Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Galectin-3 (Gal-3), a beta-galactoside-binding lectin, plays a pivotal role in various cellular processes, including immune responses, inflammation, and cancer progression.
- galectin-3
- viral infection
- host–pathogen interactions
1. Introduction
Viruses are diverse and intricate pathogens that have evolved to infect a wide range of hosts, including humans [1]. They are classified based on their genomic properties, dividing them into DNA and RNA viruses [2]. To date, 219 virus species have been identified as capable of infecting humans [3]. The yellow fever virus, an RNA virus, was the first human virus to be described in 1901 by Reed and Carroll [3]. Interestingly, more than two-thirds of viruses that infect humans can also infect other animals, and many emerging viruses that infect humans have been found to originate from mammals or birds [4]. In the complex interplay between viruses and their hosts, Gal-3 has emerged as a multifaceted player in host–pathogen interactions, playing a pivotal role in viral infections [5].
Lectins are proteins that bind to specific sugar moieties or structures and play essential roles in various cellular and physiological regulations [6]. Galectins, a type of S-type lectin, bind to β-galactosides, such as disaccharide N-acetyllactosamine, found in N-linked and O-linked glycoproteins [7]. Galectins are involved in regulating mRNA splicing, apoptosis, cell cycle, cancer formation, metastasis, immune responses, and more [8]. They are considered pattern recognition receptors and are found in various immune cells and tissues, where they play potential and significant roles in human infections caused by different types of pathogens [7][9].
Galectin-3 is a unique chimera-type galectin characterized by a C-terminal carbohydrate recognition domain (CRD) and a large N-terminal (NT) protein-binding domain (Figure 1) [10]. Galectin-3 is encoded by the LGALS3 gene and is primarily expressed in the cell cytoplasm, but it can also move into the nucleus and be secreted onto the cell surface [10][11]. It is involved in various biological activities, including cell growth, pre-mRNA splicing, differentiation, angiogenesis, inflammation, fibrosis, apoptosis, and host defense [12]. Galectin-3 is expressed abundantly during viral infections in various immune cells, fibroblasts, and epithelial and endothelial cells [12]. It not only regulates viral entry and attachment but also mediates inflammatory responses, potentially causing an imbalanced production of pro-inflammatory cytokines [13].
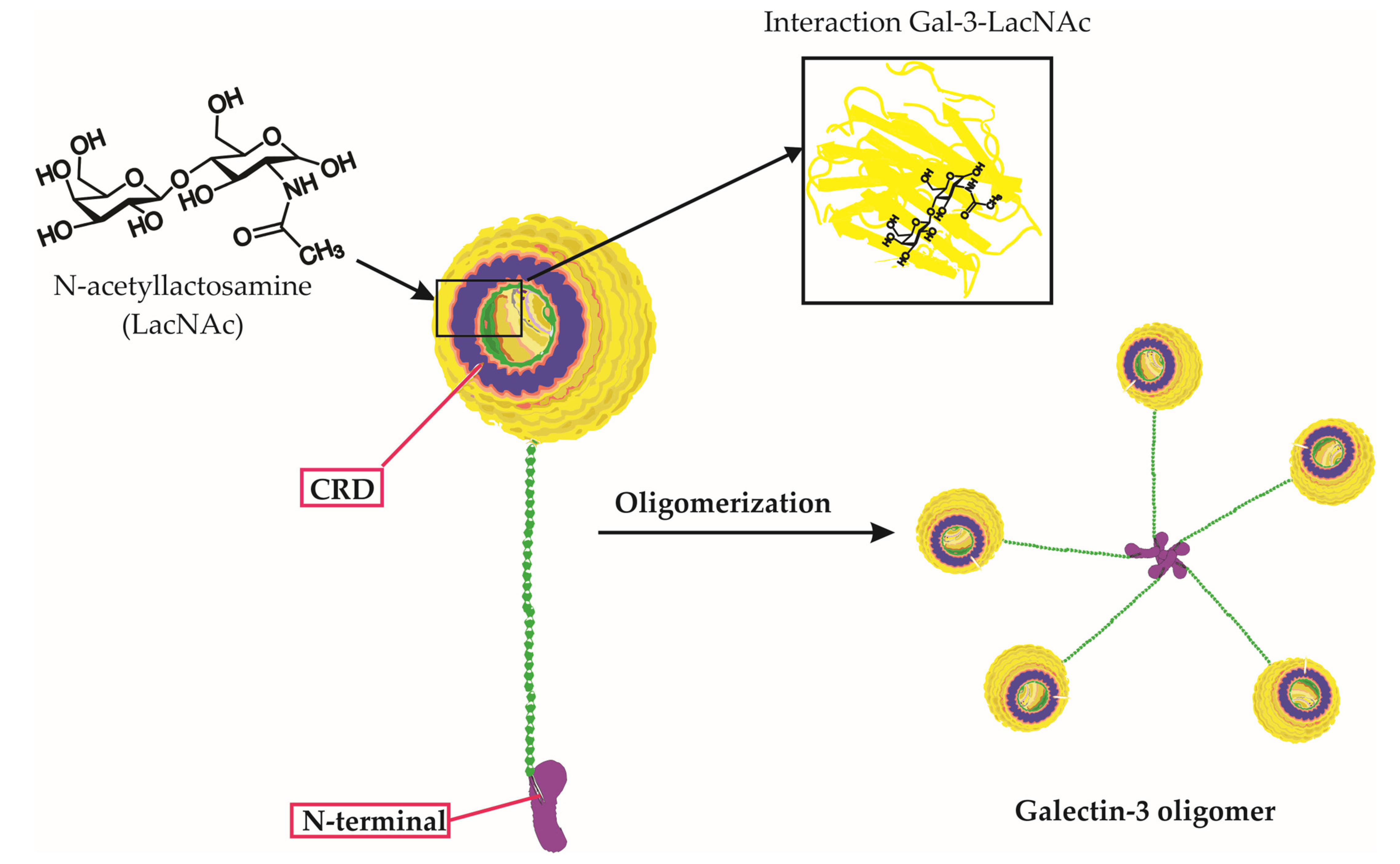
Figure 1. Galectin-3 Structure, Carbohydrate Binding, and Oligomerization. This figure presents an in-depth look at the Galectin-3 (Gal-3) molecule, emphasizing its unique chimeric structure, the specificity of its carbohydrate interactions, and the process of oligomerization. The three-dimensional representation of Gal-3 displays its distinctive carbohydrate recognition domain (CRD) and N-terminal tail. Notably, the figure highlights the binding of specific carbohydrate molecules, including lactose and N-acetyllactosamine (LacNAc), to the CRD of Gal-3. Furthermore, it depicts the oligomerization of Gal-3 molecules, wherein the N-terminal tails interact with each other to form complex higher-order structures. This interaction of Gal-3 and LacNAc within the CRD, along with its overall structure and carbohydrate binding capability, underscores Galectin-3’s critical roles in various biological processes.
2. The Comprehensive Impact of Galectin-3 on Viral Infections: From Entry to Immunity, Life Cycle, and Disease Progression
Galectin-3 is a multifaceted protein that plays a crucial role in various aspects of viral infections, with diverse and sometimes contrasting effects depending on the virus and the host’s immune response [14]. This section aims to provide a comprehensive understanding of the impact of Gal-3 on viral infections, encompassing its involvement in viral entry, modulation of immune responses, regulation of the viral life cycle, and its contribution to disease progression (Figure 2).
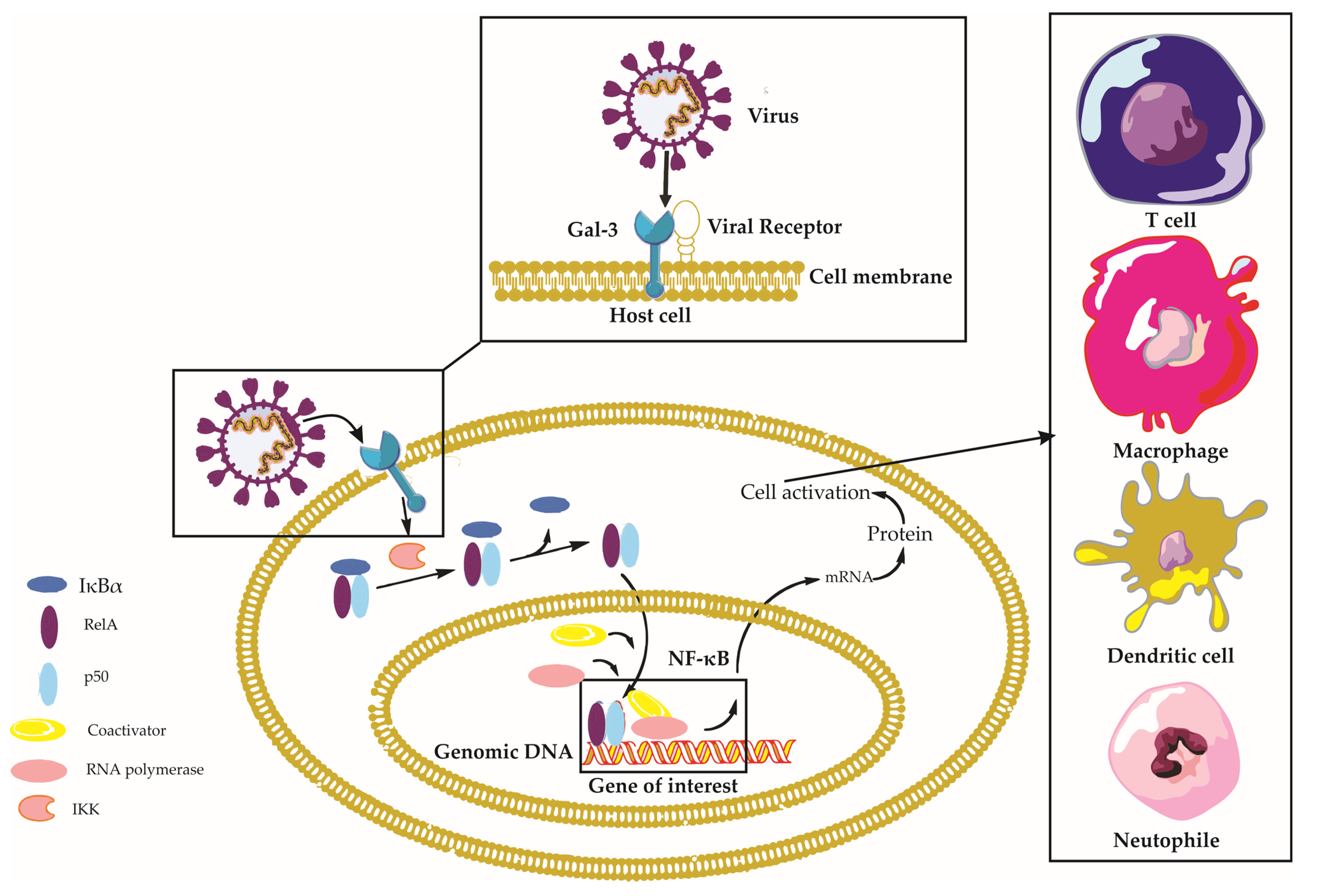
Figure 2. Galectin-3 in Viral Infections: A Comprehensive Overview. This figure depicts the intricate interplay between Galectin-3 (Gal-3), viral particles, and host cells during viral infections, emphasizing the activation of the Nuclear Factor Kappa B (NF-κB) signaling pathway. Viral particles interacting with Gal-3 on the host cell surface trigger various intracellular signaling pathways, including the NF-κB pathway. Normally, the inactive NF-κB complex—comprising RelA (p65), p50, and IκBα—is confined to the cytoplasm. The viral–Gal-3 interaction activates IκB kinase (IKK), which then phosphorylates IκBα. This phosphorylation signals IκBα for ubiquitination and subsequent proteasomal degradation, revealing the nuclear localization signal of the NF-κB dimer (RelA/p50), which translocates to the nucleus. Here, the NF-κB dimer binds to κB sites on DNA, thereby regulating the transcription of target genes with assistance from coactivators. This activity leads to diverse cellular responses, including immune response and inflammation. Concurrently, the figure illustrates immune cells, such as macrophages and T-cells, migrating towards the host cell in response to the Gal-3–virus interaction, underlining their essential roles in the immune response. Key components involved in this context include Nuclear Factor-kappa B (NF-κB), Receptor Enhancer of Nuclear Factor Kappa-B Ligand A (RelA), Inhibitor of Nuclear Factor Kappa-B Alpha (IκBα), NF-kappa-B p50 subunit (p50), I kappa B kinase (IKK), and Galectin-3 (Gal-3).
2.1. Galectin-3 and Viral Entry
Viral entry is a crucial step in the infection process, where the virus gains entry into the host cell to replicate and further spread the infection. This process usually involves the interaction between viral proteins and host cell receptors [15]. As a carbohydrate-binding protein, Gal-3 can interact with glycans present on the surface of both host cells and viral particles, which can modulate the viral entry process [16].
2.1.1. Galectin-3 as a Facilitator of Viral Attachment
The initial step in the process of viral infection is viral attachment, as it enables the virus to bind to specific receptors on the surface of host cells and initiate the process of viral replication [15]. Galectin-3, a member of the galectin family of proteins, has a carbohydrate recognition domain (CRD) that binds to β-galactosides found on host cells and viral glycoproteins [16]. In the case of human immunodeficiency virus (HIV), Gal-3 interacts with the viral envelope glycoprotein gp120, enhancing its binding to host cell surface receptors, such as CD4, and subsequently enhancing infection [17]. Additionally, Gal-3 plays a vital role in the entry of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) into host cells, and blocking it may prevent disease progression. Human Gal-3 has significant structural and sequence similarity to the N-terminal domain (NTD) of SARS-CoV-2, important for viral entry, suggesting that Gal-3 inhibitors targeting regions with structural overlap to the NTD may have double binding capabilities, providing a potential strategy to inhibit viral entry [18].
Galectin-3 has been identified as a crucial mediator in the entry and attachment of herpes simplex virus (HSV) during ocular infections and facilitating influenza binding to airway epithelial cells [19][20]. Specifically, HSV-1 directly binds human Gal-3, and targeted disruption of Gal-3 impairs HSV-1 infectivity in human corneal keratinocytes [19]. In the case of influenza, Gal-3 binds to the hemagglutinin (HA) protein of Influenza A viruses (IAVs) and desialylated airway epithelial cells, increasing Streptococcus pneumoniae adhesion [20]. These findings suggest that Gal-3 plays a critical role in viral attachment and entry for HSV-1 and influenza, making it a potential target for therapeutic intervention.
2.1.2. Galectin-3 and Viral Internalization
Following attachment, Gal-3 may also be involved in the internalization of viruses into host cells. Evidence suggests that Gal-3 can facilitate endocytosis and macropinocytosis, two common routes for viral entry [21]. For example, Gal-3 has been shown to promote HIV-1 internalization through interacting with the host CD4 receptor and the viral gp120 glycoprotein [17].
Overall, Galectin-3’s ability to interact with viral glycoproteins and host cell surface receptors highlights its potential as a target for antiviral therapies. Through inhibiting Gal-3 function or blocking its interaction with viral glycoproteins, it may be possible to disrupt viral attachment and entry, thus preventing infection and disease progression. Further research is needed to better understand the specific molecular mechanisms underlying Gal-3-mediated viral attachment and to develop targeted therapies to combat various viral infections.
2.2. Galectin-3 and Immune Responses
Galectin-3 modulates the immune response during viral infections. It can activate or inhibit immune signaling pathways, depending on the virus and the cellular context [22]. In some cases, Gal-3 promotes the production of pro-inflammatory cytokines and chemokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), enhancing the antiviral response [23].
2.2.1. Galectin-3 in Immune Cell Activation and Recruitment
Galectin-3 influences immune cell activation and recruitment during viral infection, modulating the activation of T cells, dendritic cells, macrophages, and neutrophils and affecting their cytokine production and chemotactic responses [10]. Galectin-3 promotes neutrophil migration towards infection sites via binding to specific glycan structures on their surface and recruiting neutrophils to the lungs during influenza and Streptococcus pneumoniae co-infection [7][24]. In COVID-19, there was a strong positive correlation between Gal-3 and interleukin-1 beta (IL-1β), as well as a moderate positive correlation between Gal-3, TNF-α, and IL-12, indicating a T-helper 1 (Th1) immune response [25]. Patients with severe COVID-19 had significantly higher percentages of Gal-3+ T cells, which may contribute to elevated systemic Gal-3 levels and promote inflammation [25].
In the context of Hepatitis B virus (HBV) infection, Gal-3 has been suggested to inhibit IL-10, possibly sustaining HBV replication and initiating chronic HBV infection [26]. Galectin-3 has also been linked to macrophage polarization during dengue virus infection, which is associated with infection severity [27]. The modulation of macrophage polarization by Gal-3 can impact the balance between pro-inflammatory and anti-inflammatory responses, influencing the disease outcome [27]. The complex and context-dependent roles of Gal-3 in viral infections highlight its potential as a therapeutic target but also underscore the need for a deeper understanding of its functions across various infectious diseases.
2.2.2. Galectin-3 in the Regulation of Immune Signaling Pathways
Galectin-3 plays a pivotal role in modulating immune signaling pathways during viral infections, either activating or inhibiting these pathways depending on the cellular context, which influences the immune response [22]. One key pathway influenced by Gal-3 is the Nuclear Factor-kappa B (NF-κB) signaling pathway, a critical transcription factor that regulates genes involved in immune and inflammatory responses [28]. Galectin-3 activates NF-κB, leading to the production of pro-inflammatory cytokines and enhancing the antiviral response, which contributes to the cytokine storm observed in severe COVID-19 cases [29][30].
In addition to NF-κB, Gal-3 modulates other signaling pathways such as Janus kinase/signal transducers and activators of transcription (JAK/STAT), extracellular signal-regulated kinase (ERK), and protein kinase B (AKT), which are involved in various cellular processes [31]. During viral infections, Gal-3 can dysregulate these pathways, leading to aberrant immune responses and contributing to disease pathogenesis [23]. Galectin-3 is also an agonist of Toll-like receptor 4 (TLR4) and can regulate the NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome, promoting inflammasome assembly and activation, which contributes to enhanced inflammation and tissue damage in viral infections [28][32][33].
Galectin-3 has been implicated in the regulation of suppressor of cytokine signaling 1 (SOCS1) and retinoic acid-inducible gene I (RIG-I) expression during influenza and Streptococcus pneumoniae co-infection [23]. Galectin-3 modulates SOCS1 expression, which may contribute to the overall inflammatory response, and can influence RIG-I expression during co-infection, leading to dysregulated expression and release of pro-inflammatory cytokines [23]. Overall, Galectin-3’s complex and context-dependent role in immune signaling pathways highlights the need for understanding its precise functions in various infections to identify its potential as a therapeutic target or biomarker for disease severity.
2.2.3. Galectin-3 in Modulating Apoptosis and Autophagy
Galectin-3 can also influence host cell survival and death mechanisms, such as apoptosis and autophagy [22][34]. Although the anti-apoptotic function of Gal-3 has been widely reported in various pathological processes, its role in virus-infected cells and its potential contribution to viral replication and persistence remain unclear [35]. Understanding the mechanisms through which Gal-3 impacts viral replication and persistence could lead to the development of novel antiviral therapies targeting Gal-3, providing new options for the treatment of viral diseases. Therefore, further research into the involvement of Gal-3 in virus–host interactions is crucial for the development of effective antiviral treatments.
On the other hand, Gal-3 has been implicated in the regulation of autophagy, a cellular process that can promote viral clearance through degrading viral components [34]. Notably, in adenovirus infections, Gal-3 has been demonstrated to suppress autophagic activation [36]. These findings highlight the multifaceted and intricate role of Gal-3 in host–virus interactions, particularly in the context of adenovirus infections.
2.3. Galectin-3 and Viral Life Cycle
Galectin-3, a multifunctional protein with roles in various biological processes, has emerged as an important player in the context of viral infections [10]. Researchers will explore the involvement of Gal-3 in the viral life cycle and its impact on viral replication, assembly, and release. Understanding the complex interactions between Gal-3 and viral pathogens can provide valuable insights into host–pathogen dynamics and potentially inform the development of novel therapeutic strategies.
2.3.1. Galectin-3 in Viral Replication
Galectin-3 is known to interact with viral proteins and host factors to promote viral genome replication. For example, Gal-3 was shown to facilitate HIV-1 replication via promoting cell fusion through fibronectin and Gal-3-mediated mechanisms [37]. HIV-1 Tat, an important regulatory protein for viral replication, can also induce the expression of Gal-3, which contributes to the virus’s pathogenesis through promoting replication and modulating immune responses [38]. Influenza A virus replication can be inhibited through upregulating Gal-3 expression, which enhances the expression of antiviral genes that inhibit virus replication [39]. Galectin-3 has also been shown to impact the replication of Enterovirus 71 (EV71) [40]. Galectin-3 may play a role in sustaining HBV replication through stimulating the production of cytokines and chemokines via Cluster of Differentiation 98 (CD98) interactions with macrophages [26]. In the context of SARS-CoV-2, Gal-3 inhibition could potentially influence viral RNA synthesis [41].
2.3.2. Galectin-3 in Viral Assembly and Release
Galectin-3 may also play a role in the assembly of viral particles and their release from infected host cells [22]. It has been suggested that Gal-3 can promote the budding of viral particles through interacting with viral structural proteins and host cell membrane components [42]. Galectin-3 has been shown to interact with the HIV-1 Gag protein and promote viral assembly and release. It functions via interacting intracellularly with Gag and the cellular ALG-2-interacting protein X (Alix), which are essential for viral budding and replication when new infectious virions are generated [42]. Additionally, Gal-3 plays a role in regulating virological synapse formation and facilitating intercellular HIV-1 transfer among CD4+ T cells, providing an alternative pathway for HIV-1 infection regulation [43]. Exosomes derived from HIV-1-infected dendritic cells with high Gal-3 expression have been found to facilitate HIV-1 infection and dissemination through fibronectin and Gal-3-mediated cell fusion [37]. These findings suggest that Gal-3 plays a crucial role in regulating viral assembly and release, virological synapse formation, and exosome-mediated cell-to-cell communication in HIV-1 infection. Targeting Gal-3 could represent a promising therapeutic strategy against HIV-1 infection.
3. Galectin-3 in Specific Viral Diseases
3.1. SARS-CoV-2 Virus Infection and Galectin-3
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has affected millions of people worldwide [44]. The severity of the disease varies, with some individuals developing critical conditions such as acute respiratory distress syndrome (ARDS), cytokine storm, and thromboembolic complications [45]. Identifying reliable prognostic markers and therapeutic targets is crucial for improving patient outcomes [46]. One potential candidate is Gal-3, a protein involved in inflammation, lung fibrosis, and thrombogenicity [47][48].
3.1.1. The Role of Galectin-3 in Modulating Immune Response and Inflammation in COVID-19
Galectin-3 is a multifunctional protein that plays a crucial role in various biological processes, including inflammation, cell adhesion, and immune modulation [10]. In the context of SARS-CoV-2 infection, Gal-3 has been implicated in modulating the host immune response, potentially contributing to the severity of COVID-19. Gal-3’s pro-inflammatory effects include promoting inflammation through stimulating the production and release of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 [13]. Elevated levels of these cytokines have been associated with severe COVID-19 cases, where patients may experience a “cytokine storm”, leading to ARDS and multi-organ failure [30][45].
Galectin-3 has also been shown to activate the NLRP3 inflammasome, a critical component of the innate immune response that detects and responds to pathogens [18]. Activation of the NLRP3 inflammasome by Gal-3 results in the production and release of pro-inflammatory cytokines, including IL-1β and IL-18, which contribute to the overall inflammatory response during SARS-CoV-2 infection [25]. This inflammasome activation may potentiate the severity of COVID-19, particularly in patients with pre-existing conditions or comorbidities.
Recent studies have shown a significant association between elevated Gal-3 levels and COVID-19 severity [25]. Patients with critical COVID-19 have been found to have higher serum levels of Gal-3, along with increased proinflammatory cytokines (IL-1β, TNF-α, IL-12) and chemokine C-C chemokine receptor type 5 (CCR5) expression in T cells [25]. These findings suggest that Gal-3 may contribute to the acquired proinflammatory immune response and intensify the innate pro-inflammatory immune response in the lungs, making it a valuable marker for predicting disease severity.
3.1.2. Potential Role of Human Galectin-3 in SARS-CoV-2 Viral Adherence and Entry
The N-terminal domain of the SARS-CoV-2 spike protein plays a crucial role in viral adherence and entry into host cells [18]. Recent studies have revealed that the NTD of SARS-CoV-2 shares a significant degree of similarity with human Gal-3, a lectin involved in numerous biological processes, including inflammation and cell adhesion [13]. The structural similarities between the NTD of SARS-CoV-2 and human Gal-3 suggest that they may share common interaction partners or have similar functional roles, potentially facilitating SARS-CoV-2’s ability to hijack cellular processes and enhance its infectivity [13].
3.1.3. Galectin-3 as a Potential Biomarker for COVID-19 Severity and Prognosis
Galectin-3 is a potential biomarker for predicting COVID-19 severity, prognosis, and treatment response [49]. Patients with severe COVID-19 exhibit higher levels of Gal-3, which are associated with increased inflammation, cytokine release, ARDS, and complications such as thrombosis, organ failure, and lung fibrosis [50]. Gal-3 levels correlate with inflammatory markers such as CRP, IL-6, and TNF-α, and monitoring these levels can help clinicians identify high-risk patients and guide clinical decision-making [51]. Galectin-3 also correlates with markers of thrombogenicity and clinical disease severity, suggesting its usefulness in assessing disease severity and hypercoagulability in COVID-19 patients [47].
Galectin-3 is involved in various aspects of COVID-19 pathogenesis, including promotion of inflammation, dysregulation of immune response, fibrosis and tissue remodeling, and endothelial dysfunction and thrombosis [47][52]. Understanding the role of Gal-3 in COVID-19 pathogenesis can inform the development of targeted therapies and management strategies, potentially improving patient outcomes and reducing the global burden of this devastating pandemic.
3.1.4. Comparative Roles and Interactions of Galectin-3 and Galectin-3-Binding Protein in SARS-CoV-2 Infection
Galectin-3 and Galectin-3-binding protein (Gal-3BP) both play significant roles in viral infections [14]. Galectin-3-binding protein is a multifunctional glycoprotein encoded by the LGALS3BP gene, with a wide distribution across various cell types and biological fluids [53]. With its three functional domains, Gal-3BP engages in intricate cell-to-cell and cell-to-matrix interactions [14]. It is part of a broad spectrum of biological processes, including immune response, inflammation, and tumor progression [14]. Gal-3BP’s relationship with Gal-3 is particularly noteworthy, as it forms an important axis in various immune and inflammatory processes [54]. Elevated levels of Gal-3BP are found in a range of pathological conditions, such as cancer, viral infections, and autoimmune disorders, often correlating with adverse clinical outcomes [14].
In the context of viral infections, Gal-3 and Gal-3BP share some similarities but also exhibit important differences in their roles [14]. Both proteins have been associated with unfavorable outcomes in certain viral diseases, including SARS-CoV-2 infection [55][56]. They are both involved in the innate immune response to viral infections, stimulating the production of interferon and pro-inflammatory cytokines. Their interaction is believed to modulate the expression of IL-6, a cytokine which plays a critical role in the immune response to viral infections [14].
However, while Gal-3 primarily facilitates viral entry and replication, Gal-3BP appears to exhibit antiviral activities, limiting virus expression and replication [14]. This difference suggests that while Gal-3 enhances virus propagation, Gal-3BP participates in the immune system’s defense against viral infections [14].
In the context of SARS-CoV-2 infection, the levels of both Gal-3 and Gal-3BP are elevated, with higher levels associated with disease severity. They are both thought to contribute to the pathogenesis of COVID-19, particularly through their involvement in the excessive inflammatory response observed in severe cases. However, Gal-3BP has been specifically associated with worse clinical outcomes, whereas Gal-3 levels correlate with disease severity [14].
Interestingly, the interaction between Gal-3 and Gal-3BP has been linked to the development of pulmonary fibrosis in severe cases of COVID-19. This is because Gal-3, through its interaction with Gal-3BP, enhances Transforming Growth Factor beta (TGF-β) signaling, a key driver of fibrosis. This suggests that targeting the Gal-3/Gal-3BP axis could be a potential therapeutic approach for preventing or treating pulmonary fibrosis in severe COVID-19 cases [14].
Nevertheless, the precise roles of Gal-3 and Gal-3BP in viral infections, including SARS-CoV-2, are still not fully understood, and further research is needed to elucidate their respective contributions and the potential therapeutic implications.
3.2. Human Immunodeficiency Virus (HIV) Infection and Galectin-3
The human immunodeficiency virus is a retrovirus that attacks the immune system, leading to a progressive decline in its function. Over time, this decline can result in acquired immunodeficiency syndrome (AIDS), a severe condition characterized by a weakened immune system and increased vulnerability to opportunistic infections and malignancies [57].
Galectin-3 is a protein that has been implicated in various aspects of HIV infection, including viral entry, infection, dissemination, and pathogenesis.
3.2.1. Galectin-3 in HIV-1 Entry and Infection
Research has demonstrated that Gal-3 can bind to HIV-1 gp120 and CD4 proteins, enhancing viral entry and infection. The carbohydrate recognition domain of Gal-3 interacts with HIV-1 gp120 or CD4 molecules, triggering the formation of Gal-3 oligomers, which facilitate infection [17]. Galectin-3 has been found to enhance HIV-1 entry and infection up to 20-fold, suggesting a critical role in the early stages of viral infection [37]. Additionally, differences in Gal-3 expression among various HIV-1 subtypes have been observed, implying that further investigation is necessary to understand the specific roles of galectin-3 in different viral strains [58].
3.2.2. Galectin-3 Expression and HIV-1 Tat Protein
The HIV-1 Tat protein is a crucial factor in viral replication, playing a significant role in the transactivation of the HIV-1 promoter and the efficient transcription of the viral genome [59]. Besides regulating viral gene expression, Tat also modulates host cell gene expression, promoting cell survival and proliferation and suppressing immune responses [60]. The interaction of Tat with host factors, such as specificity protein 1 (Sp1), is crucial in regulating both viral and host gene expression and underscores its importance in the pathogenesis of HIV-1 infection [61]. Notably, the expression of Gal-3 is upregulated in HIV-1-infected cells, particularly during the early stages of infection [62]. Tat has been shown to induce Gal-3 expression through the transactivation of Sp1-rich regulatory sequences upstream of the Gal-3 gene [38]. The observed interaction between Sp1 and Tat in HIV-1-infected cells suggests a potential role for Tat protein in the regulation of Gal-3 expression. Therefore, the upregulation of Gal-3 expression in Tat-expressing cells highlights the potential of viral infection to induce the expression of this protein, which may contribute to the pathogenesis of HIV infection [38].
3.2.3. Galectin-3 and HIV-1 Dissemination
Galectin-3 plays a crucial role in the process of HIV-1 viral budding, which is the final stage of the viral replication cycle [42]. This stage involves the release of newly formed virus particles from infected host cells, contributing significantly to the spread of HIV-1 infection within the host and the progression of the disease [43]. Furthermore, Gal-3 is highly expressed in exosomes derived from HIV-1-infected dendritic cells, compared to those from uninfected cells [37]. This finding suggests that Gal-3 may facilitate HIV-1 infection and dissemination. However, the role of Gal-3 in exosomes derived from HIV-1 infected T cells appears to be cell-type specific, warranting further investigation [37].
Cell-to-cell transmission of HIV-1 is an efficient mode of viral dissemination, allowing the virus to bypass extracellular defenses and evade host immune responses. Galectin-3 has been implicated in facilitating this process, contributing to the spread of the virus within the host [43]. Virological synapses are specialized cell–cell junctions that enable direct transfer of HIV-1 particles from infected cells to uninfected target cells [63]. Galectin-3, with its ability to bind glycans on cell surfaces, may promote the formation of these synapses through stabilizing cell–cell contacts and enhancing adhesion between infected and uninfected cells [36]. This interaction could lead to more efficient viral transmission and faster dissemination within the host. Additionally, the presence of Gal-3 in exosomes released from HIV-1 infected cells, particularly dendritic cells, may also contribute to cell-to-cell transmission [37]. The increased expression of Gal-3 in exosomes derived from infected cells may facilitate the transfer of viral particles and promote infection in recipient cells [37].
3.2.4. Galectin-3 and HIV-1-Infected Macrophage Cell Death
Galectin-3 has been shown to induce cell death in HIV-1-infected macrophages, which could potentially contribute to the eradication of the virus [64]. The mechanism of Gal-3-induced cell death appears to involve glycosylation changes in the Gal-3 receptor and is caspase-independent, not relying on receptor-interacting protein kinase 1 (RIPK1)- or RIPK3-dependent necroptosis [64]. Furthermore, the study found that Endo G levels were significantly increased in the nucleus and decreased in the cytoplasm in Gal-3-treated cells, suggesting that Gal-3 may act as a novel apoptosis-inducing agent in response to HIV-1 infection [64].
3.2.5. Galectin-3 and HIV-Associated Pathogenesis
Elevated expression of Gal-3 has been implicated in HIV-associated pathogenesis, potentially contributing to the development of malignancies such as Kaposi’s sarcoma [65]. Gal-3 has been associated with tumor cell growth, and its upregulation may be linked to the proliferative response observed in HIV-infected individuals [12][65]. Therefore, understanding the intricate interplay between Gal-3 expression, HIV infection, and disease progression is critical for developing novel therapeutic strategies to improve the management of HIV-infected patients.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24119617
References
- Sudhan, S.S.; Sharma, P. Human Viruses: Emergence and Evolution. Emerg. Reemerging Viral Pathog. 2020, 1, 53–68.
- Louten, J. Virus Structure and Classification. Essent. Hum. Virol. 2016, 19–29.
- Woolhouse, M.; Scott, F.; Hudson, Z.; Howey, R.; Chase-Topping, M. Human viruses: Discovery and emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2864–2871.
- Gonzalez, J.P.; Macgregor-Skinner, G. Dangerous Viral Pathogens of Animal Origin: Risk and Biosecurity: Zoonotic Select Agents. Zoonoses-Infect. Affect. Hum. Anim. 2014, 1015–1062.
- Machala, E.A.; McSharry, B.P.; Rouse, B.T.; Abendroth, A.; Slobedman, B. Gal power: The diverse roles of galectins in regulating viral infections. J. Gen. Virol. 2019, 100, 333–349.
- Mishra, A.; Behura, A.; Mawatwal, S.; Kumar, A.; Naik, L.; Mohanty, S.S.; Manna, D.; Dokania, P.; Mishra, A.; Patra, S.K.; et al. Structure-function and application of plant lectins in disease biology and immunity. Food Chem. Toxicol. 2019, 134, 110827.
- Vasta, G.R. Roles of galectins in infection. Nat. Rev. Microbiol. 2009, 7, 424–438.
- Laderach, D.J.; Compagno, D. Unraveling How Tumor-Derived Galectins Contribute to Anti-Cancer Immunity Failure. Cancers 2021, 13, 4529.
- Vasta, G.R. Galectins as pattern recognition receptors: Structure, function, and evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36.
- Díaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediat. Inflamm. 2017, 2017, 9247574.
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The role of Galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology 2018, 7, e1434467.
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379.
- Caniglia, J.L.; Asuthkar, S.; Tsung, A.J.; Guda, M.R.; Velpula, K.K. Immunopathology of galectin-3: An increasingly promising target in COVID-19. F1000Research 2020, 9, 1078.
- Gallo, V.; Arienzo, A.; Iacobelli, S.; Iacobelli, V.; Antonini, G. Gal-3BP in Viral Infections: An Emerging Role in Severe Acute Respiratory Syndrome Coronavirus 2. Int. J. Mol. Sci. 2022, 23, 7314.
- Dimitrov, D.S. Virus entry: Molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004, 2, 109–122.
- Miller, N.L.; Clark, T.; Raman, R.; Sasisekharan, R. Glycans in Virus-Host Interactions: A Structural Perspective. Front. Mol. Biosci. 2021, 8, 666756.
- Lin, C.-Y.; Wang, W.-H.; Huang, S.-W.; Yeh, C.-S.; Yuan, R.-Y.; Yang, Z.-S.; Urbina, A.N.; Tseng, S.-P.; Lu, P.-L.; Chen, Y.-H.; et al. The Examination of Viral Characteristics of HIV-1 CRF07_BC and Its Potential Interaction with Extracellular Galectin-3. Pathogens 2020, 9, 425.
- Odun-Ayo, F.; Reddy, L. Potential Roles of Modified Pectin Targeting Galectin-3 against Severe Acute Respiratory Syndrome Coronavirus-2. J 2021, 4, 824–837.
- Woodward, A.M.; Mauris, J.; Argüeso, P. Binding of transmembrane mucins to galectin-3 limits herpesvirus 1 infection of human corneal keratinocytes. J. Virol. 2013, 87, 5841–5847.
- Nita-Lazar, M.; Banerjee, A.; Feng, C.; Amin, M.N.; Frieman, M.B.; Chen, W.H.; Cross, A.S.; Wang, L.X.; Vasta, G.R. Desialylation of airway epithelial cells during influenza virus infection enhances pneumococcal adhesion via galectin binding. Mol. Immunol. 2015, 65, 1–16.
- Rotshenker, S. Galectin-3 (MAC-2) controls phagocytosis and macropinocytosis through intracellular and extracellular mechanisms. Front. Cell. Neurosci. 2022, 16, 949079.
- Liu, F.T.; Stowell, S.R. The role of galectins in immunity and infection. Nat. Rev. Immunol. 2023, 1–16.
- Nita-Lazar, M.; Banerjee, A.; Feng, C.; Vasta, G.R. Galectins regulate the inflammatory response in airway epithelial cells exposed to microbial neuraminidase by modulating the expression of SOCS1 and RIG1. Mol. Immunol. 2015, 68, 194–202.
- Snarr, B.D.; St-Pierre, G.; Ralph, B.; Lehoux, M.; Sato, Y.; Rancourt, A.; Takazono, T.; Baistrocchi, S.R.; Corsini, R.; Cheng, M.P.; et al. Galectin-3 enhances neutrophil motility and extravasation into the airways during Aspergillus fumigatus infection. PLoS Pathog. 2020, 16, e1008741.
- Gajovic, N.; Markovic, S.S.; Jurisevic, M.; Jovanovic, M.; Arsenijevic, N.; Mijailovic, Z.; Jovanovic, M.; Jovanovic, I. Galectin-3 as an important prognostic marker for COVID-19 severity. Sci. Rep. 2023, 13, 1460.
- Uluca, Ü.; Şen, V.; Ece, A.; Tan, İ.; Karabel, D.; Aktar, F.; Karabel, M.; Balık, H.; Güneş, A. Serum galectin-3 levels in children with chronic hepatitis B infection and inactive hepatitis B carriers. Med. Sci. Monit. 2015, 21, 1376–1380.
- Lee, M.S.; Tseng, Y.H.; Chen, Y.C.; Kuo, C.H.; Wang, S.L.; Lin, M.H.; Huang, Y.F.; Wang, Y.W.; Lin, Y.C.; Hung, C.H. M2 macrophage subset decrement is an indicator of bleeding tendency in pediatric dengue disease. J. Microbiol. Immunol. Infect. 2018, 51, 829–838.
- Zhou, W.; Chen, X.; Hu, Q.; Chen, X.; Chen, Y.; Huang, L. Galectin-3 activates TLR4/NF-κB signaling to promote lung adenocarcinoma cell proliferation through activating lncRNA-NEAT1 expression. BMC Cancer 2018, 18, 580.
- Darif, D.; Hammi, I.; Kihel, A.; El Idrissi Saik, I.; Guessous, F.; Akarid, K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021, 153, 104799.
- Garcia-Revilla, J.; Deierborg, T.; Venero, J.L.; Boza-Serrano, A. Hyperinflammation and Fibrosis in Severe COVID-19 Patients: Galectin-3, a Target Molecule to Consider. Front. Immunol. 2020, 11, 2069.
- Lin, C.Y.; Yang, Z.S.; Wang, W.H.; Urbina, A.N.; Lin, Y.T.; Huang, J.C.; Liu, F.T.; Wang, S.F. The Antiviral Role of Galectins toward Influenza A Virus Infection-An Alternative Strategy for Influenza Therapy. Pharmaceuticals 2021, 14, 490.
- Stojanovic, M.D.; Stojanovic, B.; Arsenijevic, N.; Stojanovic, B. The Role of TLR-4 and Galectin-3 Interaction in Acute Pancreatitis. Exp. Appl. Biomed. Res. (EABR) 2020.
- Arsenijevic, A.; Stojanovic, B.; Milovanovic, J.; Arsenijevic, D.; Arsenijevic, N.; Milovanovic, M. Galectin-3 in Inflammasome Activation and Primary Biliary Cholangitis Development. Int. J. Mol. Sci. 2020, 21, 5097.
- Liu, D.; Zhu, H.; Li, C. Galectins and galectin-mediated autophagy regulation: New insights into targeted cancer therapy. Biomark. Res. 2023, 11, 22.
- Nangia-Makker, P.; Nakahara, S.; Hogan, V.; Raz, A. Galectin-3 in apoptosis, a novel therapeutic target. J. Bioenerg. Biomembr. 2007, 39, 79–84.
- Li, F.-Y.; Wang, S.-F.; Bernardes, E.S.; Liu, F.-T. Galectins in Host Defense Against Microbial Infections. In Lectin in Host Defense Against Microbial Infections; Hsieh, S.-L., Ed.; Springer: Singapore, 2020; pp. 141–167.
- Kulkarni, R.; Prasad, A. Exosomes Derived from HIV-1 Infected DCs Mediate Viral trans-Infection via Fibronectin and Galectin-3. Sci. Rep. 2017, 7, 14787.
- Fogel, S.; Guittaut, M.; Legrand, A.; Monsigny, M.; Hébert, E. The Tat protein of HIV-1 induces galectin-3 expression. Glycobiology 1999, 9, 383–387.
- Yang, M.-L.; Chen, Y.-C.; Wang, C.-T.; Chong, H.-E.; Chung, N.-H.; Leu, C.-H.; Liu, F.-T.; Lai, M.M.C.; Ling, P.; Wu, C.-L.; et al. Upregulation of galectin-3 in influenza A virus infection promotes viral RNA synthesis through its association with viral PA protein. J. Biomed. Sci. 2023, 30, 14.
- Huang, W.C.; Chen, H.L.; Chen, H.Y.; Peng, K.P.; Lee, Y.; Huang, L.M.; Chang, L.Y.; Liu, F.T. Galectin-3 and Its Genetic Variation rs4644 Modulate Enterovirus 71 Infection. PLoS ONE 2016, 11, e0168627.
- Sigamani, A.; Mayo, K.H.; Miller, M.C.; Chen-Walden, H.; Reddy, S.; Platt, D. An Oral Galectin Inhibitor in COVID-19—A Phase II Randomized Controlled Trial. Vaccines 2023, 11, 731.
- Wang, S.F.; Tsao, C.H.; Lin, Y.T.; Hsu, D.K.; Chiang, M.L.; Lo, C.H.; Chien, F.C.; Chen, P.; Arthur Chen, Y.M.; Chen, H.Y.; et al. Galectin-3 promotes HIV-1 budding via association with Alix and Gag p6. Glycobiology 2014, 24, 1022–1035.
- Wang, S.F.; Hung, Y.H.; Tsao, C.H.; Chiang, C.Y.; Teoh, P.G.; Chiang, M.L.; Lin, W.H.; Hsu, D.K.; Jan, H.M.; Lin, H.C.; et al. Galectin-3 facilitates cell-to-cell HIV-1 transmission by altering the composition of membrane lipid rafts in CD4 T cells. Glycobiology 2022, 32, 760–777.
- Monserrat, J.; Gómez-Lahoz, A.; Ortega, M.A.; Sanz, J.; Muñoz, B.; Arévalo-Serrano, J.; Rodríguez, J.M.; Gasalla, J.M.; Gasulla, Ó.; Arranz, A.; et al. Role of Innate and Adaptive Cytokines in the Survival of COVID-19 Patients. Int. J. Mol. Sci. 2022, 23, 10344.
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92.
- Biji, A.; Khatun, O.; Swaraj, S.; Narayan, R.; Rajmani, R.S.; Sardar, R.; Satish, D.; Mehta, S.; Bindhu, H.; Jeevan, M.; et al. Identification of COVID-19 prognostic markers and therapeutic targets through meta-analysis and validation of Omics data from nasopharyngeal samples. EBioMedicine 2021, 70, 103525.
- Puccini, M.; Jakobs, K.; Reinshagen, L.; Friebel, J.; Schencke, P.-A.; Ghanbari, E.; Landmesser, U.; Haghikia, A.; Kränkel, N.; Rauch, U. Galectin-3 as a Marker for Increased Thrombogenicity in COVID-19. Int. J. Mol. Sci. 2023, 24, 7683.
- Shen, H.; Zhang, N.; Liu, Y.; Yang, X.; He, Y.; Li, Q.; Shen, X.; Zhu, Y.; Yang, Y. The Interaction Between Pulmonary Fibrosis and COVID-19 and the Application of Related Anti-Fibrotic Drugs. Front. Pharm. 2021, 12, 805535.
- Cervantes-Alvarez, E.; la Rosa, N.L.-d.; la Mora, M.S.-d.; Valdez-Sandoval, P.; Palacios-Jimenez, M.; Rodriguez-Alvarez, F.; Vera-Maldonado, B.I.; Aguirre-Aguilar, E.; Escobar-Valderrama, J.M.; Alanis-Mendizabal, J.; et al. Galectin-3 as a potential prognostic biomarker of severe COVID-19 in SARS-CoV-2 infected patients. Sci. Rep. 2022, 12, 1856.
- Baykiz, D.; Emet, S.; Ayduk-Govdeli, E.; Kaytaz, M.; Yavuz, M.L.; Karaca-Ozer, P.; Karaayvaz, E.B.; Medetalibeyoglu, A.; Elitok, A.; Genc, S.; et al. Galectin-3 as a Novel Biomarker for Predicting Clinical Outcomes in Hospitalized COVID-19 Patients. Clin. Lab. 2022, 68, 1–4.
- Özcan, S.; Dönmez, E.; Yavuz, S.T.; Ziyrek, M.; İnce, O.; Küçük, H.S.; Taşdemir, Z.A.; Yılmaz, İ.; Varol, S.; Şahin, İ.; et al. Prognostic significance of serum galectin-3 in hospitalized patients with COVID-19. Cytokine 2022, 158, 155970.
- Reddy, K.; Nichol, A.; McAuley, D.F. Galectin-3 Inhibition in COVID-19. Am. J. Respir. Crit. Care Med. 2023, 207, 118–120.
- Calabrese, G.; Sures, I.; Pompetti, F.; Natoli, G.; Palka, G.; Iacobelli, S. The gene (LGALS3BP) encoding the serum protein 90K, associated with cancer and infection by the human immunodeficiency virus, maps at 17q25. Cytogenet. Cell Genet. 1995, 69, 223–225.
- Xu, G.; Xia, Z.; Deng, F.; Liu, L.; Wang, Q.; Yu, Y.; Wang, F.; Zhu, C.; Liu, W.; Cheng, Z.; et al. Inducible LGALS3BP/90K activates antiviral innate immune responses by targeting TRAF6 and TRAF3 complex. PLoS Pathog. 2019, 15, e1008002.
- Kuśnierz-Cabala, B.; Maziarz, B.; Dumnicka, P.; Dembiński, M.; Kapusta, M.; Bociąga-Jasik, M.; Winiarski, M.; Garlicki, A.; Grodzicki, T.; Kukla, M. Diagnostic Significance of Serum Galectin-3 in Hospitalized Patients with COVID-19-A Preliminary Study. Biomolecules 2021, 11, 1136.
- Gallo, V.; Gentile, R.; Antonini, G.; Iacobelli, S. Increased Gal-3BP plasma levels in hospitalized patients infected with SARS-CoV-2. Clin. Exp. Med. 2023, 23, 151–155.
- Boasso, A.; Shearer, G.M.; Chougnet, C. Immune dysregulation in human immunodeficiency virus infection: Know it, fix it, prevent it? J. Intern. Med. 2009, 265, 78–96.
- Wang, W.H.; Yeh, C.S.; Lin, C.Y.; Yuan, R.Y.; Urbina, A.N.; Lu, P.L.; Chen, Y.H.; Chen, Y.A.; Liu, F.T.; Wang, S.F. Amino Acid Deletions in p6(Gag) Domain of HIV-1 CRF07_BC Ameliorate Galectin-3 Mediated Enhancement in Viral Budding. Int. J. Mol. Sci. 2020, 21, 2910.
- Das, A.T.; Harwig, A.; Berkhout, B. The HIV-1 Tat protein has a versatile role in activating viral transcription. J. Virol. 2011, 85, 9506–9516.
- Clark, E.; Nava, B.; Caputi, M. Tat is a multifunctional viral protein that modulates cellular gene expression and functions. Oncotarget 2017, 8, 27569–27581.
- Loregian, A.; Bortolozzo, K.; Boso, S.; Caputo, A.; Palù, G. Interaction of Sp1 transcription factor with HIV-1 Tat protein: Looking for cellular partners. FEBS Lett. 2003, 543, 61–65.
- Wang, W.-H.; Lin, C.-Y.; Chang, M.R.; Urbina, A.N.; Assavalapsakul, W.; Thitithanyanont, A.; Chen, Y.-H.; Liu, F.-T.; Wang, S.-F. The role of galectins in virus infection—A systemic literature review. J. Microbiol. Immunol. Infect. 2020, 53, 925–935.
- Feldmann, J.; Schwartz, O. HIV-1 Virological Synapse: Live Imaging of Transmission. Viruses 2010, 2, 1666–1680.
- Xue, J.; Fu, C.; Cong, Z.; Peng, L.; Peng, Z.; Chen, T.; Wang, W.; Jiang, H.; Wei, Q.; Qin, C. Galectin-3 promotes caspase-independent cell death of HIV-1-infected macrophages. FEBS J. 2017, 284, 97–113.
- Alcendor, D.J.; Knobel, S.M.; Desai, P.; Zhu, W.Q.; Vigil, H.E.; Hayward, G.S. KSHV downregulation of galectin-3 in Kaposi’s sarcoma. Glycobiology 2010, 20, 521–532.
This entry is offline, you can click here to edit this entry!
