-related transcription factor 2 (RUNX2) pathway [19].
For cardiovascular TE, PVA/CS or PVA-CS was used as a coating material for cardiovascular stents to improve their biocompatibility and reduce the risk of restenosis [20]. In addition, PVA/CS was studied as a potential material for artificial blood vessels and heart valves TE [21]. Research showed that the combination of PVA and CS can yield a composite material with enhanced properties and biocompatibility compared to any single polymer. For example, a study published in the Journal of Biomaterials Science, Polymer Edition, found that a PVA-CS composite coating on a cardiovascular stent reduced the risk of restenosis and improved endothelial cell proliferation compared with a stent coated with PVA or CS alone. Karami et al. reported that PVA-CS composite coating on a cardiovascular stent reduced the risk of restenosis and improved endothelial cell proliferation compared to a stent coated with only PVA or CS [22].
2. Drug Delivery System
Hydrogel delivery systems are used clinically and can provide therapeutically beneficial effects. The use of hydrogels allows for the precise control of the time and location of therapeutic agent delivery, including small-molecule drugs, macromolecular drugs, and cells [23].
PVA-CS was used as a drug delivery system for various therapeutic agents in TE. This combination was shown to enhance drug solubility and stability, increase drug uptake by cells, and improve drug release kinetics. The hydrophilic nature of PVA and the cationic characteristic of CS provides an ideal environment for drug loading and delivery.
In drug delivery, PVA-CS can be used in multiple forms, such as nanoparticles, microparticles, and hydrogels. The different forms have different advantages and can be tailored to meet specific drug delivery requirements. Mahato et al. developed PVA-CS lactate hydrogel and investigated it as a matrix for the continuous and gradual release of hydrophilic drugs [24]. The developed PVA-CS lactate was cross-linked, and freeze-bound water was measured to analyze the cold crystallization properties. Cell adhesion, cytotoxicity, hemolysis, and drug release properties were also investigated. In vitro cell viability of L929 cells showed that PVA-CS lactate hydrogels were compatible with cells and improved cell adhesion. Moreover, the release of ciprofloxacin from the drug-loaded PVA-CS lactate hydrogels inhibited the growth of E. coli, which provided antibacterial activity under physiological conditions [24]. Fathollahipour et al. synthesized a series of hydrogel by blending PVA-CS and adding different amounts of graphene oxide (GO) to develop composite hydrogels [25]. In this study, the drug release profile and kinetics of the drug were studied to predict the mechanism of drug release.
In recent years, PVA-CS nanoparticles were used to encapsulate various drugs, including anticancer drugs, antibiotics, and anti-inflammatory agents. They were shown to increase drug bioavailability and potentially target specific cells or regions. In 2011, Parida et al. included Cloisite 30B in the formulation of PVA-CS as a matrix material component, and curcumin was prepared at various concentrations and loaded with PVA-CS/C 30B nanocomposites to investigate the in vitro drug delivery system for anticancer drugs [26]. They studied the kinetics of the drug release in order to ascertain the type of release mechanism. The kinetics results showed that the drug release was much more significant in the basic medium than in the acidic medium [26].
Shagholani et al. has improved the interaction between PVA and CS hydrogel by magnetite nanoparticles, making them a favorable option for drug delivery and clinical applications [27]. They synthesized magnetite nanoparticles by co-precipitation with ultrasound and then coated them with CS. The CS-coated magnetite nanoparticles were then coated with PVA. These modified nanoparticles present minimal protein adsorption, making them feasible for evading opsonization during clinical applications and drug administration [27]. Cui et al. fabricated PVA-CS nanofibers containing ampicillin sodium using the electrospinning technique. This study reported that the drug release studies, and kinetic analysis of the drug delivery system fitted to the Korsmeyer–Peppas model [28].
Microparticles, microcapsules, and microspheres are common constituents of multiparticulate drug delivery systems. Microparticles are spherical particles ranging in size from 1 to 1000 µm and are used as multiparticulate drug delivery systems to improve efficacy, tolerability, and patient compliance [29]. Microparticles from PVA-CS were used to sustain drug release over an extended period. These particles were loaded with drugs and implanted into the patient’s body for controlled drug delivery [30]. Morelli et al. fabricated PVA-CS microparticles by emulsification for the purpose of encapsulation and controlled release under pH conditions. This study developed a novel technique combining cross-linking with emulsion formation to produce particles with different release profiles based on polymer composition and cross-linking. The study reported that when negatively charged drugs like sodium salicylate are encapsulated, the release of the drug is delayed, and it impacts the selective release under acidic pH conditions [31].
In 2010, the hydrogel PVA-CS was developed to deliver insulin through the nasal cavity [32]. The PVA-CS hydrogels were prepared with different formulations, and the pH was adjusted to a near-neutral value of 1.0 M NaHCO3. Insulin was incorporated into the formulated delivery system, resulting in a final solution with a concentration of 1 IU of insulin per 200 µL. The in vitro insulin release assay showed that glucose levels were maintained for 6 h, while in the in vivo experiment, the greatest reduction was observed 4 h after administration [32]. This suggests that slow release was achieved via the PVA-CS network. In 2017, a similar study was conducted to evaluate the potential of PVA-CS microspheres as a vehicle for insulin drug delivery via intranasal administration [33]. The authors developed different formulations, and morphological analysis of the optimized formulas showed that the size range was between 200 nm to 2 µm. The in vitro study showed that microspheres from PVA-CS exhibited immediate, sharp, and erratic drug release, while the in vivo investigation in rats demonstrated a reduced drug release rate and better mucoadhesive properties [33]. Thus, using PVA-CS in drug delivery systems produced favorable outcomes.
3. Wound Healing
PVA-CS was extensively studied for its potential use in wound healing, as its water retention capacity and antibacterial activity indicate that it is a perfect material for wound treatment [34][35]. CS was shown to have antimicrobial properties due to its ability to interact with bacterial cell membranes and disrupt their structure [33]. The antimicrobial activity of CS may be due to several factors, including its positive charge, which allows it to bind to negatively charged bacterial cell membranes, and its ability to form a gel-like that can physically block the growth and spread of bacteria [33][36][37]. For this reason, it was used in various forms, including hydrogel patches, films, nanofibers, and scaffolds.
PVA-CS hydrogels and films are most used as wound dressings because they can retain water, which is critical for healing. This hydrogel absorbs exudate and creates a protective barrier that shields the wound from external contaminants. Several studies reported the antibacterial activity and healing properties of PVA-CS loaded with other active ingredients [35][38][39][40][41]. Niranjan R. et al. combined PVA-CS with curcumin (CUR) and obtained as PVA-CS-CUR patches by gel casting showed antibacterial activity against the most prevalent strains found (Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis) in wound sites [38]. Furthermore, in vivo studies in albino Wistar rats on wound healing ability showed that this patch has an excellent wound healing ability and can treat all kinds of epidermal damage. Similarly, Gutha et al. developed PVA-CS-zinc oxide (ZnO) beads as a novel wound-healing agent that exhibits antibacterial properties. The antibacterial activity against Escherichia coli and Staphylococcus aureus was evaluated by the inhibition method, and the wound healing properties were tested in mice skin. The PVA-CS -ZnO showed excellent antibacterial and wound-healing activity, suggesting its potential use for wound-healing applications [35].
High cell proliferation capacity is critical for wound healing. Lin et al. reported that the combination of PVA, CS, and dextran exhibited high cell proliferation ability, making them ideal for wound dressing [39]. Recently, Feng et al. reviewed that CS plays a vital role in wound healing [42]. Wound healing processes generally involve four crucial phases: hemostasis, inflammation, proliferation, and skin remodeling. The initial three stages rely significantly on the involvement of CS during the hemostasis stage. CS helps prevent bleeding by promoting platelet and red cell aggregation and preventing fibrin disintegration. In the inflammation stage, CS helps eliminate microorganisms from the wound and finally increases skin proliferation by promoting the growth of granulation tissue in the proliferation stage [42].
Due to their high surface area and ability to mimic natural tissue structure, PVA-CS nanofibers can be used as wound dressings. Nanofibers can increase cell adhesion and migration, critical for wound healing. PVA-CS nanofibers can also be loaded with active ingredients to improve wound healing. Electrospun nanofibers are well suited as wound dressing materials because they have a high surface area ratio, variable pore size distribution, and oxygen permeability [43]. Moreover, the morphology of electrospun nanofibers is comparable to skin ECM, which stimulates cell adhesion, migration, and proliferation [43][44][45] Campa-Siqueiros et al. prepared electrospun gelatin (G) and PVA-CS nanofibers and studied their physicochemical properties and antimicrobial activity [45]. They reported that PVA-CS-G could be used as a wound dressing and combined with common bioactive chemicals or growth factors for its sustained release in treating chronic diabetic patients [45]. In contrast, Liu et al. used the solution-blowing method to prepare hydrogel nanofiber mats from PVA-CS with various ethylene glycol diglycidyl ether (EDGE) content as cross-linker [46]. SEM, FTIR, and X-ray photoelectron spectroscopy (XPS) results suggested that the PVA-CS hydrogel nanofiber mats had both the advantages of a hydrogel and a fiber mat, including excess exudate absorption, facilitation of a moist wound healing environment, permitting gas exchange, and displaying strong antibacterial properties [46].
Fatahian et al. developed a hybrid fiber mat through a co-electrospun hybrid of PVA, CS, and silk fiber mats. The hybrid fiber mat characteristics, including porosity, degradability, pore size, tensile strength, and hydrophilic properties for wound healing, were investigated in vitro and in vivo by localizing BMMSC keratinocytes on the mat [40]. Compared to PVA alone and the fiber PVA-CS, incorporating mixed CS and co-electrospun silk into the PVA-based fiber mat showed excellent cell attachment and growth. In vivo tests also showed that the composite PVA-CS + silk fiber mat incorporating keratinocytes MSCs may promote wound healing and facilitate skin tissue generation [40].
This entry is adapted from the peer-reviewed paper 10.3390/md21050304
References
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848.
- Ebhodaghe, S.O. Hydrogel—Based biopolymers for regenerative medicine applications: A critical review. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 155–172.
- Heng, B.C.; Cao, T.; Stanton, L.W.; Robson, P.; Olsen, B. Strategies for Directing the Differentiation of Stem Cells Into the Osteogenic Lineage In Vitro. J. Bone Miner. Res. 2004, 19, 1379–1394.
- Wagenbrenner, M.; Mayer-Wagner, S.; Rudert, M.; Holzapfel, B.M.; Weissenberger, M. Combinations of Hydrogels and Mesenchymal Stromal Cells (MSCs) for Cartilage Tissue Engineering—A Review of the Literature. Gels 2021, 7, 217.
- Willerth, S.M.; Sakiyama-Elbert, S.E. Combining Stem Cells and Biomaterial Scaffolds for Constructing Tissues and Cell Delivery. StemJournal 2019, 1, 1–25.
- Le, H.; Xu, W.; Zhuang, X.; Chang, F.; Wang, Y.; Ding, J. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 2020, 11, 204173142094383.
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic Differentiation of Mesenchymal Stem Cells: Challenges and Unfulfilled Expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608.
- Dashtdar, H.; Murali, M.R.; Abbas, A.A.; Suhaeb, A.M.; Selvaratnam, L.; Tay, L.X.; Kamarul, T. PVA-chitosan composite hydrogel versus alginate beads as a potential mesenchymal stem cell carrier for the treatment of focal cartilage defects. Knee Surg. Sport. Traumatol. Arthrosc. 2013, 23, 1368–1377.
- Peng, L.; Zhou, Y.; Lu, W.; Zhu, W.; Li, Y.; Chen, K.; Zhang, G.; Xu, J.; Deng, Z.; Wang, D. Characterization of a novel polyvinyl alcohol/chitosan porous hydrogel combined with bone marrow mesenchymal stem cells and its application in articular cartilage repair. BMC Musculoskelet. Disord. 2019, 20, 257.
- Nour-Eldeen, G.; Abdel-Rasheed, M.; EL-Rafei, A.M.; Azmy, O.; El-Bassyouni, G.T. Adipose tissue-derived mesenchymal stem cells and chitosan/poly (vinyl alcohol) nanofibrous scaffolds for cartilage tissue engineering. Cell Regen. 2020, 9, 7.
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2019, 12, 7.
- Abazari, M.F.; Nejati, F.; Nasiri, N.; Khazeni, Z.A.S.; Nazari, B.; Enderami, S.E.; Mohajerani, H. Platelet-rich plasma incorporated electrospun PVA-chitosan-HA nanofibers accelerates osteogenic differentiation and bone reconstruction. Gene 2019, 720, 144096.
- Wee, A.-S.; Lim, C.-K.; Tan, S.-L.; Ahmad, T.S.; Kamarul, T. TGF-β1 and -β3 for Mesenchymal Stem Cells Chondrogenic Differentiation on Poly (Vinyl Alcohol)-Chitosan-Poly (Ethylene Glycol) Scaffold. Tissue Eng. Part C Methods 2022, 28, 501–510.
- Nettles, D.L.; Elder, S.H.; Gilbert, J.A. Potential Use of Chitosan as a Cell Scaffold Material for Cartilage Tissue Engineering. Tissue Eng. 2002, 8, 1009–1016.
- Mohammadi, Y.; Soleimani, M.; Fallahi-Sichani, M.; Gazme, A.; Haddadi-Asl, V.; Arefian, E.; Kiani, J.; Moradi, R.; Atashi, A.; Ahmadbeigi, N. Nanofibrous Poly(ε-Caprolactone)/Poly(Vinyl Alcohol)/Chitosan Hybrid Scaffolds for Bone Tissue Engineering using Mesenchymal Stem Cells. Int. J. Artif. Organs 2007, 30, 204–211.
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly(vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C 2020, 107, 110347.
- Ma, P.; Wu, W.; Wei, Y.; Ren, L.; Lin, S.; Wu, J. Biomimetic gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite scaffolds for bone tissue engineering. Mater. Des. 2021, 207, 109865.
- Mathews, S.; Gupta, P.K.; Bhonde, R.; Totey, S. Chitosan enhances mineralization during osteoblast differentiation of human bone marrow-derived mesenchymal stem cells, by upregulating the associated genes. Cell Prolif. 2011, 44, 537–549.
- Chen, R.-M.; Ho, M.-H.; Liao, M.-H.; Lin, Y.-L.; Lai, C.-H.; Lin, P.-I. Improving effects of chitosan nanofiber scaffolds on osteoblast proliferation and maturation. Int. J. Nanomed. 2014, 9, 4293–4304.
- Lin, M.-C.; Lou, C.-W.; Lin, J.-Y.; Lin, T.A.; Chou, S.-Y.; Chen, Y.-S.; Lin, J.-H. Using spray-coating method to form PVA coronary artery stents: Structure and property evaluations. J. Polym. Res. 2018, 25, 101.
- Wan, W.K.; Campbell, G.; Zhang, Z.F.; Hui, A.J.; Boughner, D.R. Optimizing the tensile properties of polyvinyl alcohol hydrogel for the construction of a bioprosthetic heart valve stent. J. Biomed. Mater. Res. 2002, 63, 854–861.
- Karami, A.; Rezvani Zadeh, S.; Jafari, A.; Jahanbani, A. Preparation and characterization of a polyvinyl alcohol/chitosan composite coating for cardiovascular stents. J. Biomater. Sci. Polym. Ed. 2017, 28, 1846–1861.
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071.
- Mahato, K.K.; Yadav, I.; Singh, R.; Monika; Singh, B.N.; Singh, S.K.; Ray, B.; Kumar, M.; Misra, N. Polyvinyl alcohol/chitosan lactate composite hydrogel for controlled drug delivery. Mater. Res. Express 2019, 6, 115408.
- Fathollahipour, S.; Abouei Mehrizi, A.; Ghaee, A.; Koosha, M. Electrospinning of PVA/chitosan nanocomposite nanofibers containing gelatin nanoparticles as a dual drug delivery system. J. Biomed. Mater. Res. Part A 2015, 103, 3852–3862.
- Parida, U.K.; Nayak, A.K.; Binhani, B.K.; Nayak, P.L. Synthesis and Characterization of Chitosan-Polyvinyl Alcohol Blended with Cloisite 30B for Controlled Release of the Anticancer Drug Curcumin. J. Biomater. Nanobiotechnol. 2011, 2, 414–425.
- Shagholani, H.; Ghoreishi, S.M.; Mousazadeh, M. Improvement of interaction between PVA and chitosan via magnetite nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2015, 78, 130–136.
- Cui, Z.; Zheng, Z.; Lin, L.; Si, J.; Wang, Q.; Peng, X.; Chen, W. Electrospinning and crosslinking of polyvinyl alcohol/chitosan composite nanofiber for transdermal drug delivery. Adv. Polym. Technol. 2017, 37, 1917–1928.
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20.
- Xu, J.; Song, W.; Wu, N.; Tong, J.; Ren, L. Preparation and characterization of chitosan/polyvinyl porous alcohol aerogel microspheres with stable physicochemical properties. Int. J. Biol. Macromol. 2021, 187, 614–623.
- Morelli, S.; Holdich, R.G.; Dragosavac, M.M. Chitosan and Poly (Vinyl Alcohol) microparticles produced by membrane emulsification for encapsulation and pH controlled release. Chem. Eng. J. 2016, 288, 451–460.
- Agrawal, A.K.; Gupta, P.N.; Khanna, A.; Sharma, R.K.; Chandrawanshi, H.K.; Gupta, N.; Patil, U.K.; Yadav, S.K. Development and characterization of in situ gel system for nasal insulin delivery. Die Pharm. 2010, 65, 188–193.
- Ali Darbandi, M. Mucoadhesive Microspheres of Chitosan and Polyvinyl Alcohol as A Carrier for Intranasal Delivery of Insulin: In Vitro and In Vivo Studies. MOJ Bioequivalence Bioavailab. 2017, 3, 39–45.
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434.
- Gutha, Y.; Pathak, J.L.; Zhang, W.; Zhang, Y.; Jiao, X. Antibacterial and wound healing properties of chitosan/poly(vinyl alcohol)/zinc oxide beads (CS/PVA/ZnO). Int. J. Biol. Macromol. 2017, 103, 234–241.
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. Funct. Chitosan 2020, 457–489.
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect Hermetia illucens. Sci. Rep. 2022, 12, 8084.
- Niranjan, R.; Kaushik, M.; Prakash, J.; Venkataprasanna, K.S.; Christy, A.; Pannerselvam, B.; Venkatasubbu, G.D. Enhanced wound healing by PVA/Chitosan/Curcumin patches: In vitro and in vivo study. Colloids Surf. B Biointerfaces 2019, 182, 110339.
- Lin, S.-P.; Lo, K.-Y.; Tseng, T.-N.; Liu, J.-M.; Shih, T.-Y.; Cheng, K.-C. Evaluation of PVA/dextran/chitosan hydrogel for wound dressing. Cell. Polym. 2019, 38, 15–30.
- Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Sadeghi-Avalshahr, A. Preparation of PVA/Chitosan samples by electrospinning and film casting methods and evaluating the effect of surface morphology on their antibacterial behavior. Mater. Res. Express 2019, 7, 015401.
- Fatahian, R.; Mirjalili, M.; Khajavi, R.; Rahimi, M.K.; Nasirizadeh, N. Fabrication of antibacterial and hemostatic electrospun PVA nanofibers for wound healing. SN Appl. Sci. 2020, 2, 1288.
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598.
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Electrospun chitosan/polyvinyl alcohol nanofibre mats for wound healing. Int. Wound J. 2012, 11, 215–222.
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337.
- Campa-Siqueiros, P.; Madera-Santana, T.J.; Ayala-Zavala, J.F.; López-Cervantes, J.; Castillo-Ortega, M.M.; Herrera-Franco, P.J. Nanofibers of gelatin and polivinyl-alcohol-chitosan for wound dressing application: Fabrication and characterization. Polímeros 2020, 30, e2020006.
- Liu, R.; Xu, X.; Zhuang, X.; Cheng, B. Solution blowing of chitosan/PVA hydrogel nanofiber mats. Carbohydr. Polym. 2014, 101, 1116–1121.
This entry is offline, you can click here to edit this entry!
