Cutaneous melanoma is one of the deadliest forms of skin cancer. While advancements in systemic targeted therapies and immunotherapies have greatly improved melanoma survival in recent years, tumor resistance can limit the efficacy of these therapies. Targeting redox homeostasis in melanoma progression is a promising therapeutic approach, especially in cases of melanoma drug resistance. The role of oxidative stress in melanoma is paradoxical in that it promotes tumor initiation but prevents vertical growth and metastasis. As the disease progresses, melanoma employs adaptive mechanisms to decrease oxidative stress in the tumor environment. Thus, agents with antioxidant properties may have the greatest utility in chemoprevention whereas those with pro-oxidant properties may be better suited for treatment.
1. Introduction
Melanoma develops from the malignant transformation of melanocytes, which are dendritic pigment-producing cells located in the basal layer of the epidermis, hair follicles, inner ear, and uvea of the eye [6,7,8]. Cutaneous melanoma is characterized by a high mutational burden and is frequently associated with mutations in genes that regulate cell proliferation and survival, including BRAF, NRAS, NF1, and tumor suppressor PTEN [9,10,11,12,13,14,15]. These mutations lead to overactivation of the mitogen-activated protein kinase (MAPK) pathway and phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathways. Currently, the most common genetic cause of melanoma is the BRAFV600E mutation, which is present in approximately 50% of melanomas, and contributes to increased cell proliferation and metabolic reprogramming [10,16,17]. Other genetic alterations have been identified in cutaneous melanoma, including mutations in cell cycle regulators like CDK4, and tumor suppressor genes TP53 and CDKN2A [11,12,13,18]. These mutations contribute to alterations in telomere maintenance, histone modification and methylation, which affect gene expression and contribute to the development and progression of the disease.
2. Oxidative Stress in Melanomagenesis
Oxidative stress refers to an imbalance between the production of reactive oxygen species (ROS), reactive nitrogen species (RNS) and the antioxidant defense mechanisms that neutralize them [
30]. ROS are highly reactive molecules that can cause damage to cellular macromolecules, such as lipids, proteins, and DNA, if they accumulate to excessive levels. Endogenous sources of ROS include nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase (NOX), cyclooxygenases, lipooxygenases, and cytochrome P450, endothelial nitric oxide synthase (eNOs) and others. Exogenous sources of ROS include UV irradiation and inflammation [
31,
32]. Biomarkers for oxidative stress include DNA lesions in the form of 8-hydroxy-2′-deoxyguanosine (8-OHdG), end products of lipid peroxidation, malondialdehyde (MDA) and 4-hydroxy-2-nonenal (HNE), and derivatives of protein oxidation, advanced oxidation protein products (AOPPs) and advanced glycation end products (AGEs).
Under normal conditions, the antioxidant response system maintains a balance between ROS production and scavenging. The antioxidant response includes non-enzymatic and enzymatic molecules: glutathione (GSH), which belongs to the glutathione system, also involving glutathione reductase, glutathione peroxidase (GPX) and glutathione-s-transferase (GST), superoxide dismutase (SOD), superoxide reductase, catalase (CAT) and thioredoxin (TRX) [
33,
34]. However, under certain conditions, such as exposure to UV radiation, environmental toxins, or inflammation, the production of ROS can overwhelm the antioxidant defenses and lead to oxidative stress.
Oxidative stress and redox homeostasis are implicated in all phases of melanomagenesis as well as in the emergence of drug resistance [
10,
35]. The role of oxidative stress is somewhat paradoxical: while ROS help to promote cancer survival, proliferation, tumor vascularization and metastasis, at high levels, they can also cause DNA damage and cancer cell death [
10,
35,
36,
37]. Thus, melanoma requires adaptive strategies to resist the effects of increased ROS levels [
38,
39]. Various studies have investigated the antioxidant response of melanoma cells, and show increased levels of enzymes that protect the tumor from ROS damage [
10,
35,
40,
41,
42]. GR is associated with oxidative stress regulation in melanoma. Inhibition of GR suppresses lung metastasis and subcutaneous growth of melanoma, while also impacting other cellular behaviors like proliferation, colony formation, migration, and invasion [
43]. GPX1 expression increases with melanoma progression, correlating with heightened proliferation [
44]. GPX3, on the other hand, can act as a tumor suppressor gene and is typically downregulated in various tumors, including melanoma [
45,
46]. Low GPX3 levels are linked with higher proliferation, motility, invasiveness, and poor prognosis [
45]. GST levels decrease in metastasis derived from skin or lymph nodes but increase with overall tumor progression. GST is directly involved in melanoma invasion and the development of drug resistance, with GST π being the most expressed isoform [
47,
48]. However, GSTT and GSTM isozymes are absent in a large population portion and could influence melanoma development by decreasing oxidative stress [
49]. The role of SOD2 in melanoma is controversial, with some studies linking lower levels to metastasis [
50]. High SOD2 expression can suppress melanoma’s malignant phenotype in vitro, but serum SOD2 levels may increase in melanoma patients and correlate with disease progression [
51]. Alterations in SOD expression also relate to therapy resistance. SOD1 is involved in melanogenesis and/or differentiation, while SOD3 overexpression inhibits the growth of specific melanoma cells [
36,
52]. In melanoma, there appears to be a redox imbalance characterized by decreased catalase activity and increased SOD activity [
53]. Catalase activity may increase in stages I, II, and III, but not in stage IV, suggesting a protective role against metastasis [
54]. Mutations in the CAT gene were found in 10% of the melanoma patients, mostly leading to increased mRNA expression, but these mutations had no significant impact on survival [
34]. Transcriptional factors like Ap-1 and NF-kB and activation of the MAPK pathway and S-phase kinase-associated protein 2/Skp2/MTH1 axis have been implicated in protecting melanomas from ROS damage [
55,
56]. Overexpression of ecto-enzyme gamma-glutamyltransferase (GGT) and CD 147, a cell surface receptor for cyclophilin A, have also been shown to be involved in melanoma’s resistance to oxidative stress [
57,
58,
59]. The aldehyde dehydrogenases convert toxic aldehydes into non-toxic carboxylic acids. Upregulation of ALDH1 isozymes have been reported in several malignancies. ALDH1A1 has been implicated in tumorigenicity, invasion, and resistance to chemotherapy, in various types of cancer, including melanoma [
60]. Whereas ALDH1B1 has also been demonstrated to be upregulated in several cancers, acting as an oncogene, but its role in melanoma has not been determined [
61,
62]. Conversely, ALDH2 expression has been shown to be downregulated in melanoma, leading to acetylaldehyde accumulation, which results in poorer prognosis [
63,
64].
It should be recognized that the mechanism and impact of melanin on oxidative stress in the context of melanomagenesis has not been fully elucidated. While the production of melanin can result in oxidative stress, once formed, the melanin pigment has unpaired electrons and can interact with free radicals and other reactive species as an antioxidant [
85,
86]. However, as Slominski et al. points out, this is a double-edged sword: shielding healthy melanocytes from oxidative stress but potentially accelerating disease and limiting the response to therapies that induce oxidative stress [
7]. One study demonstrated that induction of melanogenesis in amelanotic human and hamster melanoma cells resulted in significant transformations in the cells’ metabolic condition and performance, both biochemically and molecularly [
87]. These shifts were paired with a notable surge in hypoxia inducible factor (HIF-1α) expression in the nuclear components derived from pigmented cells. Thus, it can be inferred that pigmented melanomas may be more resistant to therapies that induce oxidative stress compared to amelanotic melanomas. However, it’s important to note that the response to oxidative stress is influenced by a number of factors.
3. Preventive and Therapeutic Strategies That Target Oxidative Stress Pathways
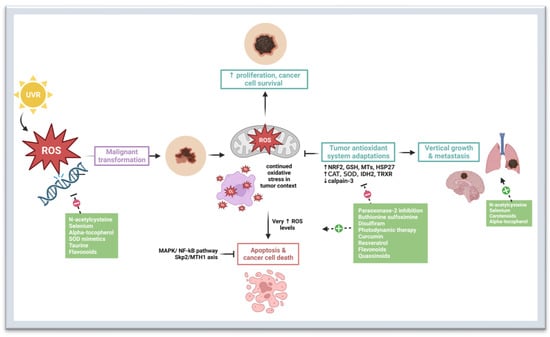
Burgeoning preventive and therapeutic approaches to melanoma exploit the complex interplay between oxidative stress, redox homeostasis, and melanomagenesis. Given the role of ROS in tumor initiation and progression, antioxidants are an interesting potential option for melanoma prevention and treatment. However, later in pathogenesis, melanoma tumors develop antioxidant mechanisms, but ROS levels are still higher compared to normal cells [
34,
88]. Therefore, further induction of oxidative stress may result in preferential demise of malignant cells. In other words, both antioxidants and pro-oxidants may have utility in the context of melanoma interventions (
Figure 1).
Figure 1. Oxidative Stress and Therapeutic Targets in Melanoma. Oxidative stress refers to an imbalance between the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and the antioxidant defense mechanisms that neutralize them [
30]. Exogenous sources of ROS include UV irradiation and inflammation [
31,
32]. Endogenous sources of ROS include nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase (NOX), cyclooxygenases, lipooxygenases, cytochrome P450, endothelial nitric oxide synthase (eNOs), and others. Oxidative stress and redox homeostasis are implicated in all phases of melanomagenesis [
10,
35]. The role of oxidative stress is somewhat paradoxical: while ROS help to promote cancer survival, proliferation, tumor vascularization, and metastasis, at high levels, they can also cause DNA damage and cancer cell death [
10,
35,
36,
37]. Thus, melanoma requires adaptive strategies to resist the effects of increased ROS levels [
38,
39]. N-acetylcysteine, selenium, alpha-tocopherol, SOD mimetics, and flavonoids have antioxidant properties that may be chemo-preventive in utility [
89,
90,
91,
92,
93,
94,
95]. However, studies have shown that antioxidants such as n-acetylcysteine, selenium, carotenoids, and alpha-tocopherol can promote growth and metastasis in later diseases by bolstering the tumor’s antioxidant system adaptations [
91,
96,
97]. Paraoxonase-2 inhibition, buthionine sulfoximine, disulfiram, photodynamic therapy, curcumin, resveratrol, flavonoids, and quassinoids inhibit tumor antioxidant system adaptations through various mechanisms, leading to increased oxidative stress and cancer cell death [
35,
98,
99,
100,
101,
102,
103,
104,
105,
106,
107,
108,
109,
110,
111,
112,
113,
114,
115,
116]. Abbreviations: UVR—ultraviolet radiation; ROS—reactive oxygen species; MAPK—mitogen-activated protein kinase; NF-kB—nuclear factor kappa B; Skp2—S-phase kinase associated protein 2; MTH1—MutT homolog 1; NRF2—nuclear factor erythroid 2-related factor 2; GSH—glutathione; CAT—catalase; SOD—superoxide dismutase; IDH2—isocitrate dehydrogenase 2; TRXR—thioredoxin reductase; MTs—metallothioneins; HSP27—Heat Shock Protein 27. Created with
BioRender.com.
3.1. N-Acetylcysteine
N-acetylcysteine (NAC) is an affordable, highly potent, orally bioavailable, and cell-permeable antioxidant [
89]. One murine study reported that pre-treatment with NAC reduced thiol depletion and blocked formation of 8-oxoguanine in mouse skin following neonatal UV treatment, and significantly delayed mean onset of UV-induced melanocytic tumors [
90]. A 2009 study in 72 individuals with a history of numerous or atypical nevi and/or personal or family history of melanoma, concluded that NAC can be safely administered to patients to modulate UV-induced oxidative stress in nevi, suggesting that NAC may be a potential chemo-preventive agent in melanoma [
89]. Ultraviolet radiation-induced glutathione depletion was attenuated in the post-drug (compared with pre-drug) nevus in approximately half of patients tested. While the above results seem promising, later studies reported that incubating human melanoma cells with NAC increased cell migration and cell division, and NAC-supplemented drinking water accelerated metastasis in murine models of malignant melanoma [
66,
117].
3.2. Selenium
Selenium is an essential micronutrient that was first studied in the context of cancer prevention nearly three decades ago [
118,
119]. The role of selenium supplementation in melanoma prevention and treatment is controversial and its efficacy is dependent on several factors. Cassidy et al. used a combination of in vitro and in vivo models to examine the utility of two unique chemical forms of selenium in these contexts. The results were mixed with topical treatment with l-selenomethionine delaying the time required for UV-induced melanoma development, but increasing the rate of growth of those tumors once they appear [
91]. Whereas oral administration of high dose methylseleninic acid significantly decreased the size of human melanoma xenografts.
3.3. Carotenoids
Carotenoids are natural pigments found in various fruits and vegetables that have antioxidant properties [
121]. They include β-carotene, lycopene, lutein, and zeaxanthin, among others. Several clinical studies have investigated the relationship between carotenoid intake and melanoma risk. These studies have primarily focused on dietary intake rather than using carotenoids as direct treatments for melanoma. The Vitamins and Lifestyle (VITAL) study, a large prospective cohort of 69,635 participants found that dietary or total intake of carotenoids was not associated with melanoma risk [
122]. The VITAL study was unable to detect an association of melanoma risk with regard to lutein and lycopene supplements as the cohort was underpowered for these carotenoids.
3.4. Alpha-Tocopherol
With its capacity to neutralize free radicals and hinder lipid peroxidation, Vitamin E (alpha-tocopherol) demonstrates potent antioxidant properties [
125]. However, Miura and Green’s meta-analysis reported that the consumption of vitamin E as food, supplements, or both did not affect the incidence of cutaneous melanoma [
120]. Whereas the SU.VI.MAX trial, a randomized, placebo-controlled investigation into the impact of antioxidant vitamins and minerals on health, reported that women consuming antioxidant supplements, including dietary supplementation with vitamin E, were at an increased risk for developing melanoma [
126]
3.5. Superoxide Dismutase Mimetics
Superoxide dismutase mimetics are a class of compounds designed to replicate the activity of the natural SOD enzymes, and studies have demonstrated their potential in melanoma prevention and adjunct therapy. One study demonstrated that an exogenous manganese superoxide dismutase isolated from
Allium sativum, a medicinal plant, was capable of modulating intracellular reactive oxygen species levels and inhibiting cell multiplication in tumor cells [
94]. A noted decrease in SOD levels during early stages of cancer development implies its potential for preventive measures. While lacking widespread clinical trials and pharmaceutical industry support, SOD liposomes and mimetics have been effective in animal models and early-phase clinical trials [
127].
3.6. Taurine
The antitumor and cytoprotective effects of taurine are linked to its ability to enhance antioxidant capacity, boost immunity, synergize with chemotherapeutic agents, and reduce chemotoxicity [
130,
131]. In B16F10 murine melanoma cells, taurine has been shown to increase the activity of SOD and GPX, leading to a decrease in ROS levels and inhibition of tumor cell growth [
95]. Monocarboxylate transporter 7 (MCT7)/Slc16a6, a novel facilitative taurine transporter, has been identified as a survival factor in melanoma [
132].
3.7. Paraoxonase-2 Inhibitors
Another promising therapeutical target in melanoma is the intracellular enzyme, paraoxonase-2 (PON2) [
35]. PON2 exerts antioxidative properties within the mitochondrial respiratory chain by binding with high affinity to coenzyme Q10 within the inner membrane, leading to a reduction of superoxide anion release during the electron transport chain [
98,
134,
135]. Interestingly, expression of PON2 has been shown to be positively correlated with Breslow thickness, Clark level, regression, mitoses, lymph node metastases, and thus, overall staging [
136].
3.8. Buthionine Sulfoximine
Buthionine sulfoximine (BSO) also acts to increase chemosensitivity. There is a growing body of evidence to suggest that depleting antioxidant defenses, particularly GSH levels, which are implicated in chemoresistance to alkylating agents, may prove to be a viable strategy in treatment resistant melanoma [
99]. Recent reports have shown encouraging results from combining chemotherapy with buthionine sulfoximine (BSO), a sulfoximine derivative that reduces GSH levels by inhibiting gamma-glutamylcysteine synthetase, the enzyme responsible for the initial step of glutathione synthesis. BSO was found to enhance melphalan cytotoxicity by 2.46-fold in SK-MEL 28 melanoma cells [
99].
3.9. Disulfiram
Disulfiram, an FDA-approved drug for the treatment of alcoholism, is a potent inhibitor of copper-zinc superoxide dismutase. Several mechanisms of its antitumor activity have been suggested, including induction of reactive oxygen species and death signaling pathways [
137]. When complexed with copper ions, the metabolite of disulfiram, diethyl dithiocarbamate (DDTC) has also been shown to inhibit the ubiquitin-proteasome system [
137,
138,
139]. It is hypothesized that the antitumor activity may be closely related to lipid peroxidation accumulation and ferroptosis mediated by the SLC7A11/GPX4 signaling pathway [
139]. Disulfiram has been shown to improve the chemosensitivity of melanoma cells to oxaliplatin treatment, according to several studies [
100,
101,
102].
3.10. Photodynamic Therapy
Photodynamic therapy (PDT) is a treatment modality that uses a photosensitizer and light of a specific wavelength to generate ROS, such as singlet oxygen, to induce cell death [
116,
140]. It has been investigated for use in various types of cancer, including melanoma. Studies have incorporated ruthenium complexes and nanoparticles to enhance the efficacy of PDT and minimize side effects [
140,
141,
142]. One study reported that homoligand polypyridyl ruthenium complexes (HPRCs) exhibited increased reactive oxygen species (ROS) generation and decreased dark cytotoxicity, mitigating damage to healthy tissue and demonstrating potential as effective antitumor PDT agents [
140].
3.11. Curcumin
Curcumin, a relative of turmeric, is a polyphenolic phytochemical that stimulates reactive oxygen species production and has been shown to induce cell death in eight melanoma cell lines [
103,
104]. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53 and is hypothesized to act through a membrane-mediate mechanism [
104] Curcumin has also been shown to block the NF-kappaB cell survival pathway and suppress the apoptotic inhibitor, x-linked inhibitor of apoptosis protein (XIAP). Kocyigit et al. were the first to highlight that ROS may be important in curcumin’s ability to cause DNA damage, cell death, and apoptosis. To test this, they studied the effects of curcumin on murine melanoma cancer cells (B16-F10) and fibroblastic normal cells (L-929) [
105].
3.12. Resveratrol
Resveratrol is an anticancer phytochemical polyphenol that naturally occurs in red grapes [
145]. Resveratrol has been shown to downregulate and inactivate Akt/protein kinase B in murine B16F10 and B16BL6 melanoma cells (derived from the former), inhibiting migratory and invasive properties of the malignant cells [
106]. Resveratrol also inhibits cell viability and α-MSH-activated matrix metalloproteinase- (MMP-)9 expression and invasion capacity of B16 melanoma cells [
107]. Resveratrol has also been shown to decrease NRF2 expression in melanoma cells, inducing increased production of ROS and inhibiting growth and proliferation by downregulating the Bcl-2 protein level and upregulating Bcl-2-related X protein expression [
108].
Oral, topical, and intraperitoneal resveratrol have all been shown to be effective in melanoma. Oral dosing of resveratrol has been associated with reduced primary tumor volume, Akt expression, and the propensity for metastasis in syngeneic mouse models of melanoma [
106]. One investigation evaluating resveratrol-loaded solid-lipid microparticulate topical gel in vitro, showed sustained release profiles, antioxidant properties, tyrosinase inhibition, cytotoxicity, and effective apoptosis in B16F10 melanoma cells [
146]. The in-vivo portion of the study in C57BL mice exhibited significant tumor reduction. Another study investigated intraperitoneal injection of resveratrol in immunocompetent mice as a potential therapeutic agent for metastatic melanoma [
147].
3.13. Flavonoids
Flavonoids are polyphenol compounds found in various vegetables, fruits, and other plants [
92]. They are well-known for their strong antioxidant properties. Apigenin, diosmin, fisetin, leteolin, quercetin, myricetin, and naringenin are among the commonly studied flavonoids for melanoma treatment [
148]. In addition to reducing ROS production, flavonoids have been shown to modulate cell growth, induce apoptosis, and repair DNA. They have also been shown to shrink cancerous tumors and prevent metastasis, and possibly contribute to lower skin cancer mortality rates. The selenocysteine (Sec)-containing mammalian thioredoxin reductase (TRXR) has evolved as a new target for anticancer drug development because TRXR and TRX are overexpressed in many aggressive tumors and the tumor cells are more dependent on the TRX system than normal cells [
109,
149].
3.14. Quassinoids
Similarly, to Flavonoids and Resveratrol, Quassinoids have been shown to downregulate NRF2. Ailanthone, an extract derived from the tree
Ailanthus altissima, has shown anti-tumor activity by downregulating NRF2 and inducing oxidative stress [
113,
114,
115].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15113038