Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The bioaccessibility of a carotenoid is defined as the maximum quantity of a carotenoid released from the food matrix that is available to be absorbed in the epithelial cells of the intestine. The fraction of an ingested compound that enters the bloodstream and performs its physiological functions is the definition of the bioavailability of a carotenoid.
- carotenoids
- gastrointestinal tract
- intestinal microbiota
1. Introduction
Carotenoids are natural pigments from the tetraterpenes family, characterized by a central chain with 40 atoms of carbon and alternating single and double bonds and various cyclic or acyclic end groups, depending on the carotenoid [1]. In terms of physicochemical properties, carotenoids are colourful lipophilic compounds [1][2], responsible for the variety of colours present in several autotrophs such as microalgae, bacteria, fungi, and plants [1][3].
Humans and animals cannot synthesize carotenoids by themselves, they can be found in their tissues due to the absorption and deposition of the carotenoids ingested in dietary food [2][4][5].
Carotenoids are natural organic pigmented compounds with structural variations, with more than 750 carotenoids being known, but only 40 of them are present in the human diet and 20 in human blood and tissues [1][6][7][8]. The 40 carotenoids present in a usual human diet [1] can be found in coloured fruits and vegetables, such as tomatoes, carrots, and spinach [9].
In terms of chemical constituents, these natural pigments can be divided into two categories: carotenes and xanthophylls [10]. If they are pure hydrocarbons, they can be classified as carotenes such as alpha(α)-carotene, beta(β)-carotene, and lycopene [8]. Xanthophylls are carotenoids with oxygenated derivatives on their terminal rings [8], with complex xanthophylls containing oxygen substituents, aldehyde groups, epoxide groups and oxo/keto groups [11]. Zeaxanthin, lutein, canthaxanthin, violaxanthin and neoxanthin are examples of complex xanthophylls.
Carotenes absorb light energy from chlorophyll and energy from singlet oxygen formed in photosynthesis, being responsible for transmitting this light and protecting the plant tissues [5]. Xanthophylls, synthesized within the plastids, work as accessory pigments, capturing the wavelengths of sunlight that chlorophyll cannot absorb [5].
Regarding functional properties, carotenoids can be classified as primary and secondary carotenoids, with the photosynthetic ones included in the primary group and playing an important role in photosynthesis [2][12].
These natural organic pigmented compounds, in terms of physicochemical properties, are associated with membrane lipid bilayers and cytosolic lipid droplets, which can affect some properties associated with the permeability and fluidity of the membrane [9].
The principal properties of carotenoids mentioned before are illustrated below, in Figure 1.
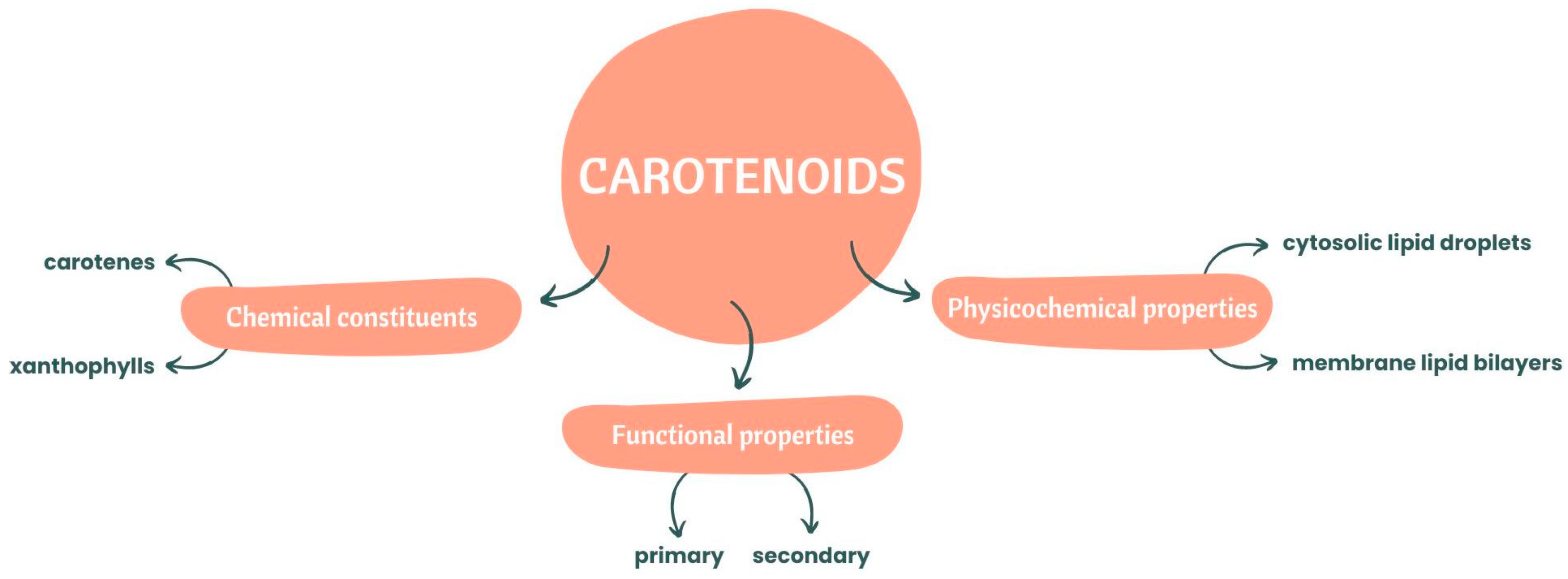
Figure 1. Chemical constituents and functional and physicochemical properties of carotenoids.
The regular consumption of fruits and vegetables is widely recommended due to their multiple health benefits such as the lower incidence of chronic diseases [13] such as cardiovascular diseases (CVDs), several types of cancers [14], and bowel diseases. Chron’s disease and ulcerative colitis are two chronic inflammatory bowel diseases (IBDs) characterized by recurring episodes of inflammation in the gastrointestinal tract (GIT) [15] that cause damage to its tissues [16].
Several studies have attributed to bioactive compounds present in the diet [14], in particular carotenoids [13][17], the responsibility for beneficial health effects in various pathologies, namely IBDs. This can be explained by their several important biological functions such as antioxidant activity [2][18], meaning that these pigments can inhibit or downregulate the unstable compounds produced by the body [2][18] in various pathologies and during oxidative stress caused by reactive oxygen species (ROS) [1][19]. In addition to this, carotenoids have other important functions such as antibacterial, immunological, and anti-inflammatory activity, and beneficial effects on the treatment of diabetes, and in infectious, eye, and neurological diseases [2][18].
Some of the more important biological functions related to human health of the three most known carotenoids are presented in Table 1.
Table 1. Principal biological functions of β-carotene, lutein, and lycopene.
| Carotenoid | Biological Functions | References |
|---|---|---|
| β-carotene | Stimulates the proliferation of lymphocytes; reduces the low-density lipoprotein (LDL) susceptibility to oxidation; activates cell communication; reduces inflammation; improves cardiovascular health. |
[2][20][21] |
| Lutein | Scavenges oxygen intermediates; blue light filter; maintenance of eye health; decreases the proliferation of breast cancer cells; reduces oxidative stress and apoptosis. |
[1][21][22][23][24] |
| Lycopene | Inhibits lipid peroxidation; eliminates reactive oxygen species (ROS); reinforces the immune system; free radical quencher; prevents skin damage. |
[2][21] |
These antioxidant phytochemicals are also important dietary sources of vitamin A and protect cells and tissues from oxidative damage, interacting with other antioxidants [11][25]. So far, only 50 carotenoids are known to have provitamin A activity [12], with α-carotene, β-carotene, gamma(γ)-carotene, and β-cryptoxanthin being the most important precursors of vitamin A in humans [12][20].
Vitamin A is important for proper visual, immune, and gastrointestinal functions, growth, and embryonal development [20]. Humans cannot synthesize vitamin A de novo, obtaining adequate amounts through dietary food, such as from orange and yellow vegetables and in vegetables with dark green leaves [20].
The recent discoveries about the health promotion properties of carotenoids have aroused interest in applying these natural pigments in diversified areas [2]. These natural pigments have several applications such as in feed, and in the food, nutraceutical, and pharmacology industries [5]. Carotenoids can be applied as colourants in food, beverages, and cosmetics, as food supplements, as feed additives, and as supplements [26].
In nature, the bioavailability of carotenoids is reduced [1][20] without processing or any type of treatment, leading to an accumulation in the colon [27], which is colonized by a large number of microorganisms [28] that play important roles in digestion and metabolism [29], as well as in maintaining normal gut physiology and health [30].
Diet is one of the most important regulators of the intestinal microbiota [31], but there is a lack of information about the relationship between carotenoids and the intestinal microbiota [32]. In addition to that, these natural pigments are hydrophobic molecules, which makes their solubility in water low and, when exposed to light, heat, oxygen, or acids, are very susceptible to multiple oxidation and isomerisation reactions [5]. Therefore, the polarity of carotenoids can change due to the polar functional groups attached to the main chain and some products with harmful or unknown effects can also be formed [1], which can affect the carotenoids’ bioaccessibility, bioavailability, and absorption.
2. Bioaccessibility and Bioavailability of Carotenoids
The bioaccessibility of a carotenoid is defined as the maximum quantity of a carotenoid released from the food matrix that is available to be absorbed in the epithelial cells of the intestine [33]. The fraction of an ingested compound that enters the bloodstream and performs its physiological functions is the definition of the bioavailability of a carotenoid [5][34].
In nature, the bioavailability of these natural pigments is reduced, since there is a resistance to digestion and degradation from the protein complexes of carotenoids and the cell walls of plants to achieve adequate release from the matrix [1][20]. In the case of β-carotene, the activity and conversion to vitamin A are high. However, the absorption from plant sources is approximately 65%, with the recommended daily intake of 2–4 mg per day not being achieved [20][35].
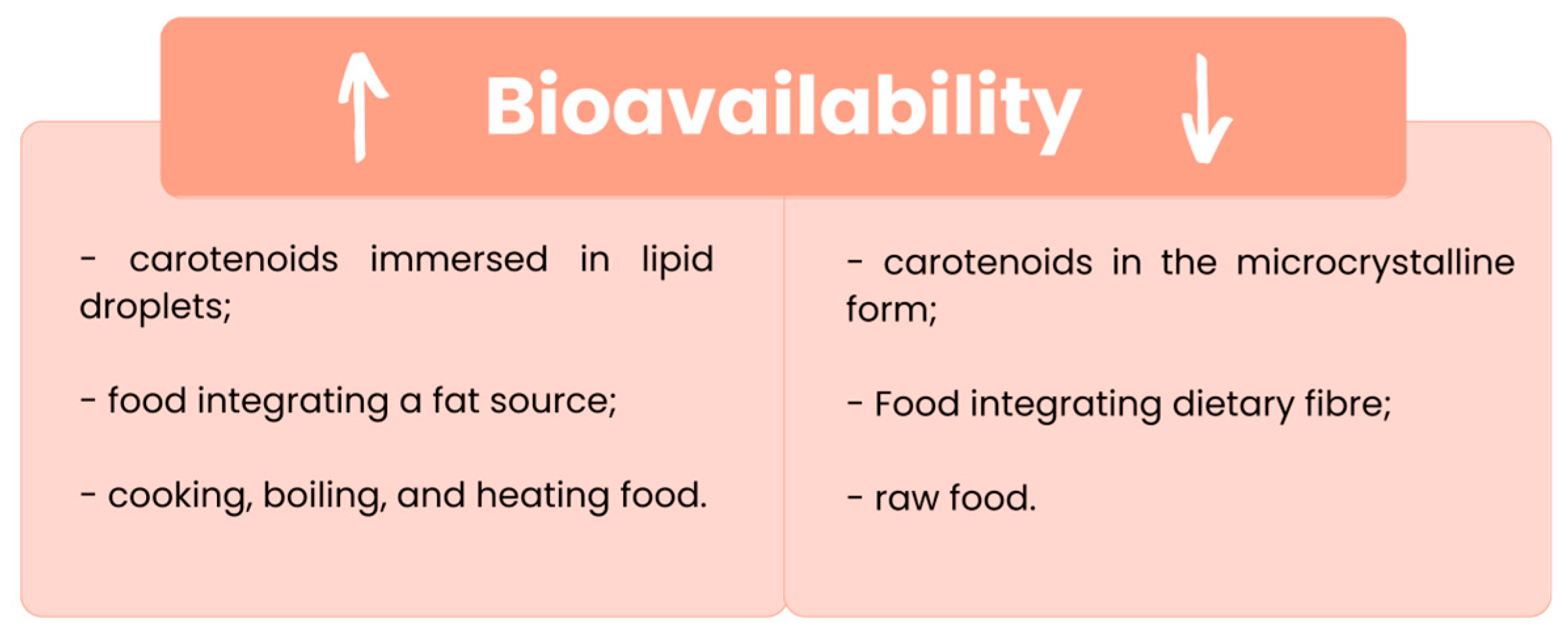
The carotenoids’ bioavailability and consequently absorption have some limitations due to factors such as the dietary sources, food composition, cooking temperature, season, the breakup of the food matrix, presence of lipids, dosage, and presence of other soluble compounds/carotenoids [18][36]. These factors can lead to the release of carotenoids from the food matrix, improving its bioavailability or transforming the carotenoids into isomers that are better absorbed by the organism [36].
The release from the food matrix depends on the state of the carotenoid, as natural pigments immersed entirely in lipid droplets are more easily released than ones in the microcrystalline form [37]. This explains the low availability of lycopene in tomatoes and β-carotene in carrots [37].
The dietary composition also has a significant effect on the bioavailability of carotenoids [36]. Carotenoids are lipophilic compounds and for this reason their bioavailability increases when they are consumed allied with a fat source [38], but decreases when they are consumed with dietary fibre such as pectin [39].
Food thermal processes such as cooking, boiling, and heating disrupt the cellular membrane, allowing the release of carotenoids from the matrix [1][20][21], and although this leads to a decrease in the carotenoid content, it raises their bioavailability and absorption when compared with uncooked food [1][8][20]. For example, in cooked tomatoes, the lycopene availability is higher than in raw tomatoes, and the more prolonged the heat treatment, the lower the carotenoid content is [21].
The principal factors affecting the carotenoids’ bioavailability, enhancing (left) or decreasing (right) it, are represented below, in Figure 2.
Therefore, different extraction technologies are required to increase carotenoids’ solubility and bioavailability [40]. The traditional methodology uses organic solvents such as hexane and acetone to extract carotenoids from food matrices, because of their hydrophobicity [40]. However, the toxicity of these organic solvents to human health, imposes the use of food-grade solvents to purify these carotenoids and use them in the food industry [40].
In the last few years, some alternative methods to recover carotenoids have been presented, such as super-critical fluid extraction (SFE), high hydrostatic pressure (HHP), and Ohmic heating (OH).
SFE is an extraction technique that reduces the toxic solvents used during the process and can generate a solvent-free extract at moderately high selectivity and yield temperatures [11]. Although it is a non-inflammable and non-toxic method, its non-polar nature demands the use of a stabiliser and a cosolvent, and carotenoid degradation and/or isomerization can occur [40][41]. This technique is advantageous insofar as the process is both environmentally benign and energy efficient and the sustainable solvent is simple to obtain. However, it presents some limitations, since it is an expensive method and the polar extracts are insoluble in the CO2 mobile phase [42][43][44].
HHP is a simpler and more efficient technique than conventional extraction methods, that contributes to improve the bioaccessibility of bioactive compounds [45]. HHP is advantageous since it is a completely solvent-free procedure that uses tomato leaf waste at a high CO2 pressure (180 bar), and at room temperature to obtain phylloquinone [41]. However, once again it is limited by its high cost, and by the necessary improvements in the associated recovery process [46].
More recently, Coelho et al. [47] proposed OH, which consists of the use of an electric current that passes across a conductor matrix (e.g., food) to generate heat from the electrical resistance of the matrix. This methodology is more advantageous than the ones mentioned before since it allows the extraction of bioactive compounds such as carotenoids and polyphenols from their matrices only using ethanol:water as a solvent [45][47], and the application of a low temperature prevents thermal losses [11].
The authors [47] showed that this method can replace traditional methods since it is selective, enabling bioactive compounds to be extracted without organic solvents. OH has some limitations given the impossibility of extracting some bioactive compounds that remain bound to dietary fibres and the lack of information about the potential antioxidant properties of these bioactive compounds, as well as how they are affected by the GIT during digestion [48].
This entry is adapted from the peer-reviewed paper 10.3390/nu15102265
References
- Bolhassani, A.; Milani, A.; Basirnejad, M.; Shahbazi, S. Carotenoids: Biochemistry, Pharmacology and treatment. Br. J. Pharm. 2017, 174, 1290–1324.
- Nabi, F.; Arain, M.A.; Rajput, N.; Alagawany, M.; Soomro, J.; Umer, M.; Soomro, F.; Wang, Z.; Ye, R.; Liu, J. Health Benefits of Carotenoids and Potential Application in Poultry Industry: A Review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1809–1818.
- Marhuenda-Muñoz, M.; Hurtado-Barroso, S.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.M. A Review of Factors That Affect Carotenoid Concentrations in Human Plasma: Differences between Mediterranean and Northern Diets. Eur. J. Clin. Nutr. 2019, 72, 18–25.
- O’Byrne, S.M.; Blaner, W.S. Retinol and Retinyl Esters: Biochemistry and Physiology. J. Lipid Res. 2013, 54, 1731–1743.
- Singh, A.; Ahmad, S.; Ahmad, A. Green Extraction Methods and Environmental Applications of Carotenoids-a Review. RSC Adv. 2015, 5, 62358–62393.
- Jomova, K.; Valko, M. Health Protective Effects of Carotenoids and Their Interactions with Other Biological Antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110.
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; da Rosa, C.G.; da Silva, M.M. Elaboration of Microparticles of Carotenoids from Natural and Synthetic Sources for Applications in Food. Food Chem. 2016, 202, 324–333.
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How Effective Are They to Prevent Age-Related Diseases? Molecules 2019, 24, 1801.
- Reboul, E. Mechanisms of Carotenoid Intestinal Absorption: Where Do We Stand? Nutrients 2019, 11, 838.
- Yaroshevich, I.A.; Krasilnikov, P.M.; Rubin, A.B. Functional Interpretation of the Role of Cyclic Carotenoids in Photosynthetic Antennas via Quantum Chemical Calculations. Comput. Theor. Chem. 2015, 1070, 27–32.
- Coelho, M.C.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Integral Valorisation of Tomato By-Products towards Bioactive Compounds Recovery: Human Health Benefits. Food Chem. 2023, 410, 135319.
- Amorim-Carrilho, K.T.; Cepeda, A.; Fente, C.; Regal, P. Review of Methods for Analysis of Carotenoids. TrAC—Trends Anal. Chem. 2014, 56, 49–73.
- Kaulmann, A.; Bohn, T. Carotenoids, Inflammation, and Oxidative Stress-Implications of Cellular Signaling Pathways and Relation to Chronic Disease Prevention. Nutr. Res. 2014, 34, 907–929.
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and Beta-Carotene Induce Cell-Cycle Arrest and Apoptosis in Human Breast Cancer Cell Lines. Anticancer Res. 2014, 34, 1377–1386.
- Meurer, M.C.; Mees, M.; Mariano, L.N.B.; Boeing, T.; Somensi, L.B.; Mariott, M.; da Silva, R.d.C.M.V.d.A.F.; dos Santos, A.C.; Longo, B.; Santos França, T.C.; et al. Hydroalcoholic Extract of Tagetes Erecta L. Flowers, Rich in the Carotenoid Lutein, Attenuates Inflammatory Cytokine Secretion and Improves the Oxidative Stress in an Animal Model of Ulcerative Colitis. Nutr. Res. 2019, 66, 95–106.
- Centers for Disease Control and Prevention. What Is Inflammatory Bowel Disease (IBD)? Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022.
- Hu, F.; Wang Yi, B.; Zhang, W.; Liang, J.; Lin, C.; Li, D.; Wang, F.; Pang, D.; Zhao, Y. Carotenoids and Breast Cancer Risk: A Meta-Analysis and Meta-Regression. Breast Cancer Res. Treat. 2012, 131, 239–253.
- Arain, M.A.; Mei, Z.; Hassan, F.U.; Saeed, M.; Alagawany, M.; Shar, A.H.; Rajput, I.R. Lycopene: A Natural Antioxidant for Prevention of Heat-Induced Oxidative Stress in Poultry. Worlds Poult. Sci. J. 2017, 74, 89–100.
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. Biomed. Res. Int. 2014, 2014, 761264.
- Donhowe, E.G.; Kong, F. Beta-Carotene: Digestion, Microencapsulation, and In Vitro Bioavailability. Food Bioproc. Tech. 2014, 7, 338–354.
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential Allies of Cardiovascular Health? Food Nutr. Res. 2015, 59, 26762.
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot, T.T.J.M. Lutein: More than Just a Filter for Blue Light. Prog. Retin. Eye Res. 2012, 31, 303–315.
- Liu, X.-H.; Yu, R.-B.; Liu, R.; Hao, Z.-X.; Han, C.-C.; Zhu, Z.-H.; Ma, L. Association between Lutein and Zeaxanthin Status and the Risk of Cataract: A Meta-Analysis. Nutrients 2014, 6, 452–465.
- Chang, J.; Zhang, Y.; Li, Y.; Lu, K.; Shen, Y.; Guo, Y.; Qi, Q.; Wang, M.; Zhang, S. NrF2/ARE and NF-ΚB Pathway Regulation May Be the Mechanism for Lutein Inhibition of Human Breast Cancer Cell. Future Oncol. 2018, 14, 719–726.
- Szabo, K.; Cătoi, A.-F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing By-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277.
- Jaswir, I.; Noviendri, D.; Hasrini, R.F.; Octavianti, F. Carotenoids: Sources, Medicinal Properties and Their Application in Food and Nutraceutical Industry. J. Med. Plant Res. 2011, 5, 7119–7131.
- Dai, Z.; Li, Z.; Shi, E.; Nie, M.; Feng, L.; Chen, G.; Gao, R.; Zeng, X.; Li, D. Study on the Interaction between Four Typical Carotenoids and Human Gut Microflora Using an in Vitro Fermentation Model. J. Agric. Food Chem. 2022, 70, 13592–13601.
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell 2012, 148, 1258–1270.
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment Dominates over Host Genetics in Shaping Human Gut Microbiota. Nature 2018, 555, 210–215.
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2020, 113, 2019–2040.
- Feng, W.; Liu, J.; Cheng, H.; Zhang, D.; Tan, Y.; Peng, C. Dietary Compounds in Modulation of Gut Microbiota-Derived Metabolites. Front. Nutr. 2022, 9, 939571.
- Chen, D.; Chen, G.; Ding, Y.; Wan, P.; Peng, Y.; Chen, C.; Ye, H.; Zeng, X.; Ran, L. Polysaccharides from the Flowers of Tea (Camellia sinensis L.) Modulate Gut Health and Ameliorate Cyclophosphamide-Induced Immunosuppression. J. Funct. Foods 2019, 61, 103470.
- Ekesa, B.; Poulaert, M.; Davey, M.W.; Kimiywe, J.; van den Bergh, I.; Blomme, G.; Dhuique-Mayer, C. Bioaccessibility of Provitamin A Carotenoids in Bananas (Musa Spp.) and Derived Dishes in African Countries. Food Chem. 2012, 133, 1471–1477.
- Sy, C.; Gleize, B.; Dangles, O.; Landrier, J.F.; Veyrat, C.C.; Borel, P. Effects of Physicochemical Properties of Carotenoids on Their Bioaccessibility, Intestinal Cell Uptake, and Blood and Tissue Concentrations. Mol. Nutr. Food Res. 2012, 56, 1385–1397.
- Haskell, M.J. The Challenge to Reach Nutritional Adequacy for Vitamin A: β-Carotene Bioavailability and Conversion—Evidence in Humans. Am. J. Clin. Nutr. 2012, 96, 1193S–1203S.
- Marques, C.S.; Lima, M.J.R.; Oliveira, J.; Teixeira-Lemos, E. Tomato Lycopene: Functional Proprieties and Health. Int. J. Agric. Biosyst. Eng. 2015, 9, 1089–1099.
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488.
- Nagao, A.; Kotake-Nara, E.; Hase, M. Effects of Fats and Oils on the Bioaccessibility of Carotenoids and Vitamin E in Vegetables. Biosci. Biotechnol. Biochem. 2013, 77, 1055–1060.
- Molteni, C.; la Motta, C.; Valoppi, F. Improving the Bioaccessibility and Bioavailability of Carotenoids by Means of Nanostructured Delivery Systems: A Comprehensive Review. Antioxidants 2022, 11, 1931.
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in Human Health. LWT 2020, 127, 109323.
- Arab, M.; Bahramian, B.; Schindeler, A.; Valtchev, P.; Dehghani, F.; McConchie, R. Extraction of Phytochemicals from Tomato Leaf Waste Using Subcritical Carbon Dioxide. Innov. Food Sci. Emerg. Technol. 2019, 57, 102204.
- Manna, L.; Bugnone, C.A.; Banchero, M. Valorization of Hazelnut, Coffee and Grape Wastes through Supercritical Fluid Extraction of Triglycerides and Polyphenols. J. Supercrit. Fluids 2015, 104, 204–211.
- Oliveira, D.A.; Salvador, A.A.; Smânia, A.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial Activity and Composition Profile of Grape (Vitis Vinifera) Pomace Extracts Obtained by Supercritical Fluids. J. Biotechnol. 2013, 164, 423–432.
- de Campos, L.M.A.S.; Leimann, F.V.; Pedrosa, R.C.; Ferreira, S.R.S. Free Radical Scavenging of Grape Pomace Extracts from Cabernet Sauvingnon (Vitis Vinifera). Bioresour. Technol. 2008, 99, 8413–8420.
- Coelho, M.C.; Pereira, R.N.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. The Use of Emergent Technologies to Extract Added Value Compounds from Grape By-Products. Trends Food Sci. Technol. 2020, 106, 182–197.
- Jha, A.K.; Sit, N. Extraction of Bioactive Compounds from Plant Materials Using Combination of Various Novel Methods: A Review. Trends Food Sci. Technol. 2022, 119, 579–591.
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of Tomato By-Products’ Bioactive Compounds Using Ohmic Technology. Food Bioprod. Process. 2019, 117, 329–339.
- Ribeiro, T.B.; Oliveira, A.; Campos, D.; Nunes, J.; Vicente, A.A.; Pintado, M. Simulated Digestion of an Olive Pomace Water-Soluble Ingredient: Relationship between the Bioaccessibility of Compounds and Their Potential Health Benefits. Food Funct. 2020, 11, 2238–2254.
This entry is offline, you can click here to edit this entry!
