1. Types of Microplastic (MP) Particles
1.1. Primary MPs
Primary microplastic particles are characterized as plastic products that are small. These plastics are commonly found in cosmetic products and face washes [
20], and there is growing evidence that they are employed in pharmacies as drug delivery systems [
21]. In the personal care and beauty goods industry, as well as the detergent industry, polyurethane main microplastics produced at least half of the micro-litter in 2017. Bath gels, shaving lubricants, eye makeup, personal care products, blushing powders, cosmetics foundation, eyeliner, shaving foam, infant goods, bubble bath creams, hair dye, nail polish, insect repellent, and moisturizers contain microplastics [
22], according to Auta et al. (2017). The presence of pellets in this class has been criticized, and virgin plastic manufactured pellets are usually 2–5 mm in size. They can also be categorized as primary microplastics under the more expansive descriptions of microplastic particles [
23]. Microplastic “scrubbers” have taken the role of previously popular organic ingredients such as powdered almonds, oats, and pumice in exfoliating hand washes and facial cleansers [
24]. The use of exfoliating cleansers with plastic ingredients has increased significantly since microplastic scrubbers used in cosmetics were patented in the 1980s [
25]. Gregory et al. discovered the presence of polystyrene spheres (<2 mm) and polyethylene and polypropylene particles (<5 mm) in one skincare product. Fendall and Sewell observed microplastics with erratic shapes in other beauty products. The microplastics had an average diameter of <0.5 mm [
26]. The annual output of primary microplastic particles in the paint industry is 220 tons, based on a European Commission report [
27].
1.2. Secondary MPs
Secondary microplastics are microscopic plastic particles that are produced as larger plastic garbage breaks down, both on land and in the sea [
28,
29]. The underlying stability of plastic trash can be compromised over time by a confluence of physical, chemical, and biological processes, leading to disintegration [
30,
31]. Long-term exposure to sunlight can cause photodegradation of plastics: the polymer structure oxidizes when exposed to the sun’s UV radiation and induces depolymerization in polymers [
32,
33]. A consequence of this deterioration could be the leaching of plastics from compounds intended to increase durability and corrosion resistance [
34]. Plastic waste on land is exposed directly to oxygen and sunshine, so it degrades swiftly, becoming brittle, cracking, and “yellowing” over time [
35,
36]. Most plastics are increasingly prone to disintegration as their structural stability deteriorates [
36]. This cycle continues, with plastic particles eventually shrinking and reaching the size of microplastics [
28]. It is believed that microplastics can further disintegrate into nanoplastics.
2. Source of MPs
Plastic pollution enters the environment because of poor human activity and/or uncontrolled waste disposal [
36]. Microplastic particles are mostly sourced from the disintegration of macroplastic items and tiny plastic granules used as abrasive scrubbers in cleansing and personal hygiene goods. The primary source is industrial and household products, such as bathrooms, hand, body, and facial cleaning agents, beauty products, small beads used as scrubbers in laundry items, powder, and resin pellets, among others, as the basic thermostatic sector feedstocks and abrasive plastic beads utilized for ship cleaning [
37,
38,
39,
40,
41,
42]. The pellets or microbeads present in cosmetics and laundry detergents are examples of primary microplastics, which are developed only for consumer use. Secondary microplastics are produced when larger plastics break down into finer particles and are ultimately introduced into the environment [
43,
44]. Both forms of microplastic (primary and secondary) are found in high amounts in terrestrial and aquatic habitats. Every year, an astonishing amount of 245 tons of microplastic particles are created, which wind up in bodies of water where they are ingested by aquatic organisms and absorbed into their bodies and tissues [
45,
46]. The bulk of plastic waste enters the environment through land-based production and processing facilities, sewage systems, and biosolid waste. Recreational activities also significantly contribute to the aquatic ecosystem plastic load, and their invasion across inland water bodies has not been properly examined. This could be the result of improperly managed landfill disposal, debris blown by the wind, storm surges, river transport, etc. Hence, the accumulation of plastics on land eventually enters oceanic and riverine ecosystems. The usage of microbeads in cosmetics and synthetic textile fibers may be an additional route for microplastic particles to reach the freshwater environment via the sewer system. Therefore, effective microplastic elimination through a wastewater treatment facility (STP) is crucial for decreasing pollutants in the environment. Maximum plastic rubbish patches are expected to be from densely inhabited areas in which a larger percentage of plastic items, such as containers, grocery bags, and hygiene supplies, are utilized [
1]. The discharge of microplastic particle-sized fibers as a consequence of fabric washing has been extensively recorded [
47,
48,
49]. This eventually makes its way into aquatic habitats, where it affects the organisms. The average consumption of polyethylene microplastic particles in liquid soap is calculated to be roughly 2.4 mg per person per day for the US population [
50].
Tourism and fishing operations, industrial sewage discharge, urban runoff [
51,
52], and marine transportation operations are manmade sources of microplastic pollution [
53]. The main element contributing to the introduction of microplastic particles into the aquatic environments in Poland and Germany is the passage of the Vistula and Oder streams into the Gulf of Gdansk and Pomeranian Bay. It is believed that riverine mobility is a significant route for microplastics to reach coastal habitats [
54]. In Singapore, human activity such as fishery and recreational use of fishing lines, food containers, plastic water bottles, and plastic detergents containers are the major sources of microplastics. Plastic waste degrades as a result of wave action, weather exposure, and ultraviolet light; finally, microplastic particles end up in Singapore’s mangroves [
55]. Microplastic contaminants have been abundantly observed in places as distant as Antarctica, and their causes include proximity to the area’s treatment facility for wastewater, ship traffic, coastal scientific research operations, and transfer by ocean circulation [
56]. According to Stolte et al. (2015), city wastes, industrial output sites, the fishing industry, and tourist industry are the most likely sources of the higher microplastic concentration [
57]. In addition to wastewater and effluent intake, Horton et al. (2017) identified highway markings composed of thermoplastic composite paint as contributors to microplastic particles in the River Thames Basin (UK) [
58]. In accordance with various studies, sewage discharge has been identified as a key contributor to microplastic particles [
59]. Owing to the inadequate eradication of microplastics during sewage treatment [
60], WWTPs are recognized contributors of microplastics in freshwater bodies. The development of microplastics has also been linked to tourism. According to a study by Retama et al. (2016), December, when tourism is at its peak along the Southern Pacific coast of Mexico, saw a higher proportion of microplastics than April, when human impact was at its lowest [
61].
Microplastics are continuously released into the atmosphere through various sources [
62]. According to Gondalia et al. (2020), tire wear caused by driving accounts for the release of 3.5 to 10 metric tons of microplastics into the atmosphere each year in Paris [
63]. Synthetic textiles, such as polyester and nylon, continuously release microplastic fibers during washing and wearing, contributing up to 71% of total microplastic particles in the atmosphere, as found by Shi et al. (2020) in Beijing [
64]. Plastic production and disposal release 0.15 to 0.84 metric tons of microplastics into the atmosphere each year, according to Cincinelli et al. (2020) in Italy [
65,
66]. Agricultural activities, such as tilling and fertilizing, also release microplastics into the atmosphere, estimated to be 0.1 to 0.8 metric tons each year, according to Barducci et al. (2020) in Switzerland [
67]. The sources and types of microplastic pollutants in terrestrial, aquatic, and atmospheric environments are shown in
Figure 1 and
Table 1.
Figure 1. Sources and types of microplastic particles.
Table 1. Source of MP pollutants in the terrestrial, aquatic, and atmospheric environments [
37,
38,
39,
40,
41,
42,
43,
44,
45,
46].
|
Source Types
|
Source of MPs Pollutants
|
|
Terrestrial sources
|
Improper waste management, composting methods in agriculture, indoor dust emissions from public buildings, use of treated wastewater from wastewater treatment plants, soil amendment in agriculture, digging of soil contaminated by plastics, construction sites, and building restoration, stormwater drain discharge, recreational boating, and freshwater recreation, use of plastic beads in medicine, municipal wastewater that has not been properly or adequately treated, industrial products, and process pollutants.
|
|
Water sources
|
Aquaculture and fishing, maritime, harbor, recreational boating, and beachside activities, maintenance of plastic-treated and plastic-painted maritime surfaces, offshore excavation and mining.
|
|
Atmosphere sources
|
Tire wear, synthetic textiles, plastic production and disposal, road dust, and agricultural activities.
|
3. Identification and Extraction of Microplastics (MPs)
The sampling is characteristic of terrestrial and aquatic habitats, sample methodologies are crucial in determining the abundance and polymeric composition of microplastic particles [
68,
69,
70,
71,
72,
73]. The water, sediment, and salt samples are typically collected using basic environmental sampling methods [
74,
75,
76].
3.1. Sampling
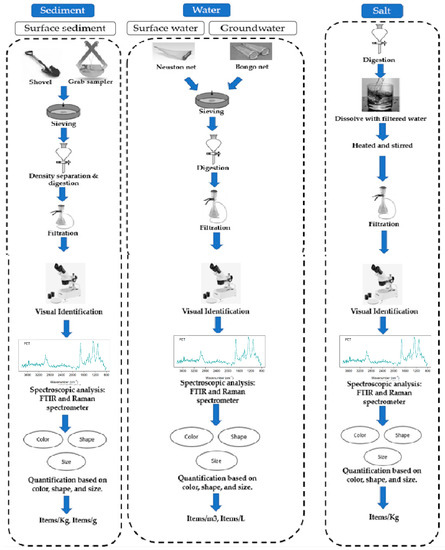
The sampling techniques utilized to obtain microplastic particles in sediment, water, and salt for global and Indian environments are given below.
3.1.1. Sediment
Many studies have examined the prevalence of MPs in sediment from coasts, islands, lakes, and beaches. For manual grab methods for sediment, use implements such as hand shovels and metal spoons. Putting a steel or wooden structure on the surface of the sediment layer, pushing it downward to a depth of 1–5 cm, sweeping out the sediment material, and extracting the samples with a steel shovel, microplastics in sediments from lakes, coastal, and beach regions has typically been used [
77]. Sampling of terrestrial and aquatic sediment was performed using a Peterson grab sampler, and the various frame dimensions utilized in India and the global scenario were 25 × 25, 30 × 30, 50 × 50, 100 × 100, and 200 × 200 cm. The sampling depths ranged from 0 to 6 cm [
78,
79]. No research has been conducted to gather data on the vertical spatial variation of MP particles [
80,
81,
82]. Microplastic-containing sediments have occasionally been collected manually or with steel tweezers [
83,
84,
85,
86]. The sample unit of microplastic analysis is generally items/kg [
87,
88,
89,
90] or items/g [
91,
92], with a few studies indicating items/m
2 [
93,
94,
95,
96,
97] as their sampling unit.
3.1.2. Water
The prevalence and variation of microplastic particles throughout global coastal and freshwater environments were examined in several studies. Several different trawling and net designs were used, including bongo, manta, neuston, and plankton nets. A trawl net is often launched from a watercraft, immersed, and lifted in the direction of travel at a slow speed for a specified time or distance [
97,
98]. Multiplying the length of the tow by the width of the trawl yields the total area sampled. To gather samples from depths ranging between 20 cm and 2–5 m, manta trawling nets with mesh sizes of 112, 200, 300, and 335 µm were utilized. Eriksen et al. (2018) employed AVANI trawl instrument for microplastic particle sampling with rectangular entrance 60 cm × 14 cm and manta trawling net with a rectangular aperture 16 cm × 61 cm [
98]. To determine the amount of water tested, a flow meter was fastened to trawl nets. Nonetheless, some studies did not indicate whether a flow meter was attached to the net, and if the net was not fully immersed or obstructed by an excessive amount of suspended and floating materials, simply measuring the distance during sampling could result in significant inaccuracies [
99]. Trawl nets are commonly used to collect marine organisms for ecological studies, but they can also capture microplastics. Therefore, trawl nets have been increasingly used as a tool for sampling microplastics in the marine environment. A study by Lusher et al. (2017) investigated the efficiency of trawl nets in collecting microplastics from the water column. The study involved deploying a small-mesh trawl net off the coast of the UK and examining the microplastics collected. The results showed that trawl nets were effective in collecting microplastics, with an average of 1.9 microplastic particles per m
3 of water sampled. The study also found that microplastics were present in a range of sizes, with particles ranging from 20 µm to 5 mm in diameter [
100]. Another study by Nelms et al. (2019) compared the efficiency of different sampling techniques for collecting microplastics in the marine environment, including trawl nets, plankton nets, and surface water pumps. The study found that trawl nets were the most efficient method for collecting microplastics, with the highest number of particles collected per unit of water sampled. However, using trawl nets for microplastic sampling can also present some limitations. One limitation is that trawl nets can potentially capture larger plastic debris, which may not accurately represent the abundance of microplastics in the water column. Additionally, trawl nets may not capture microplastics that are located deeper in the water column or that are associated with sediment [
101]. To address these limitations, other studies have suggested using a combination of sampling techniques, including trawl nets, and plankton nets, to provide a more comprehensive picture of microplastic abundance in the marine environment [
102]. Trawl nets are an effective tool for sampling microplastics in the marine environment, but they may not be the most suitable method in all situations. By combining different sampling techniques, researchers can obtain a more accurate picture of microplastic abundance in the water column. Trawl nets are commonly used in aquatic environments. However, they can potentially contaminate the samples with plastic particles. To minimize this risk, several strategies can be employed during sampling, including using alternative sampling techniques, cleaning and sterilizing nets, and avoiding areas with high levels of plastic pollution. One alternative sampling technique is the use of plankton nets, which are designed to collect smaller organisms and are less likely to accumulate plastic particles. Another option is to use gear with a smaller mesh size, which can reduce the amount of plastic that enters the net. Cleaning and sterilizing nets before use can also help to reduce the potential for contamination. This can involve using a bleach solution or high-pressure hot water to remove any particles that may be present on the net. Avoiding areas with high levels of plastic pollution can also be an effective strategy. This may involve selecting sampling sites in areas where plastic pollution is less prevalent or avoiding sampling during periods when plastic is more likely to be present in the water column, such as after storms or during periods of high wind [
99,
100,
101]. The process of grab sampling includes collecting water in a receptacle and filtering it on location. Additionally, a container with a set volume is submerged and filled with surface water for further analysis in a laboratory setting [
102]. The microplastic concentrations in the water samples were measured in units of items/L, items/km
2, and items/m
3.
3.1.3. Salt
Five studies measured the prevalence of microplastic particles in commercial salt brands and natural salt pans that are accessible to customers. Samples of unprocessed sea salt for human use were collected in Bangladesh from specific natural salt pans and glass bottles with a clean label, and a capacity of 1 L was used to collect 500 g of salts from each location and brought to the laboratory [
103]. Comparable analyses were conducted in Tuticorin on 14 salt brands made from borewells and saltwater, as well as 25 salt samples taken from various salt pans [
83]. Seth and Shriwastav [
86] employed eight commercial branded salts made in India. Kim et al. (2018) bought three commercialized kinds of sea salt in Indian stores [
85].
3.2. MP Isolation Technique
In the majority of scientific literature, MPs were separated from sediment, water, and salt samples after sampling. Smaller microplastics were retrieved employing density separation and filtration techniques, whereas larger microplastics were assessed visually and extracted utilizing tweezers.
Figure 2 shows the methods used for microplastic sampling and extraction in various environmental matrices. In laboratory settings, plastic items such as containers, pipettes, and tubes can release microplastics into the samples being tested, potentially contaminating the results. To avoid internal microplastic contamination, it is essential to follow proper handling and disposal procedures for plastic items. One of the key recommendations to avoid internal microplastic contamination is to use certified microplastic-free plastic items in laboratory settings. For example, the European Commission’s Joint Research Centre has developed a certification scheme for microplastic-free plastic products used in laboratories. This certification scheme ensures that the plastic items have been tested and verified to be free of microplastics [
104]. Another recommendation is to minimize the use of plastic items and opt for alternative materials when possible. Glass, stainless steel, and other non-plastic materials can be used as substitutes for plastic items. In addition, it is important to avoid unnecessary plastic packaging and to properly dispose of plastic items after use.
Figure 2. Methods for MP sampling and analysis in various environmental matrices are advised.
3.2.1. Sediment
Different-sized sieves were used to sieve dry sediment samples. With 63 mm [
25], 300 mm [
89,
92,
93], 0.1 mm [
105], 1 mm [
94,
95,
106], 2 mm [
107], 3 mm [
108], and 5 mm [
31,
109] are a few examples. Microplastics were removed from the sediment using the density separation technique, NaCl, and Zinc chloride. To break down the organic material, digestion with 30% H
2O
2 was performed either prior to or after density separation. With the digestion and density separation process, the sediment sample was left to dry naturally or in an oven before being passed through a range of mesh sizes of filter paper, including 0.4 mm [
105], 0.45 mm [
22,
30], 0.8 mm [
88,
89,
97], 1.2 mm [
107], 38 mm [
109] and 0.7 mm [
20,
87].
3.2.2. Water
In accordance with established techniques, collected water samples were filtered using a vacuum filtration setup and 0.45 mm Whatman cellulose filter paper before being dried at room temperature to determine the number of microplastics present [
8,
19] by Koelmans et al. (2019). In all investigations, the obtained water samples were either filtered or sieved to select the desired size. NaCl [
87,
93], ZnCl [
19], and NaI [
83,
88] were employed for density separation to isolate microplastics from the water samples, and 30% H
2O
2 digestion was utilized to eliminate organic material.
3.2.3. Salt
To disintegrate any organic matter, 100 mL of 30% H
2O
2 was added to each sample in clean glass flasks before heating in a bath maintained at 65 °C for 24 h [
75,
110]. Each container received approximately 1000 mL of filtered water and the salt was stirred with a glass rod until it was completely dissolved. Using a vacuum setup, the resulting salt solution was quickly filtered with a cellulose membrane filter (0.2 mm, 0.45 mm, 0.8 mm, and 2.7 mm pore size) [
83,
85,
86]. The filter was positioned inside a petri dish made of glass and left to dry naturally at the surrounding temperature.
This entry is adapted from the peer-reviewed paper 10.3390/w15111987