Valproic acid (VPA) and its salts (sodium calcium magnesium and orotic) are psychotropic drugs that are widely used in neurology and psychiatry. The long-term use of VPA increases the risk of developing adverse drug reactions (ADRs), among which metabolic syndrome (MetS) plays a special role. MetS belongs to a cluster of metabolic conditions such as abdominal obesity, high blood pressure, high blood glucose, high serum triglycerides, and low serum high-density lipoprotein. Valproate-induced MetS (VPA-MetS) is a common ADR that needs an updated multidisciplinary approach to its prevention and diagnosis.
- valproic acid
- metabolic syndrome
- Valproate-Induced
1. Introduction
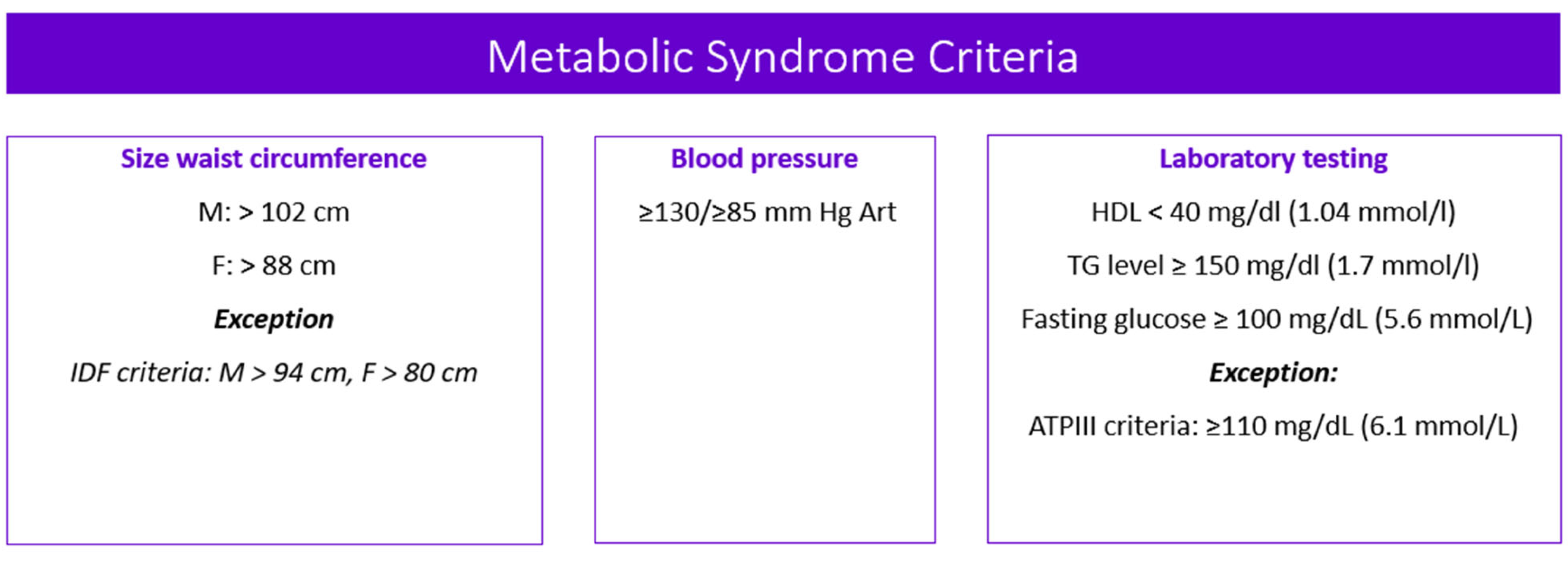
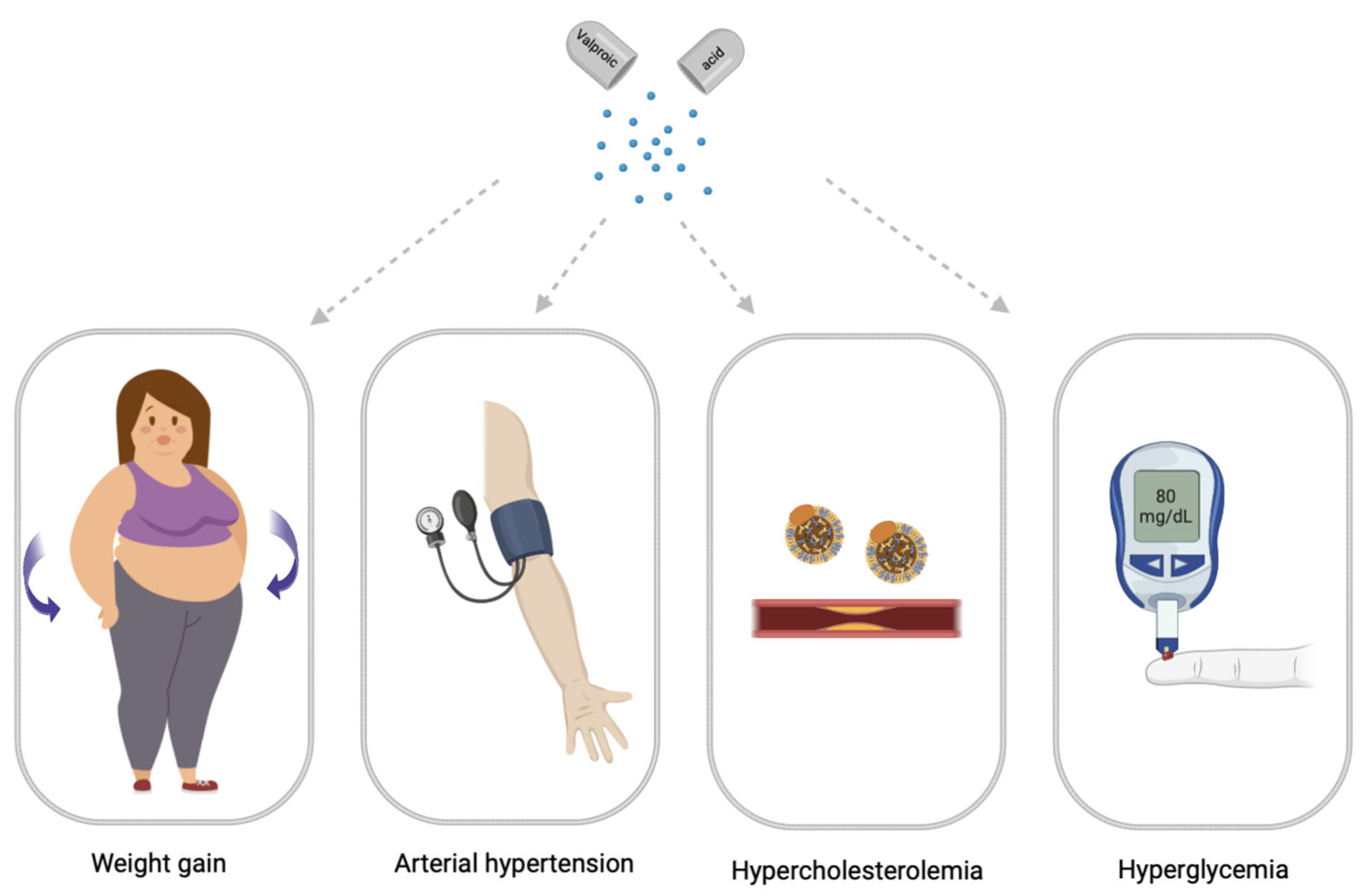
2. Main Clinical Symptoms of Valproate-Induced Metabolic Syndrome
2.1. Valproate-Induced Weight Gain
- −
-
An increase in body weight, body mass index (BMI), and waist circumference;
- −
-
An increase in the amount of fat in the abdomen, measured by the waist circumference or the ratio of waist to hips;
- −
-
Insulin resistance, which can contribute to the development of abdominal obesity.
2.2. Valproate-Induced Insulin Resistance
2.3. Valproate-Induced Arterial Hypertension
2.4. Valproate-Induced Hypercholesterolemia
2.5. Valproate-Induced Hyperglycemia
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11051499
References
- Kazamel, M.; Stino, A.M.; Smith, A.G. Metabolic syndrome and peripheral neuropathy. Muscle Nerve 2021, 63, 285–293.
- Oye-Somefun, A.; Kuk, J.L.; Ardern, C.I. Associations between elevated kidney and liver biomarker ratios, metabolic syndrome and all-cause and coronary heart disease (CHD) mortality: Analysis of the U.S. National Health and Nutrition Examination Survey (NHANES). BMC Cardiovasc. Disord. 2021, 21, 352.
- Chen, W.; Pan, Y.; Jing, J.; Zhao, X.; Liu, L.; Meng, X.; Wang, Y.; Wang, Y. Recurrent Stroke in Minor Ischemic Stroke or Transient Ischemic Attack with Metabolic Syndrome and/or Diabetes Mellitus. J. Am. Heart Assoc. 2017, 6, e005446.
- Penninx, B.W.J.H.; Lange, S.M.M. Metabolic syndrome in psychiatric patients: Overview, mechanisms, and implications. Dialogues Clin. Neurosci. 2018, 20, 63–73.
- Khasanova, A.K.; Dobrodeeva, V.S.; Shnayder, N.A.; Petrova, M.M.; Pronina, E.A.; Bochanova, E.N.; Lareva, N.V.; Garganeeva, N.P.; Smirnova, D.A.; Nasyrova, R.F. Blood and Urinary Biomarkers of Antipsychotic-Induced Metabolic Syndrome. Metabolites 2022, 12, 726.
- Available online: cadiresearch.org/topic/metabolic-syndrome/metabolic-syndrome-global/ms-criteria (accessed on 21 March 2023).
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497.
- Suplotova, L.A.; Smetanina, S.A.; Novakovskaya, N.A. Prevalence of metabolic syndrome and its components in women in different ethnic groups. Obes. Metab. 2011, 8, 48–51.
- Borisov, I.V.; Bondar, V.A.; Petrova, M.V.; Kuzovlev, A.N.; Ohlopkov, V.A.; Kanarski, M.M.; Nekrasova, J.J. Metabolic syndrome: Definition, pathogenesis and rehabilitation. Bull. All-Russ. Soc. Spec. Med. Soc. Expert. Rehabil. Rehabil. Ind. 2020, 4, 114–125.
- Chen, D.C.; Du, X.D.; Yin, G.Z.; Yang, K.B.; Nie, Y.; Wang, N.; Li, Y.L.; Xiu, M.H.; He, S.C.; Yang, F.D.; et al. Impaired glucose tolerance in first-episode drug-naïve patients with schizophrenia: Relationships with clinical phenotypes and cognitive deficits. Psychol. Med. 2016, 46, 3219–3230.
- Wofford, M.R.; King, D.S.; Harrell, T.K. Drug-induced metabolic syndrome. J. Clin. Hypertens. 2006, 8, 114–119.
- Sychev, D.A.; Ostroumova, O.D.; Pereverzev, A.P. Drug-induced diseases: Approaches to diagnosis, correction and prevention Pharmacovigilance. Pharmateka 2020, 27, 113–126.
- Shnayder, N.A.; Grechkina, V.V.; Khasanova, A.K.; Bochanova, E.N.; Dontceva, E.A.; Petrova, M.M.; Asadullin, A.R.; Shipulin, G.A.; Altynbekov, K.S.; Al-Zamil, M.; et al. Therapeutic and Toxic Effects of Valproic Acid Metabolites. Metabolites 2023, 13, 134.
- Zuo, S.; Fries, B.E.; Szafara, K.; Regal, R. Valproic Acid as a potentiator of metabolic syndrome in institutionalized residents on concomitant antipsychotics: Fat chance, or slim to none? Pharm. Ther. 2015, 40, 126–132.
- Shnaider, N.A.; Dmitrenko, D.V. Chronic valproic acid intoxication in epileptology: Diagnosis and treatment. Neurol. Neuropsychiatry Psychosom. 2016, 8, 94–99.
- Fang, J.; Chen, S.; Tong, N.; Chen, L.; An, D.; Mu, J.; Zhou, D. Metabolic syndrome among Chinese obese patients with epilepsy on sodium valproate. Seizure 2012, 21, 578–582.
- George, L.J.; Singh, P.; Aneja, S.; Singh, R.; Solanki, R.S.; Seth, A. Insulin Resistance in children on Sodium Valproate—A hospital based cross-sectional study in Indian children. Trop. Doct. 2023, 53, 91–96.
- Kim, Y.J.; Lee, Y.H.; Lee, Y.J.; Kim, K.J.; Kim, S.G. Weight Gain Predicts Metabolic Syndrome among North Korean Refugees in South Korea. Int. J. Environ. Res. Public Health 2021, 18, 8479.
- Belcastro, V.; D’Egidio, C.; Striano, P.; Verrotti, A. Metabolic and endocrine effects of valproic acid chronic treatment. Epilepsy Res. 2013, 107, 1–8.
- Romoli, M.; Mazzocchetti, P.; D’Alonzo, R.; Siliquini, S.; Rinaldi, V.E.; Verrotti, A.; Calabresi, P.; Costa, C. Valproic Acid and Epilepsy: From Molecular Mechanisms to Clinical Evidences. Curr. Neuropharmacol. 2019, 17, 926–946.
- Zhang, H.; Lu, P.; Tang, H.L.; Yan, H.J.; Jiang, W.; Shi, H.; Chen, S.Y.; Gao, M.M.; Zeng, X.D.; Long, Y.S. Valproate-Induced Epigenetic Upregulation of Hypothalamic Fto Expression Potentially Linked with Weight Gain. Cell. Mol. Neurobiol. 2021, 41, 1257–1269.
- Rehman, T.; Sachan, D.; Chitkara, A. Serum Insulin and Leptin Levels in Children with Epilepsy on Valproate-associated Obesity. J. Pediatr. Neurosci. 2017, 12, 135–137.
- Münzberg, H.; Björnholm, M.; Bates, S.H.; Myers, M.G., Jr. Leptin receptor action and mechanisms of leptin resistance. Cell Mol. Life Sci. 2005, 62, 642–652.
- Rauchenzauner, M.; Laimer, M.; Luef, G.; Kaser, S.; Engl, J.; Tatarczyk, T.; Ciardi, C.; Tschoner, A.; Lechleitner, M.; Patsch, J.; et al. Adiponectin receptor R1 is upregulated by valproic acid but not by topiramate in human hepatoma cell line, HepG2. Seizure 2008, 17, 723–726.
- Qiao, L.; Schaack, J.; Shao, J. Suppression of adiponectin gene expression by histone deacetylase inhibitor valproic acid. Endocrinology 2006, 147, 865–874.
- Brown, A.E.; Walker, M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr. Cardiol. Rep. 2016, 18, 75.
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223.
- Aly, R.H.; Amr, N.H.; Saad, W.E.; Megahed, A.A. Insulin resistance in patients on valproic acid: Relation to adiponectin. Acta Neurol. Scand. 2015, 131, 169–175.
- Jian, J.; Li, L.G.; Zhao, P.J.; Zheng, R.J.; Dong, X.W.; Zhao, Y.H.; Yin, B.Q.; Cheng, H.; Li, H.L.; Li, E.Y. TCHis mitigate oxidative stress and improve abnormal behavior in a prenatal valproic acid-exposed rat model of autism. Physiol. Genom. 2022, 54, 325–336.
- Brown, R.; Imran, S.A.; Ur, E.; Wilkinson, M. Valproic acid and CEBPalpha-mediated regulation of adipokine gene expression in hypothalamic neurons and 3T3-L1 adipocytes. Neuroendocrinology 2008, 88, 25–34.
- Khan, S.; Kumar, S.; Jena, G. Valproic acid reduces insulin-resistance, fat deposition and FOXO1-mediated gluconeogenesis in type-2 diabetic rat. Biochimie 2016, 125, 42–52.
- Rauchenzauner, M.; Laimer, M.; Wiedmann, M.; Tschoner, A.; Salzmann, K.; Sturm, W.; Sandhofer, A.; Walser, G.; Luef, G.; Ebenbichler, C.F. The novel insulin resistance parameters RBP4 and GLP-1 in patients treated with valproic acid: Just a sidestep? Epilepsy Res. 2013, 104, 285–288.
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrӧm-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Russ. J. Cardiol. 2021, 26, 4701. (In Russian)
- Aune, D.; Huang, W.; Nie, J.; Wang, Y. Hypertension and the Risk of All-Cause and Cause-Specific Mortality: An Outcome-Wide Association Study of 67 Causes of Death in the National Health Interview Survey. Biomed Res. Int. 2021, 2021, 9376134.
- Abaseynejad, F.; Akrami, R.; Mohebbati, R.; Sehab Negah, S.; Mohammad-Zadeh, M. The Effect of Sodium Valproate on Cardiovascular Responses in Pentylenetetrazol Kindling Model of Epilepsy. Biomed. J. Sci. Tech. Res. 2022, 42, 33592–33596.
- Sousa-Lopes, A.; de Freitas, R.A.; Carneiro, F.S.; Nunes, K.P.; Allahdadi, K.J.; Webb, R.C.; Tostes, R.C.; Giachini, F.R.; Lima, V.V. Angiotensin (1-7) Inhibits Ang II-mediated ERK1/2 Activation by Stimulating MKP-1 Activation in Vascular Smooth Muscle Cells. Int. J. Mol. Cell. Med. 2020, 9, 50–61.
- Zhao, Y.; Xing, B.; Dang, Y.H.; Qu, C.L.; Zhu, F.; Yan, C.X. Microinjection of valproic acid into the ventrolateral orbital cortex enhances stress-related memory formation. PLoS ONE 2013, 8, e52698.
- Rajeshwari, T.; Raja, B.; Manivannan, J.; Silambarasan, T. Valproic acid attenuates blood pressure, vascular remodeling and modulates ET-1 expression in L-NAME induced hypertensive rats. Biomed. Prev. Nutr. 2014, 4, 195–202.
- Sivananthan, M.; Mohiuddin, S. Valproate Induced Hypertensive Urgency. Case Rep. Psychiatry 2016, 2016, 1458548.
- Zárate, A.; Manuel-Apolinar, L.; Saucedo, R.; Hernández-Valencia, M.; Basurto, L. Hypercholesterolemia as a Risk Factor for Cardiovascular Disease: Current Controversial Therapeutic Management. Arch. Med. Res. 2016, 47, 491–495.
- Peters, S.A.; Singhateh, Y.; Mackay, D.; Huxley, R.R.; Woodward, M. Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: A systematic review and meta-analysis. Atherosclerosis 2016, 248, 123–131.
- Kusumastuti, K.; Jaeri, S. The effect of long-term valproic acid treatment in the level of total cholesterol among adult. Indian J. Pharmacol. 2020, 52, 134–137.
- Hamed, S.A. Atherosclerosis in epilepsy: Its causes and implications. Epilepsy Behav. 2014, 41, 290–296.
- Aziz, R.S.; Saeed, U.; Ali, L.; Arshad, M.; Abbas, R.; Mushtaq, S.; Asif Shahzad, A.; Shaukat, A. Effect on lipid profile due to prolong Valproic acid intake. Pak. J. Med. Health Sci. 2021, 15, 1497–1499.
- Guo, H.L.; Jing, X.; Sun, J.Y.; Hu, Y.H.; Xu, Z.J.; Ni, M.M.; Chen, F.; Lu, X.P.; Qiu, J.C.; Wang, T. Valproic Acid and the Liver Injury in Patients with Epilepsy: An Update. Curr. Pharm. Des. 2019, 25, 343–351.
- Verrotti, A.; Scardapane, A.; Franzoni, E.; Manco, R.; Chiarelli, F. Increased oxidative stress in epileptic children treated with valproic acid. Epilepsy Res. 2008, 78, 171–177.
- Verrotti, A.; la Torre, R.; Trotta, D.; Mohn, A.; Chiarelli, F. Valproate-induced insulin resistance and obesity in children. Horm. Res. 2009, 71, 125–131.
- Fathallah, N.; Slim, R.; Larif, S.; Hmouda, H.; Ben Salem, C. Drug-Induced Hyperglycaemia and Diabetes. Drug Saf. 2015, 38, 1153–1168.
- Felisbino, M.B.; Ziemann, M.; Khurana, I.; Okabe, J.; Al-Hasani, K.; Maxwell, S.; Harikrishnan, K.N.; de Oliveira, C.B.M.; Mello, M.L.S.; El-Osta, A. Valproic acid influences the expression of genes implicated with hyperglycaemia-induced complement and coagulation pathways. Sci. Rep. 2021, 11, 2163.
