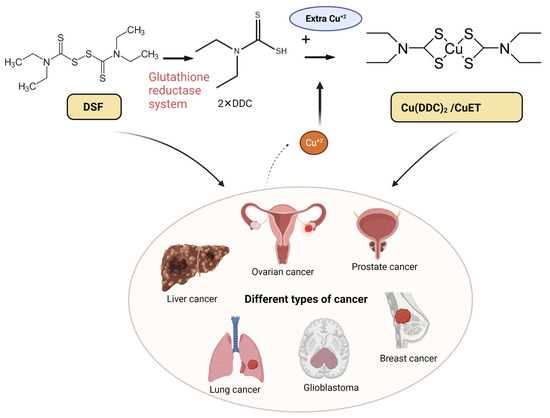
Disulfiram (DSF) is a thiocarbamate based drug. Preclinical studies have shown that DSF has anticancer efficacy, and its supplementation with copper (CuII) significantly potentiates the efficacy of DSF. However, the results of clinical trials have not yielded promising results. The elucidation of the anticancer mechanisms of DSF/Cu (II) will be beneficial in repurposing DSF as a new treatment for certain types of cancer. DSF’s anticancer mechanism is primarily due to its generating reactive oxygen species, inhibiting aldehyde dehydrogenase (ALDH) activity inhibition, and decreasing the levels of transcriptional proteins. DSF also shows inhibitory effects in cancer cell proliferation, the self-renewal of cancer stem cells (CSCs), angiogenesis, drug resistance, and suppresses cancer cell metastasis.
1. Introduction
There has been tremendous progress in the fields of drug discovery, tumor biology, nanomedicine, and targeted drug delivery for improving treatment and patient care, yet cancer remains one of the leading causes of death. The process of discovering new chemical entities and their development into anticancer drugs can be very time consuming and expensive. Drug repurposing via new drug delivery systems is a relatively less time-consuming, simple, and cost-effective strategy in treating many diseases [
1,
2]. With the advancement of computational and rapid screening technologies, new drug candidates are now available for cancer treatment. However, the efficient delivery of these drugs to cancer tumors still requires novel drug delivery devices and methods [
3,
4].
Copper stimulates the proliferation and migration of endothelial cells and is required for the secretion of several angiogenic factors by tumor cells [
5]. However, copper chelation has been reported to produce a decrease in the secretion of many of these factors [
5,
6]. Recently, Based on clinical data indicating that elevated serum copper levels associated with many cancer tumors, many copper chelators are being developed and tested in clinical trials in recent years [
7]. Although it remains to be determined the mechanism which copper chelation suppresses the growth of endothelial cells and hinders the secretion of angiogenic factors by tumors is not entirely clear, it is has been hypothesized to result from effects on copper-dependent enzymes, transporters, and chaperones [
8].
Recently, a novel cell death pathway, distinct from apoptosis, necroptosis, pyroptosis, and ferroptosis, induced by copper, has been identified, and designated as cuproptisis [
9]. Cuproptosis is significantly correlated to cellular metabolism and is frequently observed in certain types of cancer that have high levels of aerobic respiration, including melanoma, breast cancer, leukemia, and drug-resistant cancers [
10]. Copper ionophores have played a crucial role in the identification of cuproptosis and have been considered as possible anticancer treatments [
11,
12]. Diethyldithiocarbamate (DDC), an active metabolite of the drug, disulfiram (DSF), has been reported to be a copper ionophore that has in vitro and in vivo efficacy as an anticancer compound. [
11].
Disulfiram (DSF,) is a thiocarbamate derivative that has been used to treat alcoholism since 1951 [
13]. DSF inhibits the enzyme aldehyde dehydrogenase 1 (ALDH1), which significantly inhibits the biotransformation of ethanol to ethanol at the acetaldehyde stage [
14], thereby increasing the levels of ALDH1, which produces various adverse effects following the ingestion of ethanol, decreases or discourage ethanol intake [
15]. Interestingly, ALDH1 is present found in cancer cells with stem cell properties, and it catalyzes the oxidation of intracellular aldehydes and produces multidrug resistance [
16]. Subsequently, clinical data suggested that DSF had anticancer efficacy [
17]. In addition, DSF was shown to regulate the balance of reactive oxygen species (ROS) and glutathione (GSH) [
18], inhibit the activity of the ubiquitin proteasome system (UPS) [
19], and regulate intracellular signaling [
20,
21] and the activity of other enzymes, which could play a role in mediating its anticancer efficacy [
22,
23,
24]. Indeed, these mechanisms could induce cancer cell death, decrease the stemness of cancer cells [
25], decrease angiogenesis, and overcome drug resistance [
26,
27].
As mentioned earlier, trace metals are essential for the survival of cancer cells [
28,
29]. Compared with normal cells, many tumor cells have a 2–3-fold higher concentration of copper [
30]. DSF is a potent chelator of copper, and DSF can bio-transform the pro-angiogenic activity of copper to a specific compound that induces cancer cell death [
31]. Although DSF has shown promise in both laboratory and animal studies, certain clinical trials with cancer patients have been unsuccessful due to treatment requiring high doses and the expectation partially owing to the rapid biodegradation of the DSF [
32,
33]. Cu (II) chelation of the primary DSF metabolite, diethyldithiocarbamate (DDC), is crucial for inducing the death of tumor cells [
34,
35]. The copper-dependent anticancer efficacy of DSF has resulted in an increase in research related to DSF/Cu (II) [
36,
37]. DSF/Cu (II) has been reported to be efficacious in a variety of cancers, including liver cancer [
38], ovarian cancer [
39], prostate cancer [
25], lung cancer [
40], glioblastoma (GBM) [
41], and breast cancer [
42] (
Figure 1). The anticancer efficacy of DSF is most likely due to the bio-transformation of DSF to diethyldithiocarbamate (DDTC), which forms an intracellular copper–DDTC complex (Cu(DDTC)
2), which has been shown to be the most efficacious anticancer compound [
43]. DSF can induce ferroptosis and cuproptosis in different types of cancer cells [
44]. A summary of various clinical trials assessing the efficacy of DSF-based cancer is shown in
Table 1. Some clinical trials have investigated the use of DSF for treating solid tumors, with some reporting improved progression-free survival and overall survival, compared the control groups. Adverse effects were generally mild and resolved following a decrease in the dose of DSF. Two single-arm trials in glioblastoma patients showed positive effects, while a randomized controlled trial in NSCLC patients also reported an increase in patient survival [
45,
46,
47]. However, the response to DSF varied among patients, and further in vitro and animal studies are needed to explore the optimal concentration and sensitivity type. Overall, DSF appears safe and effective in prolonging survival in cancer patients [
48].
Figure 1. The chelation mechanism of DSF and Cu (II) and the use of DSF-based therapy for different types of cancer. DSF metabolizes to diethyldithiocarbamate (DDC or ET) via the glutathione reductase system; the active anti-cancer ingredient DDC further chelates with Cu (II) and forms Cu(DDC)
2 (aka CuET), which has anti-cancer efficacy. The high dose of DSF alone and low dose of DSF/Cu (II) are effective in various cancers including liver, ovarian, prostate, breast, lung cancer, and glioblastoma (GBM). Created with
BioRender.com. accessed on 19 April 2023.
|
Cancer
|
Status
|
Clinical Identifier (Clinicaltrials.Gov)
|
|
Metastatic melanoma
|
Phase I, Terminated
|
NCT00571116
|
|
Melanoma
|
Phase I/II, Completed
|
NCT00256230
|
|
Melanoma
|
Phase II, Completed
|
NCT02101008
|
|
Prostate Cancer
|
Phase I, Completed
|
NCT01118741
|
|
Prostate Cancer
|
Phase I, Recruiting
|
NCT02963051
|
|
Breast Cancer (Metastatic)
|
Phase II, Recruiting
|
NCT03323346
|
|
Refractory Breast Cancer (Metastatic)
|
Phase II, Recruiting
|
NCT04265274
|
|
Pancreatic Cancer (Metastatic, Recurrent)
|
Phase I, Recruiting
|
NCT02671890
|
|
Pancreatic Cancer (Metastatic)
|
Phase II,
Not Yet Recruiting
|
NCT03714555
|
|
Recurrent Glioblastoma
|
Phase I, Active, Not Recruiting
|
NCT02770378
|
|
Glioma Glioblastoma
|
Phase II/III, Recruiting
|
NCT02678975
|
|
Glioblastoma Multiforme
|
Phase II, Recruiting
|
NCT03363659
|
|
Solid Tumors Involving Liver
|
Phase I, Completed
|
NCT00742911
|
|
Non-small Cell
Lung Cancer
|
Phase II/III, Completed
|
NCT00312819
|
|
Glioblastoma (Recurrent)
|
Phase II, Completed
|
NCT03034135
|
|
Glioblastoma
|
Phase I/II, Recruiting
|
NCT02715609
|
|
Glioblastoma
|
Phase II, Not Yet Recruiting
|
NCT01777919
|
|
Glioblastoma
|
Phase II/III, Recruiting
|
NCT02678975
|
|
Glioblastoma
|
Early Phase I, Recruiting
|
NCT03151772
|
|
Glioblastoma
|
Early Phase I, Completed
|
NCT01907165
|
|
Germ Cell Tumor
|
Phase II, Recruiting
|
NCT03950830
|
|
Multiple Myeloma
|
Phase I, Terminated
|
NCT04521335
|
|
Refractory Sarcomas
|
Phase I, Recruiting
|
NCT05210374
|
|
Advanced Gastric Cancer
|
Phase Not Defined,
Not Yet Recruiting
|
NCT05667415
|
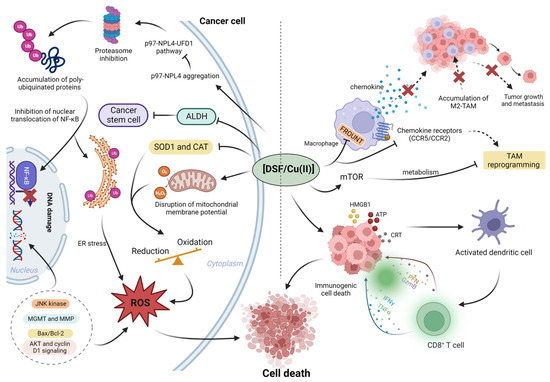
2. Anticancer Mechanisms of DSF/Cu (II)
It has been revealed that the anti-cancer mechanism of DSF/Cu (II) may be mediated by the regulation of reactive oxygen species (ROS), enzyme activity regulation, induction of DNA damage, proteasome inhibition, and transcription factors [
24] (
Figure 2). Additionally, DSF/Cu (II) also exhibits immunomodulatory effects on tumor microenvironment (TME).
Figure 2. The summary of roles of DSF/Cu (II) in cancer microenvironment. Left: DSF/Cu (II) inhibits the cancer proteasome activity via p97-NPL4 pathway; in addition, DSF/Cu (II) inhibits cancer-associated ALDH activity and inhibits cancer stem cells (CSCs). In the cancer microenvironment, aberrant enzyme activity, superoxide dismutase 1 (SOD1) and catalase (CAT), results in the elevation of ROS; the higher basal level of ROS benefits cancer proliferation. However, the further increased ROS to exceed cancer tolerance cause cancer death. Right, the DSF/Cu (II) reprograms the tumor-promoting macrophage M2 to anti-tumor type M1. In addition, DSF/Cu (II) transforms the immune-suppressive (cold) tumor microenvironment to the immune-active (hot) microenvironment via the induction of immunogenic cell death (ICD). Created with
BioRender.com. accessed on 19 April 2023.
3. The Effect of DSF/Cu (II) on Cancer
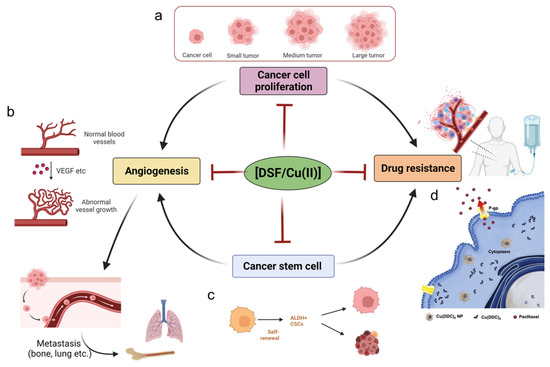
In this section, we summarize the effects of DSF/Cu (II) on cancer-associated activities such as unlimited cancer proliferation, the self-renewal activity of cancer stem cells, cancer angiogenesis, and drug resistance (Figure 3).
3.1. DSF/Cu (II) on the Inhibition of Cancer Proliferation
DSF/Cu (II) induces cell death in different cancer cell lines via different mechanisms. In human breast cancer cells, DSF/Cu (II) causes cancer cell apoptosis via increasing Bcl-2 Associated X-protein (Bax, a pro-apoptotic protein) [
50]. DSF, at a concentration of 25–50 ng/mL, produces a 4–6-fold increase in apoptosis, and co-incubation with the ROS inhibitory compound N-acetyl-cysteine (NAC) reverses DSF-induced apoptosis, suggesting that DSF-induced apoptosis is associated with an increase in ROS levels [
60]. DSF induces the disruption of the mitochondrial membrane potential and cause apoptosis in human melanoma cell lines [
60]. Xi et al. reported that DSF/Cu (II) produced significant cytotoxicity and caspase-dependent apoptosis in NSCLC cells [
106]. Additionally, DSF produced autophagy-dependent apoptosis [
103]. DSF/Cu (II) induced the apoptosis of erbB2-positive breast cancer cells by inhibiting AKT, cyclin D1, and NFκB signaling [
107]. In malignant pleural mesothelioma (MPM) cells, DSF/Cu (II) produced apoptosis by activating the proapoptotic stress-activated protein kinases (SAPKs) p38 and JNK1/2, caspase-3. Furthermore, DSF/Cu (II) increased the expression of the apoptosis transducer, cell division cycle, and apoptosis regulator 1 (CARP-1/CCAR1) and sulfatase 1 (SULF1) [
108]. The activation of NF-κB mediates cancer proliferation [
109] and the DSF/Cu (II) inhibits the activity of NF-κB, thus inhibiting hepatocellular carcinoma (HCC) growth [
110].
3.2. DSF/Cu (II) Efficacy in Cancer Stem Cells (CSCs)
CSCs have been reported to mediates cancer angiogenesis and drug resistance [
111], so we firstly discuss the functions of DSF/Cu (II) on CSCs, then, in the following two paragraphs, we will discuss the roles of DSF/Cu (II) on angiogenesis and drug resistance. Because of promotions of CSCs on activities including the activation of ABC transporters, such as P-gp [
112], and the increase in DNA repair mechanisms [
113], CSCs are resistant to conventional anticancer drugs [
37]. Furthermore, hypoxia also induces resistance of CSCs to chemotherapy. Numerous studies indicate that the presence of CSCs in patients correlates with poor prognosis [
37]. The roles of DSF/Cu (II) on CSCs can be attributed to its activity on the ALDH1 enzyme. The ALDH1 protein family (ALDH1A1, ALDH1A2, and ALDH1A3) enhance the self-renewal, survival, and proliferation of CSCs [
114]. ALDH+ CSC phenotypes have a high tumorigenic capacity [
115,
116]. DSF/Cu (II) significantly decreases MDA-MB-231 breast cancer cell proliferation by decreasing the ALDH+ CSC population [
117]. In hepatocellular carcinoma, DSF decreases CSCs by inhibiting the p38 mitogen-activated protein kinase (MAPK) pathway [
118]. DSF also inhibits CSCs in ovarian, pancreatic, pulmonary, and hematological cancers [
90,
119,
120].
3.3. DSF/Cu (II) Effects on the Inhibition of Cancer Angiogenesis
Vascular endothelial growth factor (VEGF) is a critical mediator of angiogenesis in cancer cells, and high VEGF levels are positively correlated with poor prognosis [
121]. 4HNE, a lipid peroxidation product [
122], is involved in angiogenesis [
123,
124]; and the catabolism of 4HNE is dependent on the cellular levels of glutathione-S-transferases, alcohol dehydrogenases, and ALDHs [
125]. The inhibition of ALDH2 by DSF/Cu (II) significantly decreases angiogenesis by inhibiting the hypoxia-inducible factor-1α (HIF-1α)/VEGF signaling cascade [
126]. The inhibition of SOD-1 by DSF/Cu (II) induces endothelial cell growth arrest and apoptosis and, thus, exhibits anti-angiogenesis efficacy [
127]. DSF/Cu (II) also inhibits tumor angiogenesis by inhibiting the activity of matrix metalloproteinases [
128,
129]. Copper increases the anti-angiogenic efficacy of DSF via the EGFR/Src/VEGF pathway in gliomas [
130]. CSCs were shown to modulate angiogenesis via CSC-secreted VEGF [
131]. Moreover, CSCs overexpress CXCR4, whose SDF-1/CXCL12 ligand induces VEGF production via activation of the P13K/AKT signaling pathway [
132]. Other CSC-associated factors such as SDF-1/CXCL12 also play roles in the formation of the new blood vessels [
133,
134]. Thus, the inhibition of DSF/Cu (II) in CSCs decrease angiogenesis.
3.4. DSF/Cu (II) Reverses Drug Resistance
DSF/Cu (II) overcomes drug resistance via targeting the proteasome, epithelial–mesenchymal transition (EMT), P-gp, CSC activity [
135,
136,
137]. By targeting the proteasome, DSF significantly increases the sensitivity of TMZ-resistant brain tumor-initiating cell (BTIC) variants (BT73R and BT206R) to temozolomide (TMZ) [
138]. Numerous studies have shown that EMT plays a role in mediating the resistance of cancer cells to certain anticancer drugs, such as paclitaxel in prostate (DU145-TXR) and lung cancer (A549-TXR) [
139,
140,
141]. By downregulating associated proteins such as Vimentin, DSF/Cu (II) inhibits the EMT, which consequently overcomes the paclitaxel resistance of prostate and lung cancer [
141]. In addition, DSF/Cu (II) decreases the effects of EMT in breast cancer cells via the regulation of protein kinase (ERK)/NF-κB/Snail pathway [
142]. Cancer cells expressing high levels of P-gp exhibit resistance to conventional chemotherapy drugs like doxorubicin and paclitaxel, a phenomenon referred to as multidrug resistance (MDR) [
143,
144]. However, the metabolite and active anti-cancer compound CuET is not a substrate of P-gp, and thus it is still retained inside of drug resistant cancer cells and increase the likelihood of the drug-resistant cancer cells death [
141]. DSF/Cu (II) produce efficacy in osimertinib-resistant NSCLC cells by activating macrophage-mediated innate immunity [
40]. It was discovered that the high levels of ABC protein provide the protective mechanism for CSCs to chemotherapeutics [
145]. The inhibitory effects of DSF/Cu (II) on the CSCs benefit the overcoming of drug resistance [
39]. Another important finding is that Cu(DDC)2 NP is also does not inhibit P-gp activity or expression, thus avoiding the side effects associated with P-gp inhibitors [
141].
Figure 3. Cancer cells have unlimited proliferative capacity, and DSF/Cu (II) has been shown anti-proliferation effects towards cancer cells. (
a) DSF/Cu (II) inhibits cancer cell proliferation, preventing the transformation of small cancer lesions to large tumors. Created with
BioRender.com accessed on 8 May 2023 (
b). Angiogenesis is an essential step for cancer metastasis; the VEGF in the cancer microenvironments contributes the massive and abnormal vessels in cancer lesions, and DSF/Cu (II) inhibits the angiogenesis behavior and prevents cancer metastasis to lung and bone, etc., sites. Created with
BioRender.com. accessed on 8 May 2023 (
c) Cancer stem cells aggravate the angiogenesis and drug resistance of cancer; DSF/Cu (II) inhibits cancer stem cells and thus shown potency in anti-angiogenesis and anti-drug resistance. Created with
BioRender.com accessed on 8 May 2023 (
d). Drug resistance is a typical phenomenon during the treatment of cancer; novel copper diethyldithiocarbamate nanoparticles can effectively overcome drug-resistant cancers, owing to being non-binding to P-gp and being maintained in the cancer cells. Copyright 2023, Elsevier [
141].
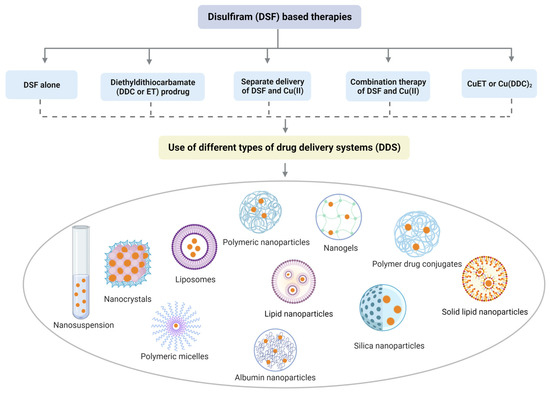
4. DSF-Based Therapies for the Treatment of Cancer
Although data from in vitro studies suggested that DSF could be an efficacious anticancer treatment, clinical trials with oral DSF plus Cu (II) in cancer patients have been equivocal [
146,
147]. The underlying reasons for the poor clinical results include: (1) the instability of DSF in the gastrointestinal environment; (2) the rapid biodegradation of DSF via first-pass metabolism; and (3) low final Cu (II) concentration at the tumor sites [
148]. The effective delivery of DSF and Cu (II) to target sites is crucial for maximizing the anticancer efficacy of DSF/Cu (II) and overcoming limitations such as poor solubility, stability, and bioavailability. Recent progress in nanotechnology has facilitated the targeted delivery of DSF, and various types of drug delivery systems based on different nanoparticles have been developed. For instance, polymeric nanoparticles, nanogels, polymer–drug conjugates, liposomes, and dendrimers have been explored as effective carriers for DSF. For instance, DSF-loaded vitamin E-TPGS-modified PEGylated nanostructured lipid carriers have gained significant attention due to their biodegradable and biocompatible properties [
149]. Nanogels are composed of nanosized particles that can entrap and release drugs in response to different stimuli, providing a promising strategy for the targeted delivery of DSF [
150]. The use of these nanoparticle-based formulations can increase the accumulation of DSF at the target site, thereby reducing the toxic effects on healthy tissues and improving the therapeutic index. Overall, nanotechnology-based strategies have shown promising results in enhancing the anticancer efficacy of DSF and can potentially overcome the limitations associated with conventional DSF-based therapies.
Figure 4 explains and summarizes this with some reported examples.
Figure 4. Divergent nanotechnology and chemical modulation-based formulations to enhance DSF anticancer effects. To sum up, DSF-based nanomedicine includes DSF alone, DDC prodrug delivery system, delivery system for Cu (II) and DSF/Cu (II), and drug delivery system for active component-CuET.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15061567