Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Asthma is a chronic respiratory disease that impacts millions of people worldwide. Several dietary factors have been recognized as potential contributors to the development and severity of asthma for its inflammatory and oxidative effects. Some food groups such as fruits and vegetables, whole grains, and healthy fats appear to exert positive effects on asthma disease. On the other hand, a high consumption of dietary salt, saturated fats, and trans-fat seems to have the opposite effect.
- asthma
- airway inflammation
- diet
- dietary patterns
1. Introduction
Asthma is associated with inflammation and oxidative stress [1], as previously mentioned, and the antioxidant and anti-inflammatory properties of several food groups and nutrients may have an influence on asthma and airway inflammation [2][3] (Figure 1).
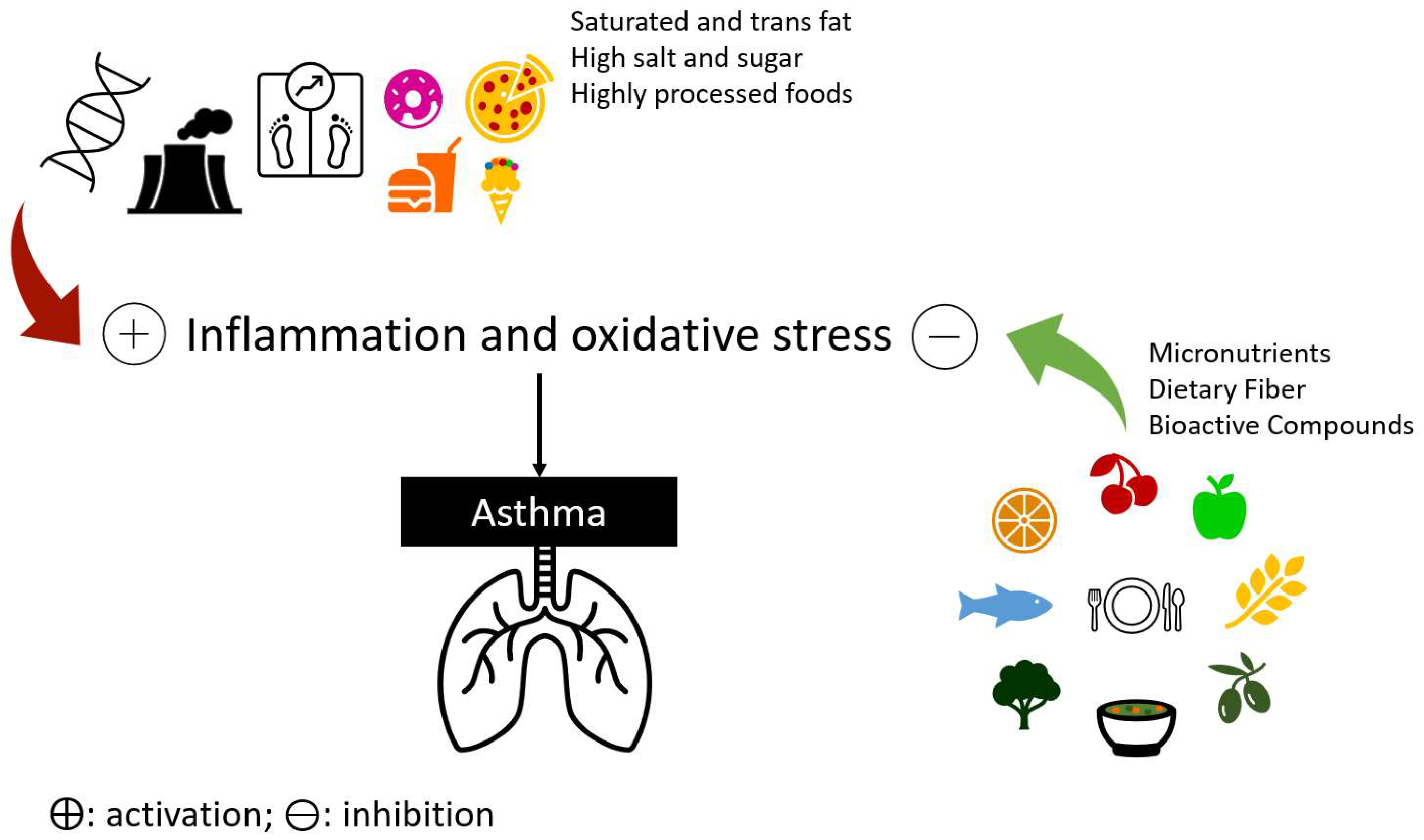
Figure 1. Deleterious or protective effects of diet on asthma genetic, environmental, and host factors.
Recent research is focusing on how epigenetic mechanisms may elucidate the connection between gene regulation and environmental factors in asthma [4]. Epigenetic mechanisms help genes adapt to changes in the environment, and these alterations can contribute to the development of disease phenotypes, being identified as one of the potential causes for the onset of diseases in susceptible individuals [5][6]. Recently, it has been demonstrated that elevated dietary acid loads may modulate asthma-related miRNAs among school-aged children [7][8].
2. Fruits and Vegetables
Fruits and vegetables are known as a fundamental component of a healthy diet and are recommended to make up half of one’s plate at each meal. They offer a variety of nutrients and bioactive compounds that can promote good health and are able to modulate inflammation and prevent chronic diseases [2].
In asthmatic children, fruit and vegetable intake was found to be negatively associated in nasal lavage with IL-8, a marker of inflammation [9]. A study suggested an inverse significant relationship between salad consumption (a source of antioxidants) and FeNO, in children [10]. It was also observed that a greater daily vegetable diversity intake was negatively associated with having self-reported asthma with an odds ratio (OR) = 0.67; 95% CI 0.47, 0.95, whereas a vegetable diversity ingestion superior to three items per day was negatively associated with levels of FeNO ≥ 35 ppb (OR = 0.38; 95% CI 0.16, 0.88) and breathing difficulties (OR = 0.39; 95% CI 0.16, 0.97) in school-aged children [11].
A longitudinal study on children up to 5 years observed that the risk of asthma was not associated with the consumption of all fruits and vegetables together, but inverse associations were seen between all leafy vegetables and asthma (Hazard Ratio (HR) = 0.87, 95% CI 0.77; 0.99), and unprocessed vegetables and non-atopic asthma (HR = 0.90, 95% CI 0.81; 0.98), respectively [12].
A cohort study demonstrated that a higher fruit intake at 8 years of age was associated with a tendency to lower the odds of prevalent asthma (T3 vs. T1, OR 0.78; 95% CI 0.60–1.01, p-trend 0.083), with reduced odds of incident asthma, and increased odds of remittent asthma (OR = 0.76; 95% CI 0.58–0.99 and OR = 1.60; 95% CI 1.05–2.42, respectively) for up to 24 years [13].
Another study conducted in adults revealed that reducing the intake of antioxidant-rich foods worsens asthma control and lung function. Furthermore, it was demonstrated that an increase in airway neutrophils, resulting from a low-antioxidant diet, can be reversed using lycopene-rich treatments (such as tomato juice) that lead to a decrease in airway neutrophil influx [14]. Wood et al. [15] compared the outcomes of a high-antioxidant diet to those of a low-antioxidant diet, with and without lycopene supplementation, in asthmatic patients, over the course of a 16-week period. It was observed that participants on the high fruit and vegetable intervention arm (high-antioxidant diet) had fewer asthma exacerbations. Several participants in the low fruit and vegetable arm (low-antioxidant diet) were given an antioxidant supplement, but this did not reduce the risk of asthma exacerbations. As improvements were only observed after increasing fruit and vegetable consumption, whole-food interventions seem to be more effective, implying that the whole food dietary component is more relevant than antioxidant supplementation [15].
Two meta-analyzes found that a higher intake of fruit and vegetables was associated with a lower risk of having asthma [16][17]. One of the meta-analyzes found that vegetable intake was negatively associated with the risk of prevalent asthma (OR = 0.95; 95% CI 0.92; 0.98) and that fruit intake was negatively associated with the risk of prevalent wheeze (OR = 0.94; 95% CI 0.91; 0.97) and asthma severity (OR = 0.61; 95% CI 0.44; 0.87) [17]. Six of the seven studies found that fruit and vegetable intake in asthmatics had a protective effect against either airway or systemic inflammation [17]. The other meta-analysis also found protective effects of the total intake of vegetables and fruits in asthmatic children with a relative risk (RR) of 0.57 (95% CI 0.42; 0.77). Fruit consumption was found to reduce the risk of wheeze and asthma (RR = 0.81, 95% CI 0.74; 0.88, and RR = 0.90, 95% CI 0.86; 0.94, respectively). Similarly, the intake of vegetables reduced the risk of wheeze and asthma (RR = 0.88, 95% CI 0.79; 0.97, and RR = 0.91, 95% CI 0.82; 1.00, respectively) [16].
Diets that have a high intake of fruits and vegetable are widely recommended for their health-promoting properties due to their mineral, vitamin, dietary fiber, and bioactive content [2].
3. Fiber
Some of the protective effects observed with fruit and vegetable consumption may also be attributed to the fiber content of these food groups [2]. Whole grains, beans, fruits, and vegetables are all sources of dietary fiber [18]. SCFAs are produced by gut bacteria following the fermentation of soluble fiber and are the principal fuel of colonocytes [19][20]. Butyrate has been demonstrated to activate the peroxisome proliferator-activated receptor alpha, (PPAR) which inhibits the NF-kB activity, a pro-inflammatory transcription factor [21]. Roduit et al. observed that oral administration of SCFAs to mice significantly reduced the severity of allergic airway inflammation [22]. Dietary fiber and SCFAs have also shown anti-inflammatory effects by activating free fatty acid receptors such as G protein-coupled receptors 41 and 43 (GPR41 and GPR43) in animal models [19][20]. GPR43 and GPR41 activation can influence the functions of immune cells, such as neutrophils and dendritic cells. This regulation can lead to a decrease in the release of pro-inflammatory mediators. GPR43 and GPR41 activate the Gi/o proteins that reduce the levels of the secondary messenger cAMP, thereby counteracting the pro-inflammatory effects of the cAMP-mediated signaling pathways [19][20].
One study assessed the acute effects of a single meal rich in soluble fiber (175 g yogurt with 3.5 g inulin and probiotics) compared with a simple carbohydrate meal (200 g of mashed potato) on asthmatic airway inflammation, including changes in free fatty acid receptor gene expression. The studied outcomes were analyzed 4 h after the meal intake. It was observed that airway inflammation biomarkers, such as sputum total cell count, macrophages, neutrophils, sputum IL-8, lymphocytes, and eNO were significantly lower in the soluble fiber group and this corresponded to increased GPR41 and GPR43 sputum gene expression and improved lung function [23].
The consumption of whole grain products, which are a source of dietary fiber, was found to be inversely associated with asthma in a study assessing 598 Dutch children [24]. For current asthma, the adjusted OR was 0.46 (95% CI 0.19; 1.10), whereas for atopic asthma with bronchial hyperresponsiveness was 0.28 (95% CI 0.08 to 0.99) [24]. Additionally, a high consumption of whole grains was associated with lower odds of asthma (OR = 0.52, 95% CI 0.33; 0.82) [25]. However, as previously observed, other nutrients found in fiber-rich foods, such as fruits and vegetables, cereals, and legumes, may also be contributing to the inverse association found between dietary fiber consumption and airway inflammation. Many fiber-rich foods contain antioxidants and other micronutrients, such as polyphenolic compounds and flavonoids, which may help to reduce the oxidative stress in asthma patients [3].
4. Nuts
Nuts are a rich source of nutrients, healthy fats, and fiber, and have been widely studied for their potential health benefits [26]. While the consumption of nuts has been associated with a lower risk of chronic disease [26], some studies suggest that nut consumption may be linked to the exacerbation of asthma symptoms, as nuts are common food allergens that may induce an allergic response in the respiratory system [27]. Nonetheless, in a cross-sectional study, a high dietary intake of nuts appeared to exert a protective role on the prevalence of wheeze in children (OR = 0.46; 95% CI 0.20; 0.98) [28]. Peanuts, another common source of food allergens, even though being a legume, from a nutritional perspective, their contents are similar to tree nuts. Du Toit, G. et al., in a randomized trial, found no significant differences in risk of asthma events at 5 years of age between infants who were randomly ascribed either to consume peanuts, or to avoid peanut intake from 4 to 60 months old (p-value = 0.230) [29]. Additionally, there were no significant differences found in the severity of asthma events (p-value = 0.839) [29]. Accordingly, Roduilt et al. demonstrated that the introduction of nuts in the first year of life was not associated with asthma at 6 years old in a cohort study (OR = 0.69; 95% CI 0.36; 1.34) [30]. Nevertheless, in the previously referred study, children with higher values of the dietary diversity score in the first year of life had a lower risk of asthma compared with those with lower score values, and there was a significant reduction of 26% for the development of asthma with each additional food item introduced in the first year of life (OR = 0.74; 95% CI 0.61; 0.89).
5. Fats and Fish
Dietary fat may modulate inflammatory responses through a variety of mechanisms [3][31][32]. Depending on the type and source of fat consumed, it may have positive [33] or negative [34] effects on asthma.
On the negative side, a high-fat diet may change the composition of the gut microbiota by altering the expansion and colonization of invasive bacteria, with a decrease in protective bacteria, and a decrease in SCFA concentration [31]. Saturated fatty acids can activate innate immune receptors including toll-like receptors (TLR)-4, resulting in altered transcription factor activity such as NF-kB, consequently stimulating the inflammatory cascade [31]. In addition, omega-6 (n-6) polyunsaturated fatty acids (PUFA) are also associated with pro-inflammatory responses, and are found specially in vegetable oils [35], which are typically linked with the western diet [31].
On the other side, omega-3 (n-3) PUFAs, namely eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), that are found in oily fish and fish oil supplements [33], were found to be beneficial in animal models of asthma [33]. EPA and DHA lipid mediators have shown to possess anti-inflammatory and inflammation-resolving properties, such as limiting neutrophil infiltration or inhibiting pro-inflammatory cytokine production [35]. Furthermore, EPA and DHA appears to directly inhibit the production of pro-inflammatory cytokines by inhibiting the activation of the nuclear transcription factor NF-kB [35]. An imbalance in n-6 PUFA intake versus n-3 PUFA intake has been proposed as a contributing factor to the rise in allergic diseases, that could be a consequence of n-6 PUFA’s pro-inflammatory activity, which promotes a type 2 helper T lymphocyte immune response [35].
Concordantly, a cross-sectional study of adult asthma patients revealed a positive association between a high n-6:n-3 PUFA ratio with higher eNO values (β = 0.05, 95% CI 0.02; 0.09) and increased odds for uncontrolled asthma (OR = 3.69, 95% CI 1.37; 9.94) [36].
A study of subjects with severe persistent asthma found that, comparatively to healthy controls, they consumed more fat and less fiber, and these intakes were associated with lower forced expiratory volume in 1 s (FEV1) and airway eosinophilia in asthmatics [3]. It was also observed that saturated fat intake was positively associated with sputum % eosinophils [3]. Another study observed that a single high-fat/high-energy meal increases neutrophilic airway inflammation and TLR4 mRNA expression in sputum cells and decreases bronchodilator responsiveness in patients with asthma [34]. However, the high-fat meals had a diverse nutritional profile, which included fats of various qualities as well as other macronutrients, which may have had an impact on the inflammatory response [34]. No changes in eNO levels were observed after the high-fat meal. However, as stated before, eNO is a marker of eosinophilic asthma, and as the inflammatory changes observed were increases in airway neutrophils, it is not surprising that eNO levels were left unaltered [34]. In the same study, another group of patients with asthma consumed a high-trans (n = 5; 5.2 g trans-fat) or non-trans (n = 5, <0.3 g trans-fat) fatty acid meal. After the high-trans fatty acid meal, sputum % neutrophils were found to be significantly higher than after the non-trans meal [34].
Higher ingestion of saturated fatty acid-rich foods, such as butter, appears to be associated with both a higher asthma symptom score and a higher degree of airway inflammation [10].
On the other side, the consumption of olive oil (a monosaturated rich type of fat) in a multi-case study of Italian adults, showed that intakes of oleic acid and of olive oil were associated with a reduced risk of current asthma. Individuals in the highest quartile of oleic acid intake had less than half the chance of having current asthma in comparison to those in the lowest quartile. Moreover, these authors uncovered that when considering olive oil intake as a continuous variable, the risk of having current asthma declined by 20% for an increase of 10 g/day in olive oil consumption [37]. Olive oil is also rich in phenolic compounds and may exert its protective effects through additional anti-inflammatory and antioxidants effects [37].
The consumption of fish (the principal source of n-3 PUFA) was found to be inversely associated to asthma in the previously referred study of 598 Dutch children [24], with the adjusted OR for current asthma being 0.34 (95% CI 0.13; 0.85) and for atopic asthma with bronchial hyperresponsiveness, 0.12 (95% CI 0.02; 0.66), respectively [24]. In another study, school children who consumed fish more often had less doctor-diagnosed asthma and less current asthma [38].
However, evidence of fish oil and fish benefits in asthmatic patients are inconsistent [39]. An inverse association between consumption and asthma risk seems to be more evident in children, as their immune system is still under development: a meta-analysis found that infant fish consumption was inversely related to the occurrence of asthma in childhood. When comparing the highest to lowest category of fish consumption, the pooled RR of asthma was 0.76 (95% CI, 0.61; 0.94) [39]. Two different studies from the previously referred meta-analysis examined the relationship between maternal fish consumption during pregnancy and the risk of asthma in offspring. However, as the exposures assessed in these studies were different, they did not combine their results through meta-analysis. In one of the studies, there was no significant association found between maternal fish consumption frequency during pregnancy and the development of asthma in offspring (OR = 1.01, 95% CI 0.85; 1.20) when comparing mothers who consumed fish more than once a week with those who consumed fish less than once a week. Similarly, the other included study reported that maternal plasma long-chain n-3 polyunsaturated fatty acid (LCn3PUFA) concentration was not associated with the risk of asthma in offspring [39]. However, the levels of LCn3PUFA in maternal expressed breast milk (EBM) were found to have a negative association with the occurrence of asthma in offspring. When comparing the group with the highest levels of LCn3PUFA to the group with the lowest levels, there was a combined RR = 0.71 (95% CI, 0.52–0.96). This suggests that higher levels of LCn3PUFA in maternal EBM are associated with a reduced risk of asthma in the children [39]. In the same meta-analysis, no statistically significant associations were found in adults among these studies [39].
A more recent meta-analysis investigating the effects of fish consumption on asthma in children, found that “all fish” (lean and fatty) consumption had an overall beneficial effect on “current asthma” (OR = 0.75, 95% CI 0.60; 0.95) and “current wheeze” (OR = 0.62, 95% CI 0.48; 0.80) in children up to 4.5 years old. In children aged 8 to 14 years old, fatty fish consumption was found to be more protective than no fish consumption (OR = 0.35, 95% CI 0.18; 0.67) [40]. However, another meta-analysis found no significant association between fish intake in infancy and childhood asthma [41].
Meta-analyzes evaluating the effect of fish oil or n-3 PUFA supplementation have not found any effects on asthma incidence or asthma symptoms [42][43]. The lack of effects observed in most studies may be due to fish oil supplementation use, which may have a different health impact than eating the whole fish and all its properties [42].
6. Salt
Dietary sodium having an influence on asthma was first proposed in 1987 based on a correlation observed between asthma mortality and table salt purchases [44]. Dietary changes associated with the development of a different, more rushed, lifestyle have been identified as one of the environmental factors that may have contributed to the rise in the prevalence of asthma, with sodium being identified as a dietary constituent that may be implicated in this phenomenon [45]. Data from a cross-sectional study found a lower potassium and a higher sodium intake in severe asthmatics patients compared to healthy controls [3]. It has been proposed that a high sodium intake may lead to hyperpolarization of the bronchial smooth muscle, causing asthma exacerbation [3], and dietary sodium intake has been shown to be positively associated with airway responsiveness [46]. However, a cross-sectional study did not corroborate these findings, as it found no significant associations between 24 h sodium urinary excretion samples and airway reactivity to methacholine in adults [47].
A meta-analysis of six randomized controlled trials that aimed to assess the effect of a dietary sodium reduction in patients with asthma found no evidence of an effect of salt intake on lung function measures in asthmatic adults [48]. Nonetheless, it suggested that there is a pattern of a small improvement in airway function and a small reduction in bronchodilator use with low-salt diets [48]. Another meta-analysis aiming to observe the effects of salt on asthma control also found no benefits of salt manipulation on asthma [49]. There was, however, some evidence that a low-sodium diet may improve lung function after exercise and possibly baseline lung function, according to the evidence from exercise-induced asthma studies, but these results were based on findings obtained from a very small number of participants [49].
7. Dairy Products
The 2015–2020 Dietary Guidelines for Americans recommend consuming three cups of dairy per day [18]. However, a case-control study compared children in the highest quartiles of dairy intake (Q3–4; OR = 1.72, 95% CI 1.19; 2.49) with children in the lowest quartiles (Q1–2; OR < 1), and found a positive association between frequent dairy consumption and odds of developing asthma (OR = 1.93, 95% CI 1.32; 2.84) [25].
A different study demonstrated that early life exposure to unpasteurized milk seems to exert a protective effect on asthma [50][51], that may be related to its bacterial composition [50] or protein components [51].
A community-based cross-sectional study of Australian young adults found that drinking whole milk was negatively associated with increased odds of doctor-diagnosed asthma (OR = 0.73, 95% CI 0.54; 0.99), while consumption of low-fat cheese was found to be positively associated (OR = 1.50, 95% CI 1.03; 2.19). Similarly, ricotta cheese intake was positively associated with current asthma (OR = 1.94, 95% CI 1.18; 3.19) [52].
Although the mechanisms by which dairy products may influence the development of asthma are unknown, there was a positive association observed between dairy consumption and concentrations of pro-inflammatory IL-17F. The authors suggest the existence of an IL-17F-dependent inflammatory pathway that can play a role in the development of asthma [25].
8. Dietary Patterns
As reviewed above, diet is an important source of nutrients and other food components that have a variety of properties that may modulate the risk of asthma, as well as other chronic respiratory diseases [53][54].
Most studies have focused on the effects of individual components or nutrients to investigate the impact of diet on asthma [31][55][56]. However, findings linking specific dietary components and eating patterns to altered airway inflammation, asthma, and lung function have rarely been consistent in human studies [33][39][42][43][48][49][57]. Considering that foods are ingested as complex combinations, which include nutrients and bioactive components, which interact with each other and the food matrix, recent efforts have concentrated on researching validated indices that assess the properties of the entire diet influencing metabolic and health effects [8][31][55][56][58].
Scores that aim to classify diet quality, such as the Healthy Eating Indexes (HEI), as well as dietary patterns such as the Mediterranean Diet (MD) and the Dietary Approaches to Stop Hypertension (DASH), mostly highlight the consumption of fruits and vegetables, whole grains, and unsaturated fat sources, recommending a lower intake of saturated fats, sodium, and refined grains [55][59][60][61]. A study using the HEI-2015, which is a measure to assess diet quality, specifically to which the dietary pattern aligns with the Dietary Guidelines for Americans, comparing dietary patterns between adults with asthma with and without asthma-related emergency room (ER) admissions, found that individuals with asthma had low vegetable and fruit intakes as well as a low HEI-2015 score (mean ± SE 52.6 ± 0.53), and those with asthma-related ER visits consumed less vegetables compared to those without (median 0.61 cup vs. 0.85 cup equivalents) [62].
Ma et al. conducted a pilot randomized controlled trial of a 6-month behavioral intervention promoting the DASH diet, which includes the intake targets for total fat, saturated fat, protein, cholesterol, fiber, magnesium, calcium, sodium, and potassium, in patients with uncontrolled persistent asthma. When compared to usual care, this intervention produced greater improvements in diet quality, asthma control, and asthma-related quality of life [63].
As for children, the evidence from eight observational studies on Mediterranean diet exposure and its association with lower asthma prevalence was examined in a systematic review. The definitions “current wheeze”, “current severe wheeze”, and “asthma ever” were used. When all studies were considered, the results of the meta-analysis revealed that an higher adherence to the Mediterranean diet during childhood is a protective factor for “current wheeze” and “asthma ever”, but not for “severe current wheeze” [64]. Another systematic review reported that increased adherence to the Mediterranean diet was negatively associated with asthma symptoms in children [65]. More recently, a systematic review indicated a protective role of the Mediterranean diet on childhood asthma [66]. On the other side, school-aged children, who follow a strict Western diet high in total and saturated fat and processed foods, seem to be at a higher risk of asthma [67].
Tarazona-Meza et al. [68] found in a cross-sectional study that better diet quality was associated with a lower odds of having asthma (OR = 0.83, 95% CI 0.72; 0.95) in children and adolescents [68]. Lower values in the Revised Brazilian Healthy Eating Index score, representing lower dietary quality, both at 18 and 22 years old, increased the odds of wheezing in the previous year, with an OR = 1.97, 95% CI 1.33; 2.91, and OR = 1.98, 95% CI 1.36; 2.87, respectively, in a longitudinal study. On the other side, remaining on a poor diet from ages 18 to 22 increased the odds of chest wheezing by more than three-fold (OR = 3.28; 95% CI 1.84; 5.84) compared to staying on a high-quality diet [69].
Findings from the PARIS birth cohort demonstrated that children in the higher tertile group of adherence to the Mediterranean diet, considered to have the higher diet quality, compared to children from the lowest tertile group, had a lower risk of having current asthma (aOR = 0.28, 95% CI 0.12; 0.64) [70]. Furthermore, adherence to the Mediterranean diet may modulate the production of several asthma inflammatory mediators: a higher adherence to this diet pattern was associated with lower IL-4 and IL-17 in asthmatic children [71].
Accordingly, a randomized controlled trial revealed that a Mediterranean diet supplemented by two meals of 150 g of cooked fatty fish weekly for six months, compared to the usual diet, reduced airway inflammation as assessed by eNO (β = −14.15 ppb, 95% CI −27.39; −0.91) in childhood asthma [72].
A study that observed associations between three different dietary scores with asthma symptoms and asthma control in French adults, found that higher dietary scores assessed by the Alternative Healthy Eating Index 2010 (AHEI-2010), the Mediterranean diet based on the literature (MEDI-LITE), and modified Programme National Nutrition Santé Guideline Score were associated with a lower asthma symptom score [55]. However, when analyzing the association of a higher diet quality with asthma symptom score according to BMI, some of the statistically significant associations were lost when BMI is ≥25 and <30 kg/m2, and most were lost when BMI is ≥30 kg/m2 [55].
Individuals with obesity may present higher circulating concentrations of many inflammatory markers as a consequence of the disease [73], and GINA guidelines recommend weight loss as part of the strategy of asthma management in obese patients [74]. Knowing that obesity is a strong risk factor for asthma, having a higher diet quality may not be enough to counterbalance the negative effects that are associated with being overweight and obese. A systematic review found that, for obese asthmatic adults, the more effective dietary intervention seems to be energy restriction, regardless of the specific dietary components or dietary pattern [75].
A meta-analysis looked at the link between high adherence to the Mediterranean diet during childhood and the risk of asthma and wheezing in children, and although there is a trend towards high adherence to the Mediterranean diet in childhood to prevent current wheeze in later life, no inverse relationship was found for asthma (OR = 0.87, 95% CI 0.72; 1.04) or severe asthma (OR = 0.97, 95% CI 0.89; 1.06) [76]. Accordingly, the US-based Nurses’ Health Study revealed that high AHEI-2010 scores were not significantly associated with a reduced risk of adult-onset asthma [77]. Visser et al. also found no effect of higher diet quality on adult-onset asthma in a cohort study [78]. Another research, in Hispanic adults, with the purpose of seeing whether a pro-inflammatory diet (as measured by the energy-adjusted dietary inflammatory index [E-DII]) or a high dietary quality (as measured by the AHEI-2010) is associated with current asthma, current asthma symptoms, and lung function, observed that a higher E-DII score (representing a more pro-inflammatory diet) was associated with current asthma (OR for quartile 4 vs. 1: 1.35, 95% CI 0.97; 1.90) and asthma symptoms (OR for quartile 4 vs. 1: 1.42, 95% CI 1.12; 1.81). However, the AHEI-2010 score was not significantly associated with any of the outcomes [79]. E-DII is composed of 45 individual food parameters, mostly micro- and macronutrients, which are scored to characterize their inflammatory potential (but only 29 parameters were available and used in the study by Han et al.). Points are assigned to each of these parameters according to whether they increase, decrease, or have no effect on six biomarkers of inflammation [79]. As for AHEI-2010, this index is a tool used to evaluate the quality of an individual’s diet, consisting of eleven components that contribute to the overall classification of diet quality. These components include the consumption of vegetables (excluding potatoes), whole fruits (not including fruit juice), whole grains, sugar-sweetened beverages and fruit juices, nuts and legumes, red and processed meats, trans fats, long-chain omega-3 fats, PUFA, sodium, and alcohol. Each component was assigned a score ranging from 0 (inadequate intake) to 10 (optimal intake). Scores of all components were then summed, resulting in a total score that ranged from 0 to 110. Higher scores indicated healthier eating habits [79].
The E-DII may be a more adequate index to characterize a pro-inflammatory diet, and as asthma is an inflammatory disease, the observed significant association of a higher E-DII with asthma may reflect the capacity of the index to identify components that contribute to asthma risk. Han et al. refer that E-DII may be a better indicator of dietary patterns leading to airway inflammation than the AHEI-2010 [79].
An overall view of the different dietary component’s effects and its interaction in relationship with inflammation are presented in Figure 2.
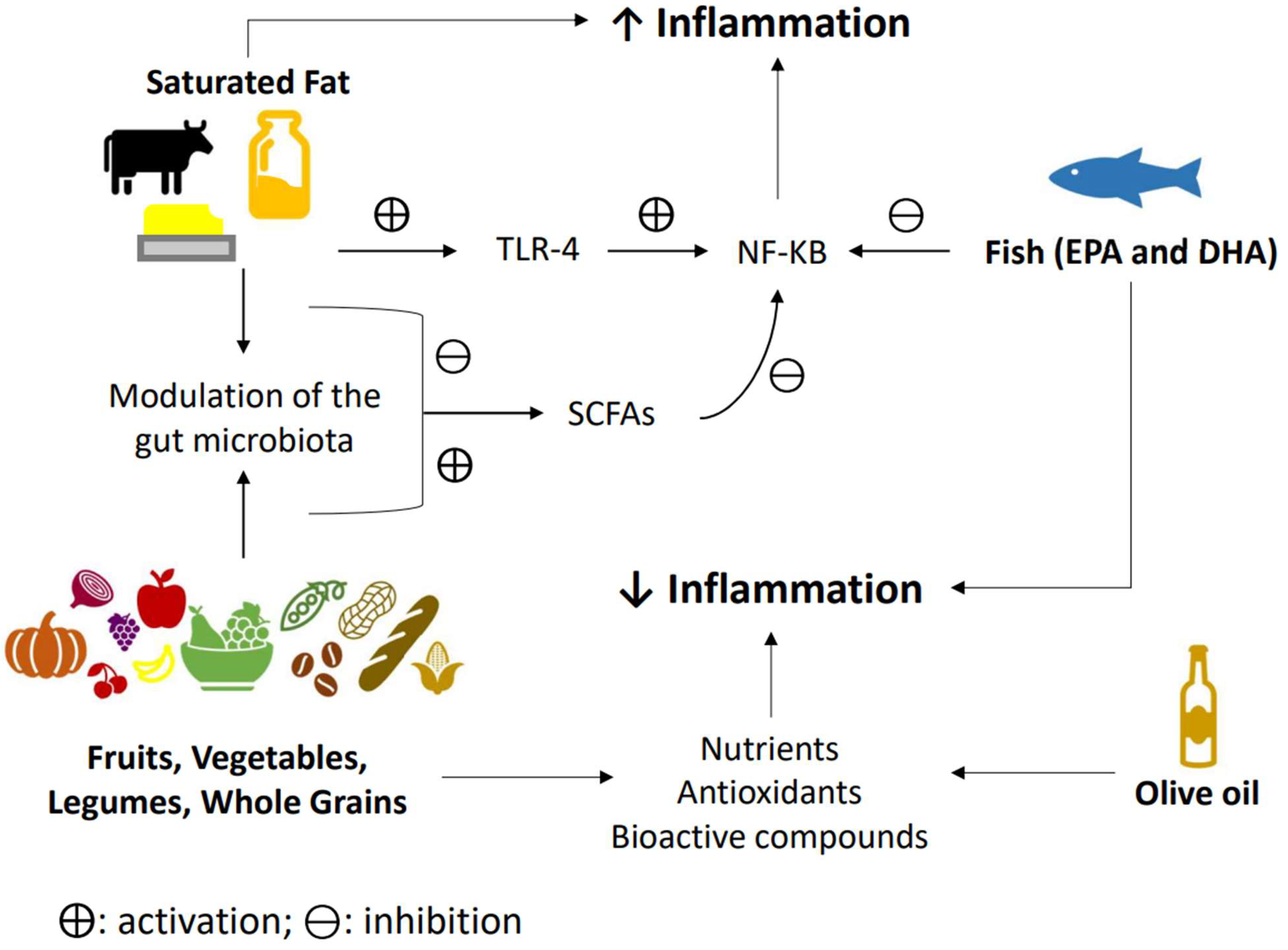
Figure 2. Diet and inflammation. The western dietary pattern promotes a pro-inflammatory environment due to a lack of consumption of antioxidants rich foods such as fruits and vegetables, leading to an increase in oxidative stress and inflammation. Fruits, whole grains, vegetables, legumes, and olive oil all have antioxidants properties that may lower inflammation. On the other side, a high intake of saturated fatty acids seems to be able to activate the innate immune system through the toll-like receptor-4 (TLR4), consequently stimulating the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) inflammatory cascade, while also dysregulating the microbiota, and consequently leading to a decrease in short-chain fatty acid production, thereby augmenting inflammation. Fruits, vegetables, legumes, and whole grains, on the contrary, are fiber-rich foods that can modulate microbiota, leading to an increase in short chain fatty acids (SCFAs) that seem to inhibit NF-kB function. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) appear to be able to inhibit the production of proinflammatory cytokines by inhibiting NF-kB activation. ⨁: activation; and ⊖: inhibition.
This entry is adapted from the peer-reviewed paper 10.3390/app13116398
References
- Bowler, R.P. Oxidative stress in the pathogenesis of asthma. Curr. Allergy Asthma Rep. 2004, 4, 116–122.
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211.
- Berthon, B.S.; Macdonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology 2013, 18, 447–454.
- DeVries, A.; Vercelli, D. Epigenetic Mechanisms in Asthma. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 1), S48–S50.
- Kabesch, M.; Michel, S.; Tost, J. Epigenetic mechanisms and the relationship to childhood asthma. Eur. Respir. J. 2010, 36, 950–961.
- Norman, R.E.; Carpenter, D.O.; Scott, J.; Brune, M.N.; Sly, P.D. Environmental exposures: An underrecognized contribution to noncommunicable diseases. Rev. Environ. Health 2013, 28, 59–65.
- Mendes, F.C.; Paciência, I.; Cavaleiro Rufo, J.; Silva, D.; Delgado, L.; Moreira, A.; Moreira, P. Dietary Acid Load Modulation of Asthma-Related miRNAs in the Exhaled Breath Condensate of Children. Nutrients 2022, 14, 1147.
- Cunha, P.; Moreira, A.; Moreira, P.; Delgado, L. Dietary diversity and childhood asthma—Dietary acid load, an additional nutritional variable to consider. Allergy 2020, 75, 2418–2420.
- Romieu, I.; Barraza-Villarreal, A.; Escamilla-Núñez, C.; Texcalac-Sangrador, J.L.; Hernandez-Cadena, L.; Díaz-Sánchez, D.; De Batlle, J.; Del Rio-Navarro, B.E. Dietary intake, lung function and airway inflammation in Mexico City school children exposed to air pollutants. Respir. Res. 2009, 10, 122.
- Cardinale, F.; Tesse, R.; Fucilli, C.; Loffredo, M.S.; Iacoviello, G.; Chinellato, I.; Armenio, L. Correlation between exhaled nitric oxide and dietary consumption of fats and antioxidants in children with asthma. J. Allergy Clin. Immunol. 2007, 119, 1268–1270.
- Mendes, F.C.; Paciência, I.; Cavaleiro Rufo, J.; Farraia, M.; Silva, D.; Padrão, P.; Delgado, L.; Garcia-Larsen, V.; Moreira, A.; Moreira, P. Higher diversity of vegetable consumption is associated with less airway inflammation and prevalence of asthma in school-aged children. Pediatr. Allergy Immunol. 2021, 32, 925–936.
- Metsälä, J.; Vuorinen, A.L.; Takkinen, H.M.; Peltonen, E.J.; Ahonen, S.; Åkerlund, M.; Tapanainen, H.; Mattila, M.; Toppari, J.; Ilonen, J.; et al. Longitudinal consumption of fruits and vegetables and risk of asthma by 5 years of age. Pediatr. Allergy Immunol. 2023, 34, e13932.
- Sdona, E.; Ekström, S.; Andersson, N.; Hallberg, J.; Rautiainen, S.; Håkansson, N.; Wolk, A.; Kull, I.; Melén, E.; Bergström, A. Fruit, vegetable and dietary antioxidant intake in school age, respiratory health up to young adulthood. Clin. Exp. Allergy 2022, 52, 104–114.
- Wood, L.G.; Garg, M.L.; Powell, H.; Gibson, P.G. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: Proof of concept. Free Radic. Res. 2008, 42, 94–102.
- Wood, L.G.; Garg, M.L.; Smart, J.M.; Scott, H.A.; Barker, D.; Gibson, P.G. Manipulating antioxidant intake in asthma: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 96, 534–543.
- Seyedrezazadeh, E.; Moghaddam, M.P.; Ansarin, K.; Vafa, M.R.; Sharma, S.; Kolahdooz, F. Fruit and vegetable intake and risk of wheezing and asthma: A systematic review and meta-analysis. Nutr. Rev. 2014, 72, 411–428.
- Hosseini, B.; Berthon, B.S.; Wark, P.; Wood, L.G. Effects of Fruit and Vegetable Consumption on Risk of Asthma, Wheezing and Immune Responses: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 341.
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. Available online: https://www.ars.usda.gov/nea/bhnrc/fsrg (accessed on 20 February 2023).
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876.
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842.
- Zapolska-Downar, D.; Siennicka, A.; Kaczmarczyk, M.; Kołodziej, B.; Naruszewicz, M. Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: The role of NF-kappaB and PPARalpha. J. Nutr. Biochem. 2004, 15, 220–228.
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809.
- Halnes, I.; Baines, K.J.; Berthon, B.S.; MacDonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Soluble Fibre Meal Challenge Reduces Airway Inflammation and Expression of GPR43 and GPR41 in Asthma. Nutrients 2017, 9, 57.
- Tabak, C.; Wijga, A.H.; de Meer, G.; Janssen, N.A.; Brunekreef, B.; Smit, H.A. Diet and asthma in Dutch school children (ISAAC-2). Thorax 2006, 61, 1048–1053.
- Han, Y.Y.; Forno, E.; Brehm, J.M.; Acosta-Pérez, E.; Alvarez, M.; Colón-Semidey, A.; Rivera-Soto, W.; Campos, H.; Litonjua, A.A.; Alcorn, J.F.; et al. Diet, interleukin-17, and childhood asthma in Puerto Ricans. Ann. Allergy Asthma Immunol. 2015, 115, 288–293.e1.
- de Souza, R.G.M.; Schincaglia, R.M.; Pimentel, G.D.; Mota, J.F. Nuts and Human Health Outcomes: A Systematic Review. Nutrients 2017, 9, 1311.
- Krogulska, A.; Dynowski, J.; Jędrzejczyk, M.; Sardecka, I.; Małachowska, B.; Wąsowska-Królikowska, K. The impact of food allergens on airway responsiveness in schoolchildren with asthma: A DBPCFC study. Pediatr. Pulmonol. 2016, 51, 787–795.
- Chatzi, L.; Apostolaki, G.; Bibakis, I.; Skypala, I.; Bibaki-Liakou, V.; Tzanakis, N.; Kogevinas, M.; Cullinan, P. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax 2007, 62, 677–683.
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813.
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064.
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and Asthma: Is It Time to Adapt Our Message? Nutrients 2017, 9, 1227.
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does Olive Oil Matter? Nutrients 2019, 11, 2941.
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484.
- Wood, L.G.; Garg, M.L.; Gibson, P.G. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J. Allergy Clin. Immunol. 2011, 127, 1133–1140.
- Venter, C.; Meyer, R.; Nwaru, B.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; Akdis, C.; Bischoff, S.; et al. EAACI Position Paper: Influence of Dietary Fatty Acids on Asthma, Food Allergy and Atopic Dermatitis. Allergy 2019, 74, 1429–1444.
- Barros, R.; Moreira, A.; Fonseca, J.; Delgado, L.; Castel-Branco, M.G.; Haahtela, T.; Lopes, C.; Moreira, P. Dietary intake of α-linolenic acid and low ratio of n-6:n-3 PUFA are associated with decreased exhaled NO and improved asthma control. Br. J. Nutr. 2011, 106, 441–450.
- Cazzoletti, L.; Zanolin, M.E.; Spelta, F.; Bono, R.; Chamitava, L.; Cerveri, I.; Garcia-Larsen, V.; Grosso, A.; Mattioli, V.; Pirina, P.; et al. Dietary fats, olive oil and respiratory diseases in Italian adults: A population-based study. Clin. Exp. Allergy 2019, 49, 799–807.
- Kim, J.L.; Elfman, L.; Mi, Y.; Johansson, M.; Smedje, G.; Norbäck, D. Current asthma and respiratory symptoms among pupils in relation to dietary factors and allergens in the school environment. Indoor Air 2005, 15, 170–182.
- Yang, H.; Xun, P.; He, K. Fish and fish oil intake in relation to risk of asthma: A systematic review and meta-analysis. PLoS ONE 2013, 8, e80048.
- Papamichael, M.M.; Shrestha, S.K.; Itsiopoulos, C.; Erbas, B. The role of fish intake on asthma in children: A meta-analysis of observational studies. Pediatr. Allergy Immunol. 2018, 29, 350–360.
- Zhang, G.Q.; Liu, B.; Li, J.; Luo, C.Q.; Zhang, Q.; Chen, J.L.; Sinha, A.; Li, Z.Y. Fish intake during pregnancy or infancy and allergic outcomes in children: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2017, 28, 152–161.
- Woods, R.K.; Thien, F.C.; Abramson, M.J. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database Syst. Rev. 2002, 2, Cd001283.
- Anandan, C.; Nurmatov, U.; Sheikh, A. Omega 3 and 6 oils for primary prevention of allergic disease: Systematic review and meta-analysis. Allergy 2009, 64, 840–848.
- Burney, P. A diet rich in sodium may potentiate asthma. Epidemiologic evidence for a new hypothesis. Chest 1987, 91 (Suppl. 6), 143s–148s.
- Mickleborough, T.D.; Fogarty, A. Dietary sodium intake and asthma: An epidemiological and clinical review. Int. J. Clin. Pract. 2006, 60, 1616–1624.
- Burney, P.G. The causes of asthma—Does salt potentiate bronchial activity? Discussion paper. J. R. Soc. Med. 1987, 80, 364–367.
- Britton, J.; Pavord, I.; Richards, K.; Knox, A.; Wisniewski, A.; Weiss, S.; Tattersfield, A. Dietary sodium intake and the risk of airway hyperreactivity in a random adult population. Thorax 1994, 49, 875–880.
- Ardern, K.D. Dietary salt reduction or exclusion for allergic asthma. Cochrane Database Syst. Rev. 2004, 3, Cd000436.
- Pogson, Z.; McKeever, T. Dietary sodium manipulation and asthma. Cochrane Database Syst. Rev. 2011, 2011, Cd000436.
- Sozańska, B.; Pearce, N.; Dudek, K.; Cullinan, P. Consumption of unpasteurized milk and its effects on atopy and asthma in children and adult inhabitants in rural Poland. Allergy 2013, 68, 644–650.
- Loss, G.; Apprich, S.; Waser, M.; Kneifel, W.; Genuneit, J.; Büchele, G.; Weber, J.; Sozanska, B.; Danielewicz, H.; Horak, E.; et al. The protective effect of farm milk consumption on childhood asthma and atopy: The GABRIELA study. J. Allergy Clin. Immunol. 2011, 128, 766–773.e4.
- Woods, R.K.; Walters, E.H.; Raven, J.M.; Wolfe, R.; Ireland, P.D.; Thien, F.C.K.; Abramson, M.J. Food and nutrient intakes and asthma risk in young adults. Am. J. Clin. Nutr. 2003, 78, 414–421.
- Berthon, B.S.; Wood, L.G. Nutrition and Respiratory Health—Feature Review. Nutrients 2015, 7, 1618–1643.
- Garcia-Larsen, V.; Del Giacco, S.R.; Moreira, A.; Bonini, M.; Charles, D.; Reeves, T.; Carlsen, K.H.; Haahtela, T.; Bonini, S.; Fonseca, J.; et al. Asthma and dietary intake: An overview of systematic reviews. Allergy 2016, 71, 433–442.
- Andrianasolo, R.M.; Kesse-Guyot, E.; Adjibade, M.; Hercberg, S.; Galan, P.; Varraso, R. Associations between dietary scores with asthma symptoms and asthma control in adults. Eur. Respir. J. 2018, 52, 1702572.
- Reyes-Angel, J.; Han, Y.Y.; Litonjua, A.A.; Celedón, J.C. Diet and asthma: Is the sum more important than the parts? J. Allergy Clin. Immunol. 2021, 148, 706–707.
- Morrissey, E.; Giltinan, M.; Kehoe, L.; Nugent, A.P.; McNulty, B.A.; Flynn, A.; Walton, J. Sodium and Potassium Intakes and Their Ratio in Adults (18–90 y): Findings from the Irish National Adult Nutrition Survey. Nutrients 2020, 12, 938.
- Cunha, P.; Paciência, I.; Cavaleiro Rufo, J.; Castro Mendes, F.; Farraia, M.; Barros, R.; Silva, D.; Delgado, L.; Padrão, P.; Moreira, A.; et al. Dietary Acid Load: A Novel Nutritional Target in Overweight/Obese Children with Asthma? Nutrients 2019, 11, 2255.
- Medina-Remón, A.; Kirwan, R.; Lamuela-Raventós, R.M.; Estruch, R. Dietary patterns and the risk of obesity, type 2 diabetes mellitus, cardiovascular diseases, asthma, and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2018, 58, 262–296.
- Blonstein, A.C.; Lv, N.; Camargo, C.A.; Wilson, S.R.; Buist, A.S.; Rosas, L.G.; Strub, P.; Ma, J. Acceptability and feasibility of the ‘DASH for Asthma’ intervention in a randomized controlled trial pilot study. Public Health Nutr. 2016, 19, 2049–2059.
- Li, Z.; Kesse-Guyot, E.; Dumas, O.; Garcia-Aymerich, J.; Leynaert, B.; Pison, C.; Le Moual, N.; Romieu, I.; Siroux, V.; Camargo, C.A.; et al. Longitudinal study of diet quality and change in asthma symptoms in adults, according to smoking status. Br. J. Nutr. 2017, 117, 562–571.
- Zhang, P.; Lopez, R.; Arrigain, S.; Rath, M.; Khatri, S.B.; Zein, J.G. Dietary patterns in patients with asthma and their relationship with asthma-related emergency room visits: NHANES 2005–2016. J. Asthma 2021, 59, 2051–2059.
- Ma, J.; Strub, P.; Lv, N.; Xiao, L.; Camargo, C.A., Jr.; Buist, A.S.; Lavori, P.W.; Wilson, S.R.; Nadeau, K.C.; Rosas, L.G. Pilot randomised trial of a healthy eating behavioural intervention in uncontrolled asthma. Eur. Respir. J. 2016, 47, 122–132.
- Garcia-Marcos, L.; Castro-Rodriguez, J.A.; Weinmayr, G.; Panagiotakos, D.B.; Priftis, K.N.; Nagel, G. Influence of Mediterranean diet on asthma in children: A systematic review and meta-analysis. Pediatr. Allergy Immunol. 2013, 24, 330–338.
- Papamichael, M.M.; Itsiopoulos, C.; Susanto, N.H.; Erbas, B. Does adherence to the Mediterranean dietary pattern reduce asthma symptoms in children? A systematic review of observational studies. Public Health Nutr. 2017, 20, 2722–2734.
- Koumpagioti, D.; Boutopoulou, B.; Moriki, D.; Priftis, K.N.; Douros, K. Does Adherence to the Mediterranean Diet Have a Protective Effect against Asthma and Allergies in Children? A Systematic Review. Nutrients 2022, 14, 1618.
- Patel, S.; Custovic, A.; Smith, J.A.; Simpson, A.; Kerry, G.; Murray, C.S. Cross-sectional association of dietary patterns with asthma and atopic sensitization in childhood—In a cohort study. Pediatr. Allergy Immunol. 2014, 25, 565–571.
- Tarazona-Meza, C.E.; Hanson, C.; Pollard, S.L.; Romero Rivero, K.M.; Galvez Davila, R.M.; Talegawkar, S.; Rojas, C.; Rice, J.L.; Checkley, W.; Hansel, N.N. Dietary patterns and asthma among Peruvian children and adolescents. BMC Pulm. Med. 2020, 20, 63.
- Menezes, A.M.B.; Schneider, B.C.; Oliveira, V.P.; Prieto, F.B.; Silva, D.L.R.; Lerm, B.R.; da Costa, T.B.; Bouilly, R.; Wehrmeister, F.C.; Gonçalves, H.; et al. Longitudinal Association Between Diet Quality and Asthma Symptoms in Early Adult Life in a Brazilian Birth Cohort. J. Asthma Allergy 2020, 13, 493–503.
- Amazouz, H.; Roda, C.; Beydon, N.; Lezmi, G.; Bourgoin-Heck, M.; Just, J.; Momas, I.; Rancière, F. Mediterranean diet and lung function, sensitization, and asthma at school age: The PARIS cohort. Pediatr. Allergy Immunol. 2021, 32, 1437–1444.
- Douros, K.; Thanopoulou, M.I.; Boutopoulou, B.; Papadopoulou, A.; Papadimitriou, A.; Fretzayas, A.; Priftis, K.N. Adherence to the Mediterranean diet and inflammatory markers in children with asthma. Allergol. Immunopathol. 2019, 47, 209–213.
- Papamichael, M.M.; Katsardis, C.; Lambert, K.; Tsoukalas, D.; Koutsilieris, M.; Erbas, B.; Itsiopoulos, C. Efficacy of a Mediterranean diet supplemented with fatty fish in ameliorating inflammation in paediatric asthma: A randomised controlled trial. J. Hum. Nutr. Diet. 2019, 32, 185–197.
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. 3), S5–S78.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2022. Available online: www.ginasthma.org (accessed on 14 March 2023).
- Forte, G.C.; da Silva, D.T.R.; Hennemann, M.L.; Sarmento, R.A.; Almeida, J.C.; de Tarso Roth Dalcin, P. Diet effects in the asthma treatment: A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1878–1887.
- Zhang, Y.; Lin, J.; Fu, W.; Liu, S.; Gong, C.; Dai, J. Mediterranean diet during pregnancy and childhood for asthma in children: A systematic review and meta-analysis of observational studies. Pediatr. Pulmonol. 2019, 54, 949–961.
- Varraso, R.; Chiuve, S.E.; Fung, T.T.; Barr, R.G.; Hu, F.B.; Willett, W.C.; Camargo, C.A. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: Prospective study. BMJ 2015, 350, h286.
- Visser, E.; de Jong, K.; Pepels, J.J.S.; Kerstjens, H.A.M.; Ten Brinke, A.; van Zutphen, T. Diet quality, food intake and incident adult-onset asthma: A Lifelines Cohort Study. Eur. J. Nutr. 2023, 62, 1635–1645.
- Han, Y.Y.; Jerschow, E.; Forno, E.; Hua, S.; Mossavar-Rahmani, Y.; Perreira, K.M.; Sotres-Alvarez, D.; Afshar, M.; Punjabi, N.M.; Thyagarajan, B.; et al. Dietary Patterns, Asthma, and Lung Function in the Hispanic Community Health Study/Study of Latinos. Ann. Am. Thorac. Soc. 2020, 17, 293–301.
This entry is offline, you can click here to edit this entry!
