Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The sensitivity of semiconductor metal oxide sensors can be significantly increased by using nanostructured sensitive layers based on two-component materials, consisting of metal oxides with different electronic characteristics and chemical properties.
- mixed metal oxide sensor
- nanoparticle interaction
- morphology
1. Introduction
Sensors based on semiconductor metal oxides are currently widely used to detect various gases in the environment. The principles for production of highly efficient sensors, based on metal oxides, for the detection of various substances have been investigated for more than four decades (see, for example, reviews [1][2][3][4][5]). The operation of metal oxide sensors is based on the change in their conductivity, under the influence of the analyzed gases. Depending on the oxygen content in the environment, this process is described by two different models. The ion-sorption model describes the sensor process at a high oxygen concentration. The chemisorbed oxygen molecules present in the sensitive layer of the particle capture electrons from the conduction band of the metal oxide, creating oxygen anion centers, such as O−, O2−, and O2− on the surface. As a result, a negatively charged layer is formed on the surface of the nanoparticles, and the near-surface region is depleted of conduction electrons (the depletion region), due to their escape to the surface from the near-surface layer [6].
The resistance of the sensor depends on the parameters of the barrier that the electrons overcome during the transition between particles, and the concentration of electrons in the layers near the surface of the particles. The distribution of electrons in a nanoparticle is determined by the equilibrium conditions (see Section 3 in originial paper). Correspondingly to the change in the concentration of electrons in the layers near the surface of the particles, the sensor resistance increases.
The interaction of the molecules of the analyzed gas, adsorbed on the surface of metal oxide nanoparticles with the active oxygen centers, results in the release of trapped electrons, which subsequently return to the conduction band of the semiconductor. There occurs an increase in the conductivity of electronic (n) semiconductors and a decrease for hole (p) semiconductors.
At low oxygen concentrations, for example, to detect impurities of reducing gases in an inert gas, the sensor process proceeds in accordance with the vacancy model [7][8]. In this case, the reducing gas reacts with the oxygen ions of the O2− lattice of the metal oxide on the surface of the sensitive layer nanoparticles, to form oxygen vacancies containing weakly bound electrons, which also increases the conductivity of the sensor [8].
The type of oxygen ions adsorbed on the surface of a semiconductor metal oxide depends on temperature (see Figure 1). At temperatures in the range of 200–400 °C, at which metal oxide sensors mainly “work” for the detection of reducing gases, oxygen anions (O−) are the active centers [8][9][10].
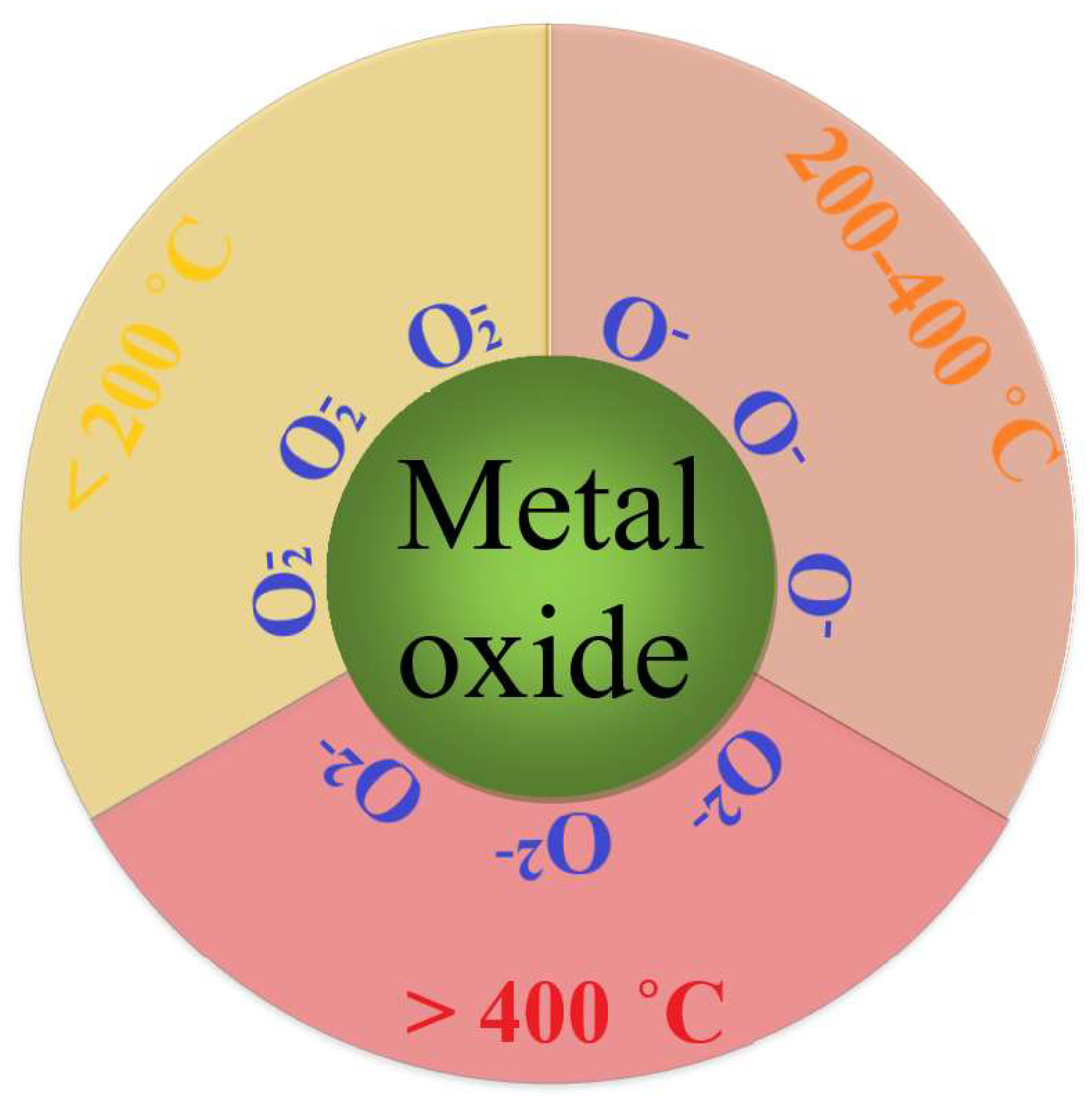
Figure 1. Schematic representation of the priority formation of active oxygen species on the surface of metal oxide nanoparticles at different temperatures.
As for the O2− ions, there are no convincing experimental data on the state and behavior of these particles on the metal oxide surface, and the calculations in some previous studies [11][12] give contradictory results. In addition, these calculations do not consider surface oxygen vacancies, which play an important role in the sensor process. All of the above support the sensor response model involving the radical ion O−.
A generally accepted approach to improving the sensitivity, rate, and selectivity of the sensors is to use composite systems that combine metal oxides with different electronic characteristics and chemical properties (see, for example, [13][14][15][16][17][18][19][20]). Until recently, only systems containing small additives (no more than 5–10%) of modifying oxides were considered in the study of sensor characteristics of metal oxide composites. A detailed study of the behavior of composites containing measurable amounts of different oxides began with the studies in Refs [20][21][22]. In the last 5–6 years, studies by other authors have appeared, that considered sensor systems consisting of mixtures of oxides over a wide range of compositions (see, for example, [23][24]).
The sensor properties of metal oxide composites are determined by the electronic structure of the nanoparticles, which, in turn, is affected by the interaction between the components of the composite. It is this interaction that determines the sensor activity of metal oxide nanocomposite sensors.
Depending on the relative content of metal oxides, it is reasonable to classify the two-component systems into single-phase and two-phase systems. A single-phase composite is a solid solution in which an ion of the doping oxide enters the lattice of nanocrystals of another oxide. Such solutions are formed only in the range of doping oxide concentrations not exceeding 10%. The difference in the sizes and charges of the ions forming the lattice produces additional deformations and defects in the lattice. This stimulates the adsorption of oxygen and detected gases on the surface of nanocrystals, which increases the sensor effect.
Binary two-phase systems, formed at comparable concentrations of oxides, consist of a solution of a small addition of one oxide (up to 10%) in the lattice of another oxide, without nanocrystal of the first component in the system. The influence of the interaction of components on the sensitivity of the sensor system is due to the contact between nanocrystals of different phases.
An analysis of the available literature shows that the sensor effect of nanostructured metal oxide systems is determined mainly by two factors [25][26]. The first is the chemisorption of the detected gas and oxygen on the surface of sensor particles, and the subsequent interaction of the chemisorbed gas with active oxygen centers. The second factor is the change in the electronic structure of metal oxides during the sensor reaction, which affects the conductivity of the sensitive layer. It is important to note that in a system consisting of metal oxides with different electronic and physicochemical properties, both of the above factors depend on the interaction between these components. This interaction largely determines the behavior of metal oxide nanocomposite sensors and makes it possible to purposefully change their performance characteristics.
Since the metal oxides used in sensors are ionic compounds, their contact in mixed oxide composites can lead to the transition of metal ions of one component into the nanoparticles of another (doping of nanoparticles). This causes the transfer of electrons between particles, their mutual charging, and changes in the structure of the nanoparticles [27][28]. The introduction of small concentrations (up to 5–7%) of metal ions with a higher charge into the metal oxide results in the appearance of an additional positive charge.
Compensation of this charge occurs due to a decrease in the concentration of positively charged oxygen vacancies and an increase in the concentration of conduction electrons. Thus, when In2O3 is doped with tin oxide, In+3 ions are replaced by Sn+4 ions, which are accompanied by an increase in conductivity and a decrease in the sensor response to hydrogen [19]. Replacing lattice ions with lower valence ions creates localized negative charges on metal ions embedded in the lattice. In this case, the concentration of positively charged oxygen vacancies in the lattice increases [29], leading to a decrease in the concentration of conduction electrons in the metal oxide.
An important role in the sensor process is also played by the transfer (spillover) of chemisorbed molecules and products of their reactions with metal oxides between the contacting nanoparticles. Thus, a noticeable sensor effect in a system of SnO2 nanofibers, coated with a layer of catalytically active Pd nanoparticles, is explained by the spillover of oxygen atoms formed on the surface of Pd nanoparticles, upon dissociation of O2 [30]. Oxygen atoms transfer to SnO2 nanofibers, capturing conduction electrons from them, and, as a result, are converted into O− radical anions.
The sensor process proceeds according to a similar scheme in a system containing comparable concentrations of ZnO and In2O3 [22]. An increase in the sensor effect upon the addition of ZnO occurs because of the spillover of O− radical anions formed during the dissociation of oxygen, on catalytically active ZnO, into tin oxide nanoparticles. These O− radical anions determine the conductivity of the composite.
2. Mechanisms of Sensitivity of Semiconductor Metal Oxide Sensors in the Detection of Reducing Gases
The sensor effect, which characterizes the sensitivity of the sensor, is mainly determined by three factors: the specific surface area of the sensitive nanostructured layer; the adsorption of oxygen and analyzed compounds on the surface of nanoparticles; and the reactivity of adsorbed compounds with respect to active oxygen centers [2].
The sensitivity of semiconductor metal oxide sensors can be significantly increased by using nanostructured sensitive layers based on two-component materials, consisting of metal oxides with different electronic characteristics and chemical properties (see, for example, [13][14][15][16]). The mechanisms for the increase in sensitivity depend on the interaction between the metal oxide components, which is affected by their phase state.
As noted above, a single-phase, two-component system is a solid solution in which metal ions of one component enter the lattice of nanocrystals of another component. The presence in the lattice, of contacting metal ions of different sizes and charges, contributes to the formation of additional deformations and defects in the crystal (in particular, oxygen vacancies), which stimulate the adsorption of oxygen and analyzed gases on the surface of nanocrystals and increase the sensor effect.
However, metastable solid solutions in a two-component metal oxide system are formed only in the region of low doping oxide concentrations, which depend on the size and valence of metal ions, and do not exceed 10%. At higher concentrations, segregation of solid solutions occurs when the particles of the segregated material coexist with the particles of the solid solution [14][19][31].
In binary two-phase systems, the influence of the interaction of components on the sensitivity of the sensor system is due to contacts between nanocrystals of different phases. The transfer of electrons between nanocrystals with different work functions, during the contact of oxides, leads to mutual charging of the nanocrystals [27], which, in turn, affects the adsorption of molecules, especially dipole types.
Of particular interest are contacts between catalytically active metal oxides and electron-rich nanoparticles that form the conduction paths of a sensor system (see, for example, [14][19][32]). In this case, the increase in sensitivity is due to the transition into conducting nanocrystals of atomic and molecular particles, arising from the dissociation of oxygen molecules and the adsorption of the analyzed gas.
2.1. Mechanisms of Increasing the Sensitivity of Two-Component, Single-Phase Metal Oxides
2.1.1. Change in the Lattice of Particles during Doping—Dependence of the Interaction of doping Ions with Ions of Main Lattice on Their Valence
The formation of binary metal oxide systems, by coprecipitation of components from solutions containing a mixture of salts or organometallic precursors or by impregnation of crystals of one metal oxide with another metal, can be accompanied by the incorporation of one of the components into the crystal structure of the second oxide, i.e., doping [13][16][33]. In this case, the metal ions of the base oxide are replaced by doping ions. There are two types of doping, namely isovalent doping, in which the valences of the main ions that make up the crystal lattice and the doping ions are the same, and heterovalent doping, when the valences of the metal ions are different.
The change in the structure and properties during isovalent doping is due to the deformation of bonds in the main crystal when doping ions are introduced into its lattice, the size of which differs from the size of the main ions. The energy of ionic bonds (EMO) in the metal oxide crystal depends on the surface charge density of the cation in the lattice and increases with decreasing cation size.
The influence of lattice deformations on the concentration of oxygen vacancies depends on the method of formation of a two-component system. A typical example is In2O3 nanocrystals doped with aluminum oxide. During the formation of Al2O3-In2O3 nanocrystals from a mixed solution of indium and aluminum salts by electrospinning, the In+3 ions (radius 0.80 Å) are partially replaced by the smaller Al+3 ions (radius 0.54 Å). This leads to the contraction of the lattice of In2O3 nanocrystals and their rearrangement into a stronger rhombohedral phase, which is accompanied by an increase in EMO and a decrease in the concentration of oxygen vacancies [34].
A similar result was obtained for SnO2 nanocrystals synthesized by electrospinning (Sn+4 radius 0.71 Å) and doped with Ti+4 ions (radius 0.64 Å) [35]. At the same time, Al2O3-In2O3 nanocrystals formed by the hydrothermal method retain the weaker cubic structure of In2O3 crystals. Lattice compression upon the doping of crystals with aluminum ions leads to the formation of defects and an increase in the concentration of oxygen vacancies [36]. These vacancies form centers of oxygen chemisorption, which increase the sensor effect in the detection of reducing compounds [36][37].
An increase in the concentration of oxygen vacancies and the associated increase in the sensor effect are more pronounced because of lattice expansion upon the isovalent doping of metal oxide nanocrystals with metal ions of larger size than the main lattice ions. This is clearly seen in the example of In2O3 nanocrystals with In3+ ions (radius 0.80 Å) doped with La3+ ions (radius 1.03 Å) [38][39], which is explained by the weakening of bonds in the In2O3 lattice under the influence of La3+ ions.
The most significant effect on the sensitivity of two-component, single-phase metal oxides is provided by heterovalent doping. Due to the difference in the valence of metal ions, not only local deformations but also new local charges arise in the lattice of a nanocrystal. The change in the sensor properties of the metal oxide during doping depends on the ratio of the charges of the doping ions to the main ions of the crystal lattice, which determines the conductivity.
The introduction of up to 5–7% of metal ions with a larger charge into the basic oxide lattice produces an additional positive charge in the system. The concentration of positively charged oxygen vacancies decreases and the concentration of conduction electrons increases. As a result, when In2O3 is doped with SnO2 and In3+ ions are replaced by Sn4+ ions, an increase in conductivity and a decrease in the sensor response to hydrogen are observed [19][33].
When lattice ions are replaced by ions with a lower valence, localized negative charges appear on metal ions built into the lattice, the concentration of positively charged oxygen vacancies increases [29], and the concentration of conduction electrons decreases. Such a process is considered in the example of the formation of the SnO2-In2O3 composite [29][33]. Since the size of In+3 is larger than that of Sn+4, the substitution of In+3 for Sn+4 results in an increase in interplanar distances in the SnO2 lattice and an increase in the response to hydrogen [33][40].
When lattice ions are replaced by doping ions, in contrast to their incorporation into the intercrystallite space, the emerging vacancies can form complexes with these ions. Such complexes were characterized for In2O3 nanocrystals doped with Mn+2 and Co+2 ions [41]. The vacancies formed when these ions are included in the In2O3 lattice, and enter the first coordination sphere of oxygen anions surrounding the doping ion [42].
Many metal ions form specific bonds with organic molecules. Such complexes, in which the centers of oxygen chemisorption (oxygen vacancies) are associated with the centers of adsorption of the detected gas on embedded metal ions, create especially favorable conditions for the sensor process. Thus, the impregnation of nanocrystalline In2O3 by divalent cobalt, which is accompanied by the incorporation of Co+2 ions into the In2O3 lattice, produces a material with an exceptionally high response to CO (Figure 2) [43]. Impregnation of In2O3 nanoparticles by other metals also increases the sensor effect, but much less so than in the case of Co+2 ion incorporation.
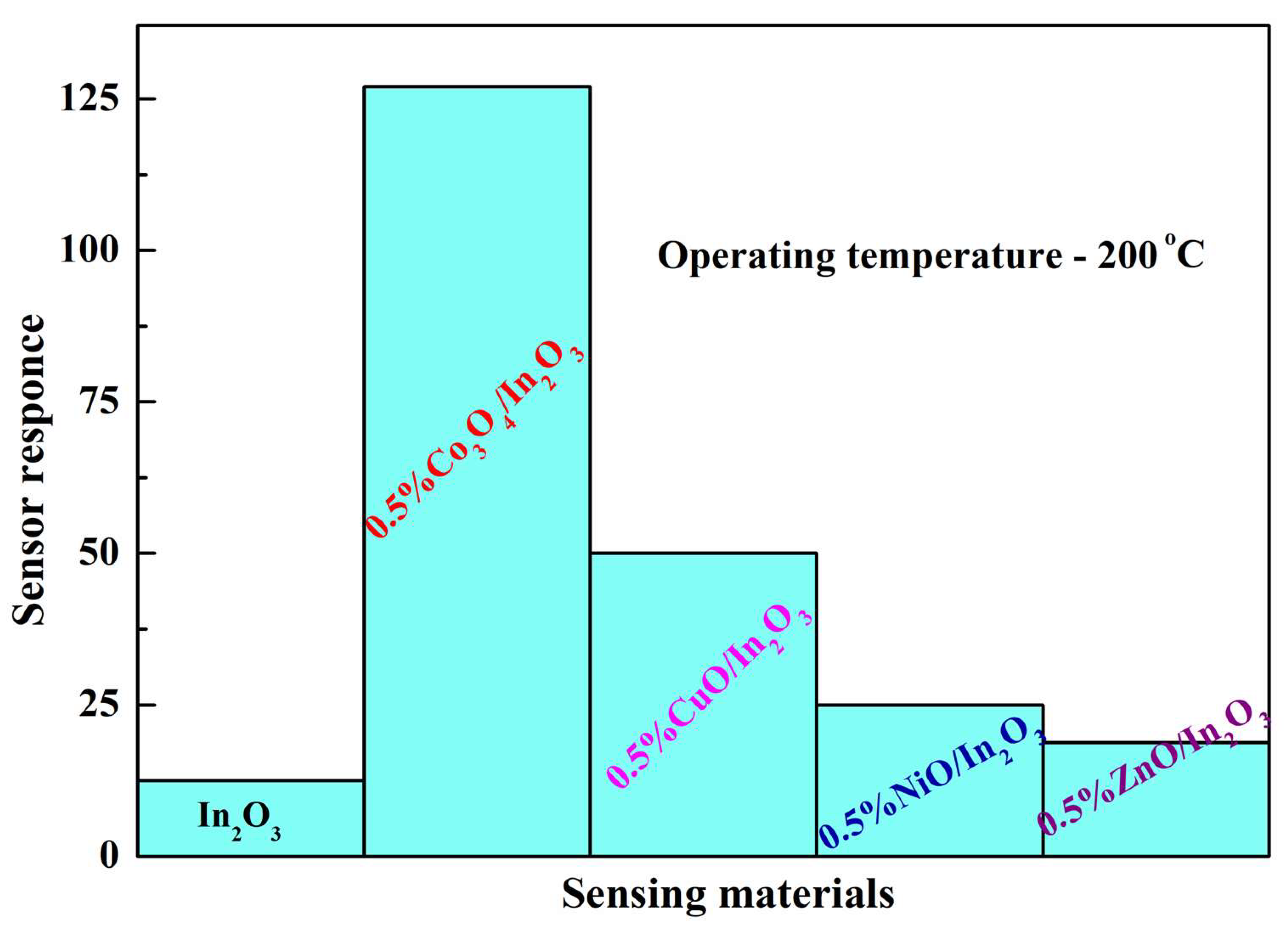
Figure 2. Sensor response of In2O3-based nanocomposites impregnated by various metal oxides in the detection of 1000 ppm CO.
The activity of In2O3 doped with Co+2 ions is attributed to the fact that complexes of cobalt ions with oxygen vacancies are active centers of the sensor reaction [41]. The bonds of these ions with CO molecules [44] increase the adsorption of CO on such a complex.
2.1.2. Effect of Doping on Adsorption of Detected Gases
A change in the structure of metal oxide nanocrystals, due to their doping with metal oxides, leads to the formation of new adsorption centers. Two types of adsorption bonds between adsorbed organic molecules and metal oxide nanocrystals of the sensitive layer can be distinguished: acid–base bonds [45] and bonds between π-electrons of adsorbed molecules and d-electrons of metal ions in metal oxides [46][47].
Acid–base bonds are characteristic of adsorption on metal oxides with polar functional groups containing nitrogen or oxygen atoms. Doping of metal oxide nanocrystals with basic metal ions, including alkaline earth metal ions, creates adsorption centers for acidic compounds in their lattice. Such compounds are alcohols, aldehydes, ketones, etc., related to Brönsted acids [48][49]. Doping not only increases the number of adsorbed molecules but also can change the strength of their bond with nanocrystals, depending on the acidity of the molecules.
The influence of doping of In2O3 nanocrystals with Ca, Sr, and Ba ions on sensor effects in the detection of formaldehyde, ethanol, and acetone by sensors based on these nanocrystals has been studied [50]. The sensor effect in the detection of formaldehyde decreases from Ca to Ba [48]. This is due to a drop in the electronegativity of metal ions and a decrease in their ability to attract electrons from the surrounding oxygen ions of an oxide. Their electron donation and the energy of bonds between the metal ions and adsorbed acid-type molecules also increase [51].
The sensor effect for In2O3 nanocrystals doped with alkaline earth metal ions increases sharply [48] with an increase in the acidity of the analyzed compounds in the series acetone (pKa = 20), ethanol (pKa = 15.9), and formaldehyde (pKa = 13.27). Thus, there is a correlation between the acid–base bonds of the detected molecules with the sensitive layer and the magnitude of the sensor effect.
The nature of the adsorption bonds of π-electron molecules with a sensitive metal oxide layer depends on the electronic structure of the cations that make up this layer. Such bonds have been the most studied in relation to the CO molecule [52]. For effective detection, CO bonds with metal ions must be strong enough to ensure reliable CO adsorption on the surface of the sensor layer, at temperatures of 200–300 °C.
These bonds should not interfere with the sensor reaction of adsorbed molecules with oxygen active sites, mainly O−. In this respect, the doping of oxides with divalent metal ions, in particular, Mg2+, Mn2+, Fe2+, Co2+, Ni2+, and Zn2+, which are characterized by reversible binding to CO molecules, is of particular interest [44]. Simultaneously, upon doping with these ions, oxygen vacancies appear in the system, which are the active centers of the sensor reaction. Ions in which d-electron shells are either absent (Mg2+) or completely filled (Zn2+) have the lowest binding energy with CO molecules. This bond is due to the electrostatic attraction between the metal ion and the lone pair of electrons of the 5σ orbital of the C atom in the CO molecule; the energy of such a bond is about 8 kcal/mol [46][53].
When CO interacts with metal cations with unfilled d-electron shells (Co2+, Ni2+), the electron pair of the 5σ-orbital of carbon is connected with the d-electrons of the metal. Concurrently, due to the overlapping of the unoccupied d-orbitals of the metal ion by the antibonding π*-orbitals of CO, the π*-d interaction appears [47]. This leads to an increase in the binding energy of the ion with CO, which, for Co2+ and Ni2+, reaches about 13 kcal/mol [44].
In a series of CO sensors obtained by the hydrothermal method from indium oxide doped with Cu2+, Pd2+, and Ni2+ ions, sensors containing Ni2+ have the highest sensor effect, which is six times the effect in the absence of additives [54]. Doping of In2O3 with Zn2+ ions also increases the sensor effect approximately 3.5 times [55].
A similar effect of the interaction energy of CO with doping ions on the sensor effect was observed in sensors based on SnO2 doped with Zn2+ and Ni2+ ions [56]. Thus, as in systems with acid–base bonds between the analyzed gas and the sensitive layer, an increase in the energy of CO adsorption bonds, due to the π*-d interaction of CO with doping ions, causes an increase in the sensor effect.
2.1.3. Influence of the Nature of Doping Ions on the Reactivity of Oxygen Anionic Centers
In the reactions of anions localized on the surface of a metal oxide sensitive layer, the activation energy that determines the sensor effect depends on the binding energy of the anion with the metal oxide. The effect of these bonds was demonstrated in the detection of formaldehyde by a sensor based on In2O3, doped with ions of various metals [57][58][59]. The Fermi level (see Figure 3) of the sensitive layer increased or decreased depending on the nature of the doping ions.
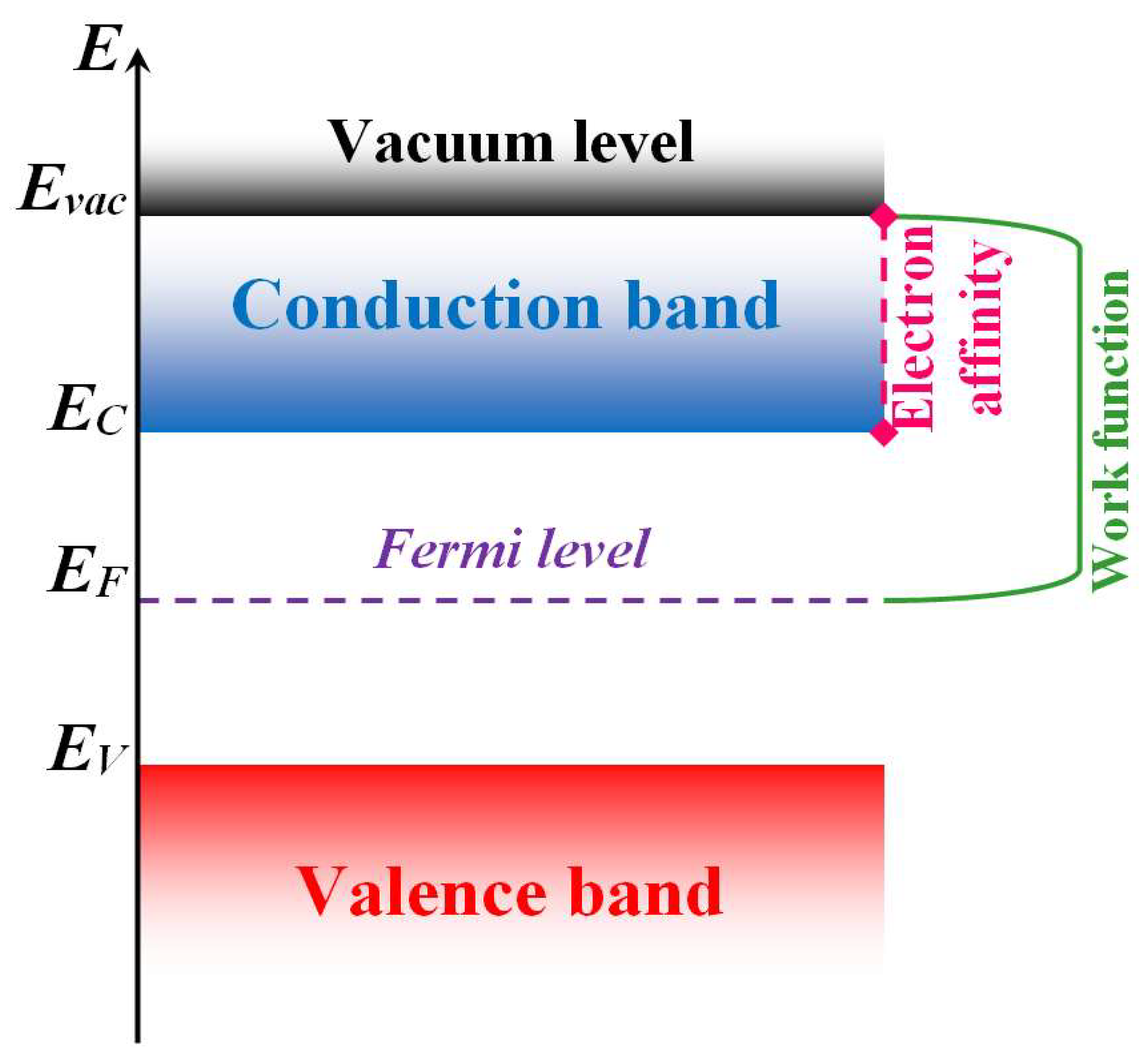
Figure 3. Semiconductor band structure. Here, Evac is minimal vacuum energy of electron, EC is bottom of the conduction band, EF is Fermi level and EV is valence band ceiling.
An increase in the Fermi level leads to an increase in the sensor effect in the detection of formaldehyde and other reducing compounds [52]. This trend can be attributed to the increase in the reactivity of active oxygen anions, due to a reduction in their binding energy to the surface. The reduced binding energy is caused by a decrease in the electron affinity χe of the metal oxide sensitive layer from the increase in the Fermi level.
An increase in the Fermi level of the sensitive layer leads to the increase in the concentration of adsorbed oxygen anions. The change in concentration is due to the difference between the Fermi level of the metal oxide and the energy of electrons captured by adsorbed oxygen atoms or molecules, as well as the increase in the number of electrons passing from the semiconductor to oxygen, to achieve equilibrium. An increase in χe, on the contrary, causes a decrease in the sensor effect [57]. A similar dependence of the sensor effect on the Fermi level (and χe) of the sensitive layer was observed in [48][50].
The influence of the metal oxide layer on the reactivity of the active oxygen particles associated with this layer was considered, as well as the dependence on the average characteristics, such as the electron affinity related to the entire layer [57]. In reality, oxygen molecules are adsorbed on certain lattice defects, where the coordination between metal ions and oxygen ions of the metal oxide lattice is disrupted. In this case, bonds of coordinating-unsaturated metal ions with environmental molecules can be formed. Essentially, such defects are oxygen vacancies [37], whose properties depend on the structure of the crystal lattice and the location of the vacancy. Accordingly, the chemical activity of a vacancy in the sensor process is changed [60].
The properties of vacancies depend on the conditions of metal oxide annealing during the formation of the sensitive layer. Thus, nanocrystalline SnO2, obtained by the hydrothermal method of tin hydroxide annealing, contains both isolated oxygen vacancies and clusters of oxygen vacancies, in combination with vacancies of tin ions [61]. The number of vacancies and the ratio between vacancies of different types depends on the medium in which the annealing is carried out—in air, in oxygen, or in helium. In accordance with this, the sensor response to acetone, ethanol, and formaldehyde is changed [61].
As noted in the previous section, the chemical activity of the metal oxide, and the sensor effect in the detection of reducing agents, increase with a decrease in the bond energy between oxygen ions and metal ions. To clarify this mechanism, the sensitivity of MnO2 nanofibers doped with niobium ions to CO was studied [62]. The Mn–O–Nb sequences formed in doped nanofibers contain oxygen ions weakly bound with the ions of these metals. Therefore, the reaction, CO + Mn–O–Nb → CO2 + Mn–VO–Nb, with the formation of oxygen vacancies (VO) proceeds efficiently, even at room temperature. This reaction leads to a sharp drop in the resistance of nanofibers, but the reasons for this are not discussed in [62].
It can be assumed that, since the electron levels of the formed vacancies lie near the conduction band of the nanofibers [37][58], electrons from oxygen vacancies easily pass into them. As a result, the conductivity of the composite increases, leading to a sensor effect. After replacing the air mixture, with CO, with pure air, the vacancies are filled with oxygen, and the resistance of the sensor system returns to its original value.
This system has exceptionally high sensitivity to CO, which makes it possible to detect the gas, even at its concentration in air of 2 ppm [62]. A high selectivity of CO detection is thus achieved in the presence of organic compounds, which can be explained by the selective adsorption of CO on Mn–O–Nb structures [63]. These structures are very stable. Therefore, the formation of oxygen vacancies under the influence of CO and their death upon interaction with oxygen are completely reversible processes.
A similar increase in the reactivity of oxygen ions was found for cerium oxide doped with samarium ions [64]. The Ce–O–Sm structures formed in this material also contain labile oxygen ions weakly bonded to the lattice. As a result, the rate of CO oxidation with the formation of oxygen vacancies increases, and the maximum of oxidative activity shifts to lower temperatures. All this testifies to the key role of oxygen vacancies and complexes of vacancies with metal ions in the sensor process.
2.2. Mechanisms for Increasing Sensitivity of Two-Component, Two-Phase Metal Oxide Composites
2.2.1. Effect of Conduction Pathways on Sensor Properties of Binary Two-Phase Nanocomposites
The conductivity of the sensor layer is determined by the aggregates of metal oxide particles that are in contact with each other and form current flow paths. In a two-component system, in which components A and B form different crystalline phases, there are three types of intercrystalline contacts, namely homophase contacts A-A and B-B, and heterophase contacts A-B. As a rule, nanoparticles A and B have different work functions (W), such that, upon their contact, electron transfer occurs at WA > WB from B to A, and at WB > WA, in the opposite direction. Such an electron transfer between nanoparticles corresponds to their mutual charging [27].
An internal electric field develops at heterogeneous contacts. In this case, the potential barrier, that prevents the passage of current through a heterogeneous contact between particles, depends on the mutual direction of the internal and external fields [65]. In a disordered system of nanoparticles, which includes heterogeneous contacts, when the direction of the external field is opposite to the direction of the field in the contact, high barriers inevitably arise on the current path. The current flow path “bypasses” such contacts, choosing the homogeneous contacts with lower barriers for electron transfer [65].
Thus, in two-phase nanocomposites consisting of particles of components A and B, two parallel homophase conduction paths can be realized through the contacting nanoparticles A-A or B-B. The current flows through chains of contacting nanoparticles, which form conducting clusters that connect the electrodes of the measuring system (“endless” clusters [65][66]). The nanoparticles of the second component, that did not enter the conduction pathways, are modifiers, the interaction with which changes the chemical and electronic characteristics of the conducting nanocrystals, and, consequently, the conductivity and sensor effects.
In a disordered two-phase conglomerate from particles of two components, the conduction paths are determined by percolation theory [66], and depend on the relative content of the components and the ratio between the sizes of the corresponding nanocrystals [65].
In nanostructured composites ranging from n- and p-modifications of TiO2, three conduction regions are possible [65], based on the percolation model, depending on the relative content of the components. These are regions I and II, with conducting clusters of nanoparticles of only one type, and region III, in which conducting clusters of both types coexist. In region III, the conductivity of the composite depends on the ratio between the concentrations of components, the size of the particles, and the concentration of current carriers (electrons or holes) in the components [65].
In a nanostructured composite of metal oxides with different work functions, the magnitude and direction of the sensor effect are determined by the electron transfer between the conducting clusters and the modifier. A characteristic example is the result obtained from the study of the conductivity and sensor effect in a composite of SnO2 nanofibers with nanoclusters of TiO2 or WO3 modifiers located on the surface of the nanofibers [67]. In SnO2 nanofibers and modifier nanoclusters, the work function is different; therefore, the effect of modifiers on conductivity and sensor properties depends on the difference between the WSnO2 and W of the modifier. Modification of nanofibers with TiO2 nanoclusters (WTiO2 < WSnO2) leads to an increase in the conductivity of the composite, due to electron transfer from TiO2 to SnO2, while modification with WO3 nanoclusters (WWO3 > WSnO2) reduces the conductivity, due to electron transfer from SnO2 to WO3 [67].
The modification of SnO2 nanofibers also changes sensor effects, in addition to the conductivity. An increase in conductivity under the influence of TiO2 causes an increase in the sensor effect for oxidizing gases but has almost no effect on the sensor effect for reducing agents. The decrease in conductivity caused by WO3 nanoclusters also leads to an increase in the sensor effect in the detection of reducing gases [67].
A similar increase in the sensor response to reducing gases, accompanied by a decrease in the conductivity of the composite, is observed for the deposition of WO3 nanoclusters on the surface of In2O3 nanofibers. In contrast, the deposition of In2O3 on WO3 nanofibers increases their conductivity and sensor response to oxidizing gases but reduces the sensitivity of the composite to reducing agents [68].
In numerous reviews on two-phase nanocomposites (see, for example, [69][70]), as in the studies considered above, only systems with conduction paths from particles of one component are mostly discussed. Particles of the second component, interacting with conductive particles, change their properties and affect sensor effects.
Binary nanocomposites with different conduction paths have different sensitivity to detected gases. The authors of [71][72] studied systems consisting of metal or metal oxide nanoparticles in combination with thin nanolayers of reduced graphene oxide (rGO). With minimal coverage of rGO layers by Pd particles, these layers are current flow paths, and the conductivity decreases in the presence of hydrogen. This is due to the transformation of Pd nanoparticles into PdHx, followed by electron transfer from PdHx to rGO and a decrease in the concentration of charge carriers—holes—in the p-semiconductor rGO [71].
With an increase in the concentration of Pd particles, the response to H2 decreases, due to a change in the conduction paths—from rGO layers to clusters of Pd nanoparticles. This occurs during the formation of a continuous Pd film on the surface of the rGO layers. Due to the change in the structure of the Pd film, under the influence of hydrogen dissolved in it, the response increases [71]. In the absence of Pd nanoparticles, the conductivity of rGO layers largely does not react to the presence of nonpolar molecules in the air, for example, H2. The effect of current flow paths in nanocomposites combining rGO layers with metal oxide nanoparticles on conductivity and sensor properties was studied in detail using the response to ammonia as an example [73].
As rGO was added to nanostructured SnO2, the type of conductivity of the composite changed from electronic conductivity, characteristic of pure SnO2, to hole conductivity, characteristic of rGO. Due to the difference in the shape of the components (thin, extended rGO nanolayers and quasi-spherical SnO2 nanoparticles), the percolation transition from n- to p-composites occurs already at small (about 0.5 wt.%) additions of rGO to SnO2.
These experimental results agree with the calculations [73]. The response of the sensor to ammonia is due to its reaction with oxygen anions on the surface of SnO2 particles. In n-type nanocomposites, where the current flows through clusters of SnO2 nanoparticles, the addition of ~0.5 wt.% rGO leads to an increase in the electronic conductivity of the composite [73]. Since WrGO (5.2 eV) of a completely reduced graphene oxide film [74] is larger than WSnO2 (4.9 eV), electrons pass from SnO2 to rGO. As a result, the electron concentration in conducting clusters of SnO2 nanoparticles decreases, which increases the response to reducing gases [67][75].
In connection with the mutual charging of contacting SnO2 nanoparticles and rGO nanolayers, during the transition of electrons from SnO2 to rGO, favorable conditions are created for the adsorption of polar molecules in the contact region in the nanocomposite. This also applies to NH3 molecules and additionally increases the sensitivity of the composite sensor to ammonia. An increase in rGO concentration to 1 wt.% leads to a percolation transition from conduction through SnO2 particles to conduction paths through rGO layers; that is, to hole conduction [73].
The paths of hole and electron conduction are different in nanostructured metal oxides [3][70][76]. The electron current flows through contacting nanoparticles, crossing their surface layers where potential barriers are formed between the particles, due to oxygen ionosorption accompanied by the capture of electrons from the conduction band. The resistance of the surface layers is significantly increased, compared to the resistance in the bulk of the particles. The height of such barriers on the current path is determined by the number of electrons captured by oxygen, which decreases when O– interacts with a reducing gas. This leads to an increase in conductivity.
In the case of hole conduction, the current flows mainly in the near-surface layers of p-nanoparticles, wherein, the negative charge of O− on the surface is compensated by the positive charge of holes in the near-surface layer. The concentration of holes in this layer is naturally higher than in the volume of the particles [76][77]. The total resistance to the hole current in the volume of the particles and surface layers is determined by the morphology of the nanostructured metal oxide [78].
At the hole conduction, the total resistance (Rsen) of the sensor layer includes a volume component which is largely independent of the processes on the particle surface. Therefore, the sensitivity to reducing gases in such systems is significantly less than in the case of electronic conduction, when the change in resistance is determined by the reactions of the analyzed gases with O− on the surface of the particles. SnO2-rGO sensors to ammonia, with hole conduction along the paths formed by rGO layers, are characterized by greater stability and a shorter response time to ammonia, and relaxation time after its removal [73].
The conductivity and sensor properties of nanocomposite fibers containing n-SnO2 and p-Co3O4 nanoparticles were studied in [23]. The resistance of the xSnO2 – (1 – x)Co3O4 system and its sensor response to CO, C6H6, and C3H6O reach their maximum values at x = 0.5. The nanofiber of this composition has electronic conductivity. Since the arrangement of nanoparticles in a fiber is chaotic, the conductivity of a nanocomposite fiber is determined by the percolation model [65][66].
According to this model, the electron current in such a system flows along n-type homophase paths, excluding heterophase n-p contacts, the inclusion of which in the current path would lead to the occurrence of large potential barriers. Heterophase contacts between n- and p-nanoparticles of composite lead to the loss of electrons, due to electron-hole recombination. As a result, the electron concentration in the conducting channels decreases. In some cases, this leads to an increase in the response to reducing gases [76].
Similar effects are also characteristic of other n-p nanocomposites [79][80], in which the maximum response is achieved in the systems with electronic conductivity. The sensitization of the sensor response by p-nanoparticles contacting with n-nanoparticles of conducting pathways is due to a decrease in the electron concentration in conducting pathways, resulting from electron-hole recombination. At n-n-contacts, the concentration of conduction electrons does not decrease, but the electrons pass into a lower-energy conductivity band, forming an “accumulation layer” of electrons in acceptor centers [76][81]. Such a transition also reduces the concentration of conduction electrons in the conducting paths of the composite, but to a lesser extent since the transition of electrons is hindered by the resulting electric field. Therefore, n-p contacts are more effective for sensitizing the composites undergoing the electronic conductivity.
2.2.2. Effect of Interaction between Conducting and Modifying Nanocrystals on Sensor Properties of a Composite
In binary systems, the interaction of nanoparticles, constituting current flow paths with modifier nanoparticles surrounding these paths, is a source of sensor sensitization. There are two types of sensitizations: chemical and electronic [13][82].
Chemical sensitization is caused by catalytically active particles of the modifier, which chemisorb oxygen and molecules of detected gases with the formation of reactive particles, in particular, atoms or radicals. The most common variant of chemical sensitization is the adsorption and dissociation of oxygen molecules on modifier particles with a subsequent spillover of oxygen atoms into conducting clusters. After that, oxygen atoms capture electrons from the conduction band of nanoparticles constituting conducting clusters, forming O− radical anions.
Since the reaction of reducing gases with O− on the surface of conducting clusters “releases” trapped electrons and increases the conductivity of the composite, the concentration of adsorbed oxygen should be increased to raise the sensitivity. This is clearly facilitated by the catalytic activity of modifier nanoparticles [14][83].
The detection of several π-electron compounds, by sensors based on SnO2 nanofibers modified with Au, Pd, and Pt nanocrystalline particles, was studied to determine the optimal conditions for achieving the maximum sensitivity [84]. The study indicated that the adsorption bonds, of π-electron molecules with metal particles, are determined by the location of the metal d-electron band, relative to the π-electron orbitals of the molecules of the analyzed gases. Therefore, by varying the electronic structure of the contact between a gas molecule and a metal particle on the surface of SnO2 nanofibers, chemisorption can be increased. The spillover of adsorbed molecules from metal particles onto SnO2 fibers increases the gas concentration on nanofibers and the sensitivity of the composite. The conditions were determined for the most efficient chemical sensitization of the sensor effect by Au, Pd, and Pt particles in the detection of CO, benzene, and toluene [84].
Chemical sensitization depends both on the structure of the detected molecule and the properties of the catalytically active particle. A typical example is the sensitization of CO and H2 detection by a sensor based on nanocrystalline In2O3, containing nanoparticles of the well-known catalyst, ZrO2. The particles of ZrO2 increase the sensor effects in the detection of H2, but practically, do not affect the detection of CO [85].
The effect of chemical sensitization of the sensor response to CO on this system could be expected, due to the dissociation of O2 on the surface of ZrO2 nanoparticles with the subsequent formation of O− active centers. However, the dissociation of O2 requires a temperature above 800 °C [86]. The efficiency of H2 detection is probably ensured by the dissociation of H2 molecules under the influence of ZrO2. As a result of the spillover of the formed hydrogen atoms onto In2O3 particles, H atoms react with O− radical anions localized on the In2O3. In this case, the electrons captured by oxygen return to In2O3.
The conductivity of a nanofiber network Is determined by potential barriers to electron transfer between contacting nanofibers, and depends on the electron concentration in their surface layers (ns) [76]. The decoration of SnO2 fibers, with electron-donating TiO2 (WTiO2 < WSnO2) or electron-withdrawing WO3 (WWO3 > WSnO2) nanoclusters, leads to a redistribution of conduction electrons between conducting channels and decorating clusters [67]. The transfer of electrons from SnO2 nanofibers to WO3 nanoclusters reduces ns and increases the barriers for electron transfer between contacting nanofibers [76]. All this affects the sensor effect [61], and can be considered as electronic sensitization.
The sensor properties of nanocomposites depend on the method of their formation, which determines their morphology. In this regard, the indicators are the results of studies of composites obtained by physical mixing of oxide nanopowders or by laser sputtering of one oxide on the surface of crystals of another [21][22][87][88][89]. In such composites based on electron acceptor SnO2 or ZnO oxides, electron-donating additives of In2O3 or TiO2 nanoparticles do not decrease the sensor effect for reducing gases, as in [67][90][91], but increase it.
It should be noted that the sensor characteristics of binary composites obtained by mixing nanocrystalline powders, in contrast to the systems considered in [67][90][91], are mainly determined by the contact of particles with different electron affinities and clearly marked interphase boundaries [87], where the charges, formed as a result of mutual charging of such particles, are localized. Charges in the interfacial regions of the nanocomposite, as mentioned above [73], can increase the chemisorption of oxygen and detected gases and, thus, increase the sensor effect.
The effect of interfacial contacts on the sensor properties of nanocomposites was not considered in [67][68]. This is probably due to the small size of electron-donor and electron-acceptor clusters located on the surface of conducting nanofibers, because of which, the distribution of electrons in the region of these contacts is largely blurred.
It can be assumed that the differences in the sensor properties of nanocomposites obtained by different methods are due to the structure of the interfacial regions in such composites, which determines the adsorption of gases and their activity in sensor reactions. The largest increase in the sensor effect is achieved due to the joint effect of chemical and electronic sensitization by chemically active modifiers with a high work function [83].
Such sensitization of the sensor effect occurs, in particular, as a result of the modification of the surface of conducting metal oxide particles by nanoclusters of catalytically active metals, Au, Pd, and Pt [84]. Nanoclusters of these metals, as shown above, catalyze the formation of highly reactive atoms and radicals on the surface of metal oxide nanocrystals, which corresponds to chemical sensitization. These nanoclusters, which have a higher work function than conductive metal oxide nanocrystals, capture the electrons of the nanocrystals, lowering ns. Thus, there is also an electronic sensitization of the sensor effect.
A characteristic example of the synergism of electronic and chemical sensitization is the detection of ethanol vapor by sensors based on In2O3 nanotubes, decorated by nanoclusters, Co3O4, Fe2O3 or a combination of these nanoclusters [92]. The deposition of nanoclusters, of even a single metal oxide on the surface of nanotubes, increases the sensor effect due to electron sensitization, since the work function of these metal oxides is higher than that of In2O3. In this case, Co3O4 clusters have a greater influence, although W for Fe2O3 (5.3 eV [93]) is higher than for Co3O4 (4.8 eV [94]).
Co3O4 nanoclusters are both electronic and chemical sensitizers, which is due to the catalytic activity of Co3O4 in the dissociation reactions of adsorbed oxygen molecules with the formation of O−. The largest increase in the sensor effect occurs with the joint application of Co3O4 and Fe2O3, when high chemical sensitization of the sensor effect under the action of Co3O4 is combined with high electronic sensitization under the influence of Fe2O3 [92].
The synergism of the sensitizing action of different agents is also manifested in sensors based on porous In2O3 nanospheres, modified with NiO and Au nanoclusters [95]. The interaction of NiO clusters with In2O3 leads to the formation of oxygen vacancies on the surface of In2O3 in such systems, due to the difference in the charges of Ni+2 and In+3 ions. Concurrently, Au nanoclusters catalyze the dissociation of these molecules, with the capture of electrons and the formation of O− active centers.
The combined action of NiO and Au clusters on In2O3 nanoparticles produces high sensitivity in the detection of reducing compounds, in particular, toluene [95]. Using such a sensor, toluene can be detected, even at concentrations of the order of 100 ppb, since Au clusters adsorb toluene and thus increase the sensor effect. A similar synergistic effect of electronic and chemical sensitization of the sensor effect was also found for In2O3 nanotubes containing NiO and PdO nanoclusters on the surface [32].
The results presented in this section have shown that the sensor response for two-component, two-phase metal oxide composites can differ markedly for various gases. Table 1 summarizes the results of previous investigations and makes it possible to compare the response magnitudes to various gases of sensors synthesized with different methods under diverse conditions.
Table 1. Sensor response to various gases of two-component two-phase metal oxide composites.
| Material | Synthesis Method | Sensor Response (Gas Concentration) | Temperature | References |
|---|---|---|---|---|
| 0.5SnO2-0.5Co3O4 | electrospinning method | 18.7 (1 ppm C6H6) | 350 °C | [23] |
| WO3-SnO2 | sputtering high-purity Ti or W targets on SnO2 | 140 (1 ppm H2) | 300 °C | [67] |
| TiO-SnO2 | 232 (1 ppm O2) | |||
| WO3-In2O3 | sol-gel method | 27 (200 ppm NO2) | 300 °C | [68] |
| graphene-Pd/SnO2 composites | grapheme by CVD method, SnO2 gas phase synthesis method | 14.8% (1% C2H5OH) | 200 °C | [72] |
| rGO-SnO2 | rGO by hydrothermal treatment of aqueous dispersion of GO, rGO-SnO2 composite by mixing | 1.3 (50 ppm NH3) | 22 °C | [73] |
| 0.5SnO2-0.5NiO | electrospinning process | 36 (10 ppm NO2) | 300 °C | [79] |
| SnO2-2.78CuO | sol-gel route | 200% (400 ppm CO) | 350 °C | [80] |
| In2O3-Co3O4 | Electrospinning method | 39 (200 ppm HCHO) | 260 °C | [81] |
| Pt-SnO2 | photolithographic process and γ-ray radiolysis method | 40 (1 ppm C7H8) | 300 °C | [84] |
| 10%Co3O4-90% In2O3 | Mixing metal oxides | 1300 (1100 ppm H2) | 250 °C | [85] |
| 20%ZrO-80% In2O3 | 280 (1100 ppm H2) | 315 °C | ||
| TiO2/SnO2 | hydrothermal process | 52.3 (100 ppm triethylamine) | 260 °C | [89] |
| ZnO@In2O3 | hydrothermal method | 28.6 (100 ppm C2H5OH) | 160 °C | [90] |
| Au-NiO/In2O3 | solvothermal method | 80.6 (10 ppm toluene) | 250 °C | [95] |
The consideration of the processes of chemical and electronic sensitization of sensor reactions has shown that such a division is rather arbitrary. For example, oxygen molecules dissociate on the surface of nanoparticles with the formation of chemically active centers, which corresponds to chemical sensitization, wherein, the formed oxygen atoms actively capture electrons from the near-surface layer, thereby depleting it; this is already characteristic of electron sensitization. Thus, in various sensor processes, both types of sensitization mechanisms can occur simultaneously, but the chemical or electronic nature will be manifested to a greater extent.
2.3. Interaction of Nanocomponents in Core–Shell Sensor Systems
Two-phase structures of the core–shell type have been intensively studied in the last decade. Such structures consist of a core of metal oxide covered by a layer of another semiconductor oxide (see, for example, reviews [14][96][97][98][99]).
With the right choice of core and shell materials, such structures can achieve exceptionally high sensitivity and selectivity in the detection of various substances. This also makes it possible to significantly lower their operating temperature [14][97][98][100][101][102]. These materials have generally different morphologies, but the highest sensitivity is exhibited by structures formed by composite nanofibers (see Figure 4). The sensor characteristics of such a material depend on the path of current flow. According to the percolation model of conductivity in a disordered system of semiconductor particles with different electron work functions [65][66], the current in the sensitive layer passes through the fiber shell, overcoming the barriers of the homocontacts, but bypassing the potential barriers of heterocontacts in the core–shell system [103].
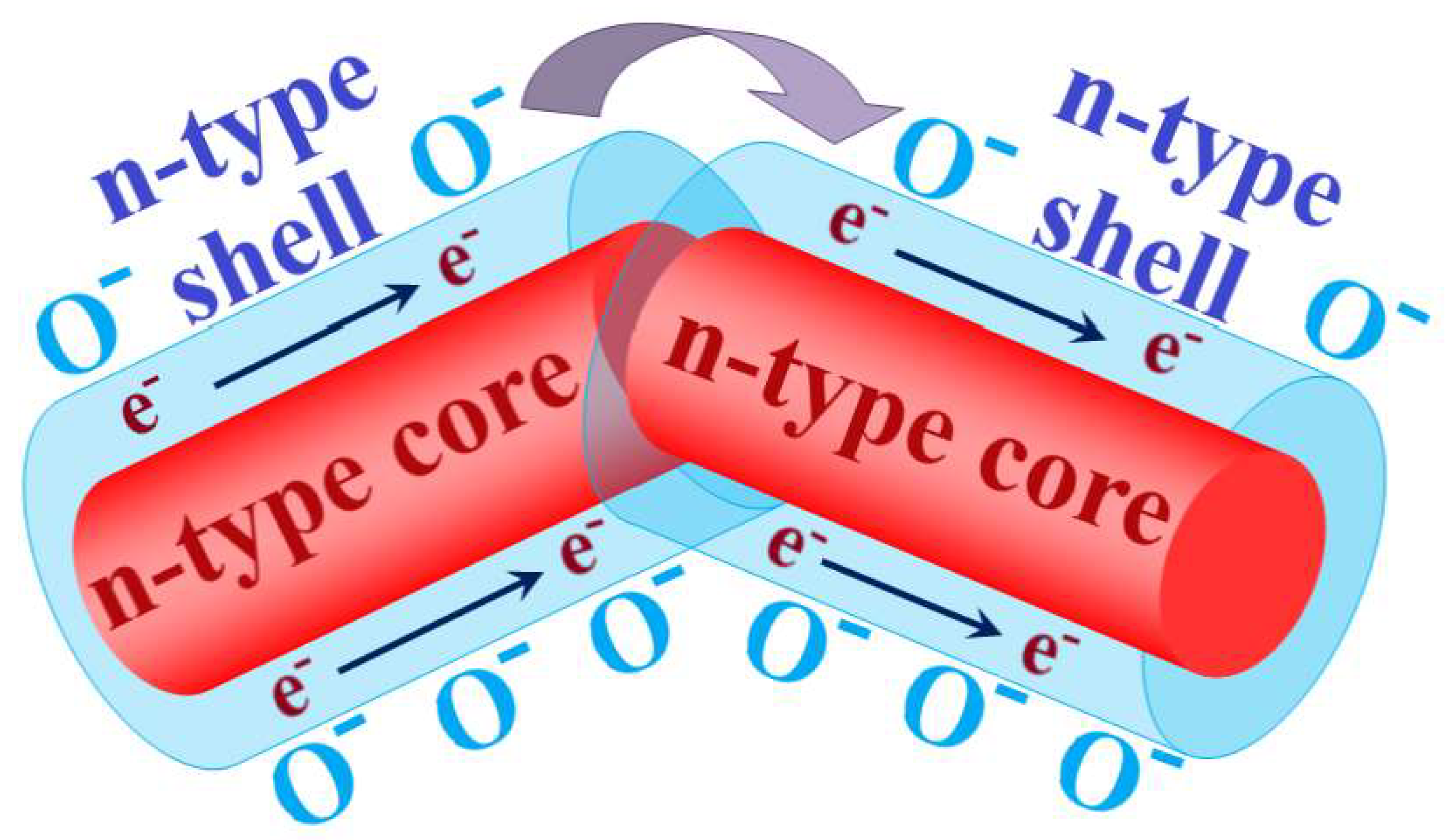
Figure 4. Scheme of the sensitive layer of composite nanofibers of the core–shell type. «e− → e−» indicates the current flow in the shell of nanofibers.
Such a scheme has been confirmed by the results obtained from the detection of a reducing agent (benzene) and an oxidizing agent (NO2) by composites of n-ZnO–p-CuO nanofibers [104] (here and hereinafter, the first oxide is the core, and the second is the shell). The conductometric sensor response to these compounds in the sensitive layer of n-ZnO–p-CuO nanofibers indicates hole conductivity through the nanofiber shells without crossing the fibers. In this case, the value of the sensor response, due to the features of hole conductivity [105], is much lower than in the sensitive layer from pure zinc oxide nanofibers with electronic conductivity [104]. An increase in the sensor response, when p-CuO nanoparticles are included in such a layer, is due to a change in the electronic conductivity as a result of contacts between n-ZnO and p-CuO nanoparticles and electron transfer from ZnO to CuO.
The resistance of the sensitive layer, as in other nanostructured metal oxide semiconductors, is determined by the resistance of contacts between nanoparticles (in this case, nanofibers), which in systems with electronic conductivity increases with a decrease in the electron concentration in the shells.
Two factors affect the concentration of electrons in the shell nanofiber. The first factor is the capture and localization of conduction electrons by electron-withdrawing centers on the outer and inner surfaces of the shell, which leads to the depletion of the shell in electrons. On the outer surface, such centers are adsorbed forms of oxygen, which, at the “operating” temperatures of the sensors above 200 °C, are mainly oxygen atoms. On the inner surface separating the nanofiber shell from the core, electrons are trapped by lattice defects that arise during the formation of the composite, due to the difference in the structures of the core and shell [106].
Another factor is associated with the presence of electron heterojunctions between the core and shell. Such a transition is determined by the difference in the values of the electron work function W for the core WC and the shell WSh of the fiber. If WSh < WC, conduction electrons pass from the shell to the core. In this case, the shell of the composite fiber is even more depleted of electrons.
According to existing concepts, the most promising are sensors based on core–shell nanofibers, which contain weakly conductive catalytically active layers of SnO2 or ZnO. Modification of core–shell metal oxide systems with noble metal nanoparticles can have a significant effect on their sensor response and selectivity. Sensors based on such nanofibers make it possible to detect even traces of various organic compounds in the air (see, for example, [107][108][109][110]).
The response to 0.1 ppm CO of core–shell sensors, based on SnO2-ZnO nanofibers with Au nanoclusters located on the surface of ZnO shells, is 25, while for pure SnO2 and ZnO nanofibers, it is only 3 and 2, respectively [107][111]. The maximum sensor response in the detection of 100 ppb benzene, by SnO2-ZnO nanofibers with Pd nanoclusters on the surface of ZnO shell, is 71 [109]. This is much higher than the sensor effect for SnO2 (1.9) and ZnO (2.1) nanofibers, as well as for SnO2-ZnO sensors that do not contain Pd clusters (11.2). It is also interesting that the deposition of silver nanoparticles on the surface of a nanosystem consisting of tungsten oxide (W18O49), included in a polypyrrole shell, leads to an increase in the sensor response to ammonia and an improvement in the selectivity of this system [108].
To determine the mechanism of sensor effects in composite nanofibers, it is important to find out how the electrical and sensor characteristics of fibers change when the material of the core and shell are swapped. Sensor effects in the detection of CO and aromatic hydrocarbons were determined for core–shell ZnO-SnO2 nanofibers [111]. A comparison with data for similar layers of SnO2-ZnO nanofibers [102] makes it possible to elucidate important features of the resistance and sensor effects in these systems.
In both cases, shells were formed on monocrystal nanorods by deposition of atomic layers. According to [101][102], the interaction between the core and shell in SnO2-ZnO nanofibers leads to an increase in the resistance of the sensor system, due to the contacts between the layers. The sensor effect is also increased.
The values of resistance and the sensor effect of core–shell composites largely depend on the thickness of the shell. For the SnO2-ZnO nanocomposite, the maximum values of these parameters are achieved at a shell thickness of about 30 nm; that is, close to the Debye charge screening length in ZnO (λZnO), which is 22–35 nm [109]. A similar dependence on the shell thickness was also observed in another sensor system of the same type.
The maximum sensor response of the Nb2O5-ZnO system of the core–shell type to 1% H2 at 300 °C is observed at a ZnO shell thickness of about 50 nm, which is close to 2λZnO [112]. These data indicate the presence of electron-withdrawing defects in the Nb2O5-ZnO system at the interface core–shell, which reduce the electron concentration in the shell. Therefore, the length of the screening of charges on oxygen centers, formed on the surface of the shell during oxygen chemisorption, increases. Meanwhile, the reaction of these centers with the analyzed reducing agent and the “release” of trapped electrons, respectively, causes a sensor response, as evidenced by an increase in conductivity.
Judging by the data on the resistance of SnO2-ZnO and ZnO-SnO2 nanofiber layers [107][110], the concentration of conduction electrons in the shells of these nanofibers decreases mainly due to defects at the boundary between SnO2 and ZnO. The transfer of electrons between SnO2 and ZnO increases or decreases the electron concentration in the shells, depending on which of these materials forms the core or shell of the nanofibers. The value of the sensor effect, in the detection of reducing compounds (CO, C6H6, C7H8) by SnO2-ZnO and ZnO-SnO2 sensors, changes according to the change in the resistance of the sensitive layer [107][110].
The attribution of the large value of the sensor effect in core–shell nanofibers, when both the core and the shell have catalytic activity, to only the influence of the system resistance, is not sufficiently substantiated. Indeed, in [109][110][111], a significant increase in the sensor effect was observed when nanoclusters of noble metals were located on the surface of shells, although such additives only reduce the resistance of the film. In addition, a very noticeable sensitivity and selectivity was found in the detection of ethanol by In2O3-ZnO nanofibers [113] containing In2O3 core with a very high electron concentration, which can pass into the ZnO shell, since the work function of the electron for In2O3 is lower than for ZnO. The sensor effect for composite nanofibers is 10 times that for In2O3 nanofibers. In this case, In2O3, as in mixed systems of nanoparticles, is an electron supplier, while ZnO is a catalytically active agent.
The sensor layer based on core–shell nanofibers was also investigated for the ZnO-SnO2 system, which has a slightly different crystal structure. These fibers consist of a monocrystal ZnO core and a continuous SnO2 shell, formed during the thermal reaction of (CH3)4Sn with oxygen on the ZnO surface [114]. The sensitivity of such a layer to reducing gases (CO, CH4, H2, C2H5OH) at 200 and 300 °C is very low and almost does not differ from the sensitivity of a layer of ZnO nanofibers. An increase in the sensor effect, under the influence of the SnO2 shell located on the surface of ZnO nanofibers, manifests itself only when the temperature rises to 400 °C. Therefore, in ZnO-SnO2 nanofibers [114], conduction electrons, trapped by defects at the interface between the core and shell, are held more strongly in defects than in the ZnO-SnO2 and SnO2-ZnO nanofibers considered above, with shells formed by the deposition of atomic layers [107][110].
Thus, the sensor properties of composite metal oxide nanofibers of the core–shell type strongly depend on the composition and method of synthesis of the nanofibers (Table 2) and, accordingly, on their morphology. A detailed study of such systems will elucidate the role of the interaction between the core and shell in the sensor process, and will contribute to the development of new, highly efficient sensor materials.
Table 2. Sensor responses of core–shell materials to various gases.
| Material | Synthesis Method | Sensor Response (Gas Concentration) | Temperature | References |
|---|---|---|---|---|
| Ag–α-Fe2O3 Core–shell composites |
two-step reduction-sol gel approach, including Ag nanoparticles | 9 (500 ppm) | 250 °C | [98] |
| Au-ZnO core–shell nanoparticles | facile low-temperature solution route | 103.9 (100 ppm H2) | 300 °C | [100] |
| SnO2-ZnO core–shell nanowires | two-step process | 25 (10 ppm NO2) 75 (10 ppm C7H8) 83 (10 ppm C6H6) 77 (10 ppm CO) |
300 °C | [101] |
| SnO2-ZnO core–shell nanofibers | two-step process | 6.5 (1 ppm CO) 48 (1 ppm NO2) |
300 °C | [103] |
| ZnO-CuO core–shell nanowires | facile three-step process | 29 (10 ppm NO2) | 350 °C | [104] |
| In2O3/ZnO core–shell nanowires | thermal evaporation of indium powder in an oxidizing atmosphere, followed by the atomic layer deposition of ZnO | 196 (1000 ppm C2H5OH) | 300 °C | [106] |
| SnO2-ZnO core–shell nanowires functionalized by Au nanoparticles | Vapor-liquid-solid growth method | 26.6 (100 ppb CO) | 300 °C | [107] |
| Ag functionalized W18O49@PPy core–shell nanorods | polymerizing the uniform PPy shell film on surface of W3 nanorods with AgNO3 as oxidant and DBSA as modifier. | 2.5 (20 ppm NH3) | 40 °C | [108] |
| Pd-functionalized SnO2-ZnO core-shell nanowires | two-step growth technique. Pd functionalized SnO2-ZnO by using the ray radiolysis technique |
71 (100 ppb C6H6) | 300 °C | [109] |
| SnO2-ZnO core–shell nanowires functionalized Pt nanoparticles | two-step growth technique. Pt functionalized SnO2-ZnO by using the ray radiolysis technique |
279 (100 ppb C7H8) | 300 °C | [110] |
| ZnO-SnO2 core–shell nanowires | two-step growth technique | 41.13 (10 ppm CO) 39.48 (10 ppm C7H8) 40.34 (10 ppm C6H6) |
300 °C | [111] |
| Nb2O5/ZnO core–shell nanorod | two-step growth process | 156 (100 ppm H2) | 300 °C | [112] |
| In2O3-ZnO core–shell nanowires | two-step growth process | 265 (400 ppm C2H5OH) 7 (2000 ppm H2 |
350 °C | [113] |
| ZnO-SnO2 core–shell nanowires | two-step vapor growth method | 66.3 (10 ppm NO2) | 200 °C | [114] |
This entry is adapted from the peer-reviewed paper 10.3390/chemosensors11060320
References
- Degler, D.; Weimar, U.; Barsan, N. Current understanding of the fundamental mechanisms of doped and loaded semiconducting metal-oxide-based gas sensing materials. ACS Sens. 2019, 4, 2228–2249.
- Degler, D. Trends and advances in the characterization of gas sensing materials based on semiconducting oxides. Sensors 2018, 18, 3544.
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B 2014, 204, 250–272.
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B 2007, 121, 18–35.
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75.
- Liu, J.; Liu, X.; Zhai, Z.; Jin, G.; Jiang, Q.; Zhao, Y.; Luo, C.; Quan, L. Evaluation of depletion layer width and gas-sensing properties of antimony-doped tin oxide thin film sensors. Sens. Actuators B 2015, 220, 1354–1360.
- Sharma, A.; Rout, C.S. Advances in understanding the gas sensing mechanisms by in situ and operando spectroscopy. J. Mater. Chem. A 2021, 9, 18175–18207.
- Gurlo, A.; Riedel, R. In situ and operando spectroscopy for assessing mechanisms of gas sensing. Ang. Chem. Intern. Ed. 2007, 46, 3826–3848.
- Morrison, S.R. The Chemical Physics of Surfaces; Springer Science & Business Media: New York, NY, USA, 2013; Available online: https://scholar.google.com/scholar_lookup?title=The%20Chemical%20Physics%20of%20Surfaces&publication_year=1990&author=S.R.%20Morrison (accessed on 1 January 2020).
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceramics 2001, 7, 143–167.
- Sopiha, K.V.; Malyi, O.I.; Persson, C.; Wu, P. Chemistry of oxygen ionosorption on SnO2 surfaces. Appl. Mater. Interfaces 2021, 13, 33664–33676.
- Yamazoe, N.; Suematsu, K.; Shimano, K. Extention of receptor function theory to include two types of adsorbed oxygen for oxide semiconductor gas sensors. Sens. Actuators B 2012, 163, 128–135.
- Yamazoe, N.; Kurorawa, Y.; Seiyama, T. Effects of additives on semiconductor gas sensors. Sens. Actuators B 1983, 4, 283–289.
- Gerasimov, G.N.; Gromov, V.F.; Ilegbusi, O.J.; Trakhtenberg, L.I. The mechanisms of sensory phenomena in binary metal-oxide nanocomposites. Sens. Actuators B 2017, 240, 613–624.
- Korotcenkov, G.; Cho, B.K. Instability of metal oxide-based conductometric gas sensors and approaches to stability improvement (short survey). Sens. Actuators B 2011, 156, 527–538.
- Schwarz, A.; Contescu, C.; Contescu, A. Methods for preparation of catalytic materials. Chem. Rev. 1995, 95, 477–510.
- Korotcenkov, G.I.; Cornet, B.; Cirera, J.R.; Golovanov, V.; Boris, I.; Lychcovsky, V.; Karkotsry, Y.; Rodriguez, A. The influence of additives on gas sensing and structural properties of In2O3-based ceramics. Sens. Actuators B Chem. 2007, 120, 657–664.
- Lin, C.-Y.; Fang, Y.-Y.; Lin, C.-W.; Tunney, J.J.; Ho, K.-C. Fabrication of NOx gas sensors using In2O3-ZnO composite films. Sens. Actuators B 2010, 146, 28–34.
- Gerasimov, G.N.; Ikim, M.I.; Gromov, V.F.; Ilegbusi, O.J.; Trakhtenberg, L.I. Chemical modification of impregnated SnO2-In2O3 nanocomposites due to interaction of sensor components. J. Alloys Compd. 2021, 883, 160817.
- Trakhtenberg, L.I.; Gerasimov, G.N.; Gromov, V.F.; Belysheva, T.V.; Ilegbusi, O.J. Effect of composition and temperature on conductive and sensing properties of CeO2 + In2O3 nanocomposite films. Sens. Actuators B 2015, 209, 562–569.
- Trakhtenberg, L.I.; Gerasimov, G.N.; Gromov, V.F.; Belysheva, T.V.; Ilegbusi, O.J. Effect of composition on sensing properties of SnO2 + In2O3 mixed nanostructured films. Sens. Actuators B 2012, 169, 32–38.
- Trakhtenberg, L.I.; Gerasimov, G.N.; Gromov, V.F.; Belysheva, T.V.; Ilegbusi, O.J. Conductivity and sensing properties of In2O3 + ZnO mixed nanostructured films: Effect of composition and temperature. Sens. Actuators B 2013, 187, 514–521.
- Kim, J.-H.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Optimization and gas sensing mechanism of n-SnO2-p-Co3O4 composite nanofibers. Sens. Actuators B 2017, 248, 500–511.
- Yin, X.-T.; Li, J.; Dastan, D.; Zhou, W.-D.; Garmestani, H.; Alamgir, F.M. Ultra-high selectivity of H2 over CO with a p-n nanojunction based gas sensors and its mechanism. Sens. Actuators B 2020, 319, 128330.
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B 1991, 5, 7–19.
- Yamazoe, N.; Shimanoe, K. Receptor function and response of semiconductor gas sensor. J. Sens. 2009, 138, 875704.
- Nagaev, E.L. Small metal particles. Soviet Phys. Uspekhi 1992, 35, 747–782.
- Trakhtenberg, L.I.; Gerasimov, G.N.; Grigoriev, E.I.; Zavialov, S.A.; Zagorskaja, O.V.; Zufman, V.Y.; Smirnov, V.V. Nanoheterogeneous metal-polymer composites as a new type of effective and selective catalysts. Stud. Surf. Sci. Catal. 2000, 130, 941–946.
- Maier, J.; Göpel, W. Investigations of the bulk defect chemistry of polycrystalline tin (IV) oxide. J. Solid State Chem. 1988, 72, 293–302.
- Kolmakov, A.; Klenov, D.O.; Lilach, Y.; Stemmer, S.; Moskovits, M. Enhanced gas sensing by individual SnO2 nanowires and nanobelts functionalized with Pd catalyst particles. Nano Lett. 2005, 5, 667–673.
- Aragon, F.H.; Coaquira, J.A.H.; Hidalgo, P.; daSilva, S.W.; Brito, S.L.M.; Gouvêa, D.; Morais, P.C. Evidences of the evolution from solid solution to surface segregation in Ni-doped SnO2 nanoparticles using Raman spectroscopy. J. Raman Spectrosc. 2011, 42, 1081–1086.
- Luo, Y.; An, B.; Bai, J.; Wang, Y.; Cheng, X.; Wang, Q.; Li, J.; Yang, Y.; Wu, Z.; Xie, E. Ultrahigh-response hydrogen sensor based on PdO/NiO co-doped In2O3 nanotubes. J. Colloid Interface Sci. 2021, 599, 533–542.
- Gerasimov, G.N.; Gromov, V.F.; Ikim, M.I.; Ilegbusi, O.J.; Ozerin, S.A.; Trakhtenberg, L.I. Structure and gas-sensing properties of SnO2-In2O3 nanocomposites synthesized by impregnation method. Sens. Actuators B 2020, 320, 128406.
- Zhu, X.; Li, Y.; Zhang, H.; Song, L.; Zu, H.; Qin, Y.; Liu, L.; Li, Y.; Wang, F. High-performance field effect transistors based on large ratio metal (Al, Ga, Cr) doped In2O3 nanofibers. J. Alloys Compd. 2020, 830, 154578.
- Sakthiraj, K.; Balachandrakumar, K. Influence of Ti addition on the room temperature ferromagnetism of tin oxide (SnO2) nanocrystal. J. Magn. Magn. Mater. 2015, 395, 205–212.
- Shen, J.; Li, F.; Yin, B.; Sun, L.; Chen, C.; Wen, S.; Chen, Y.; Ruan, S. Enhanced ethyl acetate sensing performance of Al-doped In2O3 microcubes. Sens. Actuators B 2017, 253, 461–469.
- Al-Hashem, M.; Akbar, S.; Morris, P. Role of oxygen vacancies in nanostructured metal-oxide gas sensors: A review. Sens. Actuators B 2019, 301, 126845.
- Zeng, X.; Liu, L.; Lv, Y.; Zhao, B.; Ju, X.; Xu, S.; Zhang, J.; Tian, C.; Sun, D.; Tang, X. Ultra-sensitive and fast response formaldehyde sensor based on La2O3-In2O3 beaded na-notubes at low temperature. Chem. Phys. Lett. 2020, 746, 137289.
- Lemos, S.C.S.; Nossol, E.; Ferrari, J.L.; Gomes, E.O.; Andres, J.; Gracia, L.; Sorribes, I.; Lima, R.C. Joint theoretical and experimental study on the la doping process in In2O3: Phase transition and electrocatalytic activity. Inorg. Chem. 2019, 58, 11738.
- Aragon, F.H.; Coaquira, J.A.H.; Villegas-Lelovsky, L.; da Silva, S.W.; Cesar, D.F.; Nagamine, L.C.C.M.; Cohen, R.; Men’endez-Proupin, E.; Morais, P.C. Evolution of the doping regimes in the Al-doped SnO2 nanoparticles prepared by a polymer precursor method. J. Phys. Condens. Matter 2015, 27, 095301.
- An, Y.; Yang, D.; Ma, G.; Zhu, Y.; Wang, S.; Wu, Z.; Liu, J. Role of Co cluster and oxygen vacancies in the magnetic and transport properties of Co-doped In2O3 films. J. Phys. Chem. C 2014, 118, 10448–10454.
- Liu, X.; Zhang, S.; Wu, Z.; An, Y. Manganese-vacancy complexes induced room temperature ferromagnetism in Mn/Mg co-doped In2O3 diluted magnetic semiconductors. Superlattices Microstruct. 2019, 132, 106174.
- Yamaura, H.; Moriya, K.; Miura, N.; Yamazoe, N. Mechanism of sensitivity promotion in CO sensor using indium oxide and cobalt oxide. Sens. Actuators B 2000, 65, 39–41.
- Bloch, E.D.; Hudson, M.R.; Mason, J.A.; Chavan, S.; Crocellà, V.; Howe, J.D.; Lee, K.; Dzubak, A.L.; Queen, J.W.L.; Zadrozny, M.; et al. Reversible CO binding enables tunable CO/H2 and CO/N2 separations in metal-organic frameworks with exposed divalent metal cations. J. Am. Chem. Soc. 2014, 136, 10752–10761.
- Jinkawa, T.; Sakai, G.; Tamaki, J.; Miura, N.; Yamazoe, N. Relationship between ethanol gas sensitivity and surface catalytic property of tin oxide sensors modified with acidic or basic oxides. J. Mol. Catal. A 2000, 155, 193–200.
- Lupinetti, A.J.; Fau, S.; Frenking, G.; Strauss, S.H. Theoretical analysis of the bonding between CO and positively charged atoms. J. Phys. Chem. A 1997, 101, 9551–9559.
- Hocking, R.K.; Hambley, T.W. Database analysis of transition metal carbonyl bond lengths: Insight into the periodicity of π back-bonding, σ donation, and the factors affecting the electronic structure of the TM-CtO moiety. Organometallics 2007, 26, 2815–2823.
- Liang, Q.; Zou, X.; Chen, H.; Fan, M.; Dong, G.-L. High-performance formaldehyde sensing realized by alkaline-earth metals doped In2O3 nanotubes with optimized surface properties. Sens. Actuators B 2020, 304, 127241–127246.
- Gao, L.; Fu, H.; Zhu, J.; Wang, J.; Chen, Y.; Liu, H. Synthesis of SnO2 nanoparticles for formaldehyde detection with high sensitivity and good selectivity. J. Mater. Res. 2020, 35, 2208–2217.
- Zhao, Y.; Zou, X.; Chen, H.; Chu, X.; Li, G.-D. Tailoring energy level and surface basicity of metal oxide semiconductors by rare-earth incorporation for high-performance formaldehyde detection. Inorg. Chem. Front. 2019, 6, 1767–1774.
- Dimitrov, V.; Komatsu, T. Correlation among electronegativity, cation polarizability, optical basicity and single bond strength of simple oxides. J. Solid State Chem. 2012, 196, 574–578.
- Mahajan, S.; Jagtap, S. Metal-oxide semiconductors for carbon monoxide (CO) gas sensing: A review. Appl. Mater. Today 2020, 18, 100483.
- Hurlburt, P.K.; Rack, J.J.; Luck, J.S.; Dec, S.F.; Webb, J.D.; Anderson, O.P.; Strauss, S.H. Nonclassical metal carbonyls: + and +. J. Am. Chem. Soc. 1994, 116, 10003–10014.
- Kim, S.-J.; Hwang, I.-S.; Kang, Y.C.; Lee, J.-H. Design of selective gas sensors using additive-loaded In2O3 hollow spheres prepared by combinatorial hydrothermal reactions. Sensors 2011, 11, 10603–10614.
- Singh, N.; Comini, E.; Ponzoni, A.; Lee, P.S. Chemical sensing investigation on Zn-In2O3 nanowires. Sens. Actuators B 2012, 171–172, 244–248.
- Zhou, Q.; Chen, W.; Xu, L.; Kumar, R.; Gui, Y.; Zhao, Z.; Tang, C.; Zhu, S. Highly sensitive carbon monoxide (CO) gas sensors based on Ni and Zn doped SnO2 nanomaterials. Ceram. Int. 2018, 44, 4392–4399.
- Chen, H.; Zhao, Y.; Shi, L.; Li, G.-D.; Sun, L.; Zou, X. Revealing the relationship between energy level and gas sensing performance in heteroatom-doped semiconducting nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 29795–29804.
- Chen, H.; Sun, L.; Li, G.-D.; Zou, X. Well-tuned surface oxygen chemistry of cation off-stoichiometric spinel oxides for highly selective and sensitive formaldehyde detection. Chem. Mater. 2018, 30, 2018–2027.
- Wang, Z.; Hou, C.; De, Q.; Gu, F.; Han, D. One-step synthesis of Co-doped In2O3 nanorods for high response of formaldehyde sensor at low temperature. ACS Sens. 2018, 3, 468–475.
- Bonu, V.; Das, A.; Prasad, A.K.; Krishna, N.G.; Dhara, S.; Tyagi, A.K. Influence of in-plane and bridging oxygen vacancies of SnO2 nanostructures on CH4 sensing at low operating temperatures. Appl. Phys. Lett. 2014, 105, 243102.
- Liu, L.; Shu, S.; Zhang, G.; Liu, S. Highly selective sensing of C2H6O, HCHO, and C3H6O gases by controlling SnO2 nanoparticle vacancies. ACS Appl. Nano Mater. 2018, 1, 31–37.
- Kumar, R.; Jaiswal, M.; Singh, O.; Gupta, A.; Ansari, M.S.; Mittal, J. Selective and reversible sensing of low concentration of carbon monoxide gas using Nb-doped OMS-2 nanofibers at room temperature. IEEE Sens. J. 2019, 19, 7201–7206.
- Genuino, H.C.; Seraji, M.S.; Meng, Y.; Valencia, D.; Suib, S.L. Combined experimental and computational study of CO oxidation promoted by Nb in manganese oxide octahedral molecular sieves. Appl. Catal. B Environ. 2015, 163, 361–369.
- Kuntaiah, K.; Sudarsanam, P.; Reddy, B.M.; Vinu, A. Nanocrystalline Ce1-xSmxO2-δ (x = 0.4) solid solutions: Structural characterization versus CO oxidation. RSC Adv. 2013, 3, 7953–7962.
- Savage, N.; Chwieroth, B.; Ginwalla, A.; Patton, B.R.; Akbar, S.A.; Dutta, P.K. Composite n-p-semiconducting titanium oxide as gas sensors. Sens. Actuators B 2001, 79, 17–27.
- Efros, A.L. Physics and Geometry of Disorder: Percolation Theory; Mir: Moscow, Russia, 1986; Available online: https://archive.org/details/physics-of-disorder (accessed on 20 May 2023).
- Choi, S.-W.; Katoch, A.; Kim, J.-H.; Kim, S.S. Striking sensing improvement of n-type oxide nanowires by electronic sensitization based on work function difference. J. Mater. Chem. C 2015, 3, 1521–1527.
- Nam, B.; Ko, T.-K.; Hyun, S.-K.; Lee, C. NO2 sensing properties of WO3-decorated In2O3 nanorods and In2O3-decorated WO3 nanorods. Nano Converg. 2019, 6, 40.
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217.
- Sowmya, B.; Athira, J.; Panda, P.K. A review on metal-oxide based p-n and n-n heterostructured nanomaterials for gas sensing applications. Sens. Int. 2021, 2, 100085.
- Mirzaei, A.; Yousefi, H.R.; Falsafi, F.; Bonyani, M.; Lee, J.-H.; Kim, J.-H.; Kim, H.W.; Kim, S.S. An overview on how Pd on resistive-based nanomaterial gas sensors can enhance response toward hydrogen gas. Int. J. Hydrogen Energy 2019, 44, 20522–20571.
- Dhall, S.; Kumar, M.; Bhatnagar, M.; Mehta, B.R. Dual gas sensing properties of graphene-Pd/SnO2 composites for H2 and ethanol: Role of nanoparticles-graphene interface. Int. J. Hydrogen Energy 2018, 43, 17921–17927.
- Feng, Q.; Li, X.; Wang, J. Percolation effect of reduced graphene oxide (rGO) on ammonia sensing of rGO-SnO2 composite based sensor. Sens. Actuators B 2017, 243, 1115–1126.
- Sygellou, L.; Paterakis, G.; Galiotis, C.; Tasis, D. Work function tuning of reduced graphene oxide thin films. J. Phys. Chem. C 2016, 120, 281–290.
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators B 2008, 128, 566–573.
- Li, T.; Zeng, W.; Wang, Z. Quasi-one-dimensional metal-oxide-based heterostructural gas-sensing materials: A review. Sens. Actuators B 2015, 221, 1570–1585.
- Hübner, M.; Simion, C.E.; Tomescu-Stanoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens. Actuators B 2011, 153, 347–353.
- Pokhrel, S.; Simion, C.E.; Quemener, V.; Bârsan, N.; Weimar, U. Investigations of conduction mechanism in Cr2O3 gas sensing thick films by ac impedance spectroscopy and work function changes measurements. Sens. Actuators B 2008, 133, 78–83.
- Kim, J.-H.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. (n)-SnO2—(p)-NiO composite nanowebs: Gas sensing properties and sensing mechanisms. Sens. Actuators B 2018, 258, 204–214.
- Zhou, W.; Dastan, D.; Yin, X.; Nie, S.; Wu, S.; Wang, Q.; Li, J. Optimization of gas sensing properties of n-SnO2/p-xCuO sensors for homogenous gases and the sensing mechanism. J. Mater. Sci. Mater. Electron. 2020, 31, 18412–18426.
- Zhang, R.; Cao, S.; Zhou, T.; Fei, T.; Wang, R.; Zhang, T. Rational design and tunable synthesis of Co3O4 nanoparticle-incorporating into In2O3 one-dimensional ribbon as effective sensing material for gas detection. Sens. Actuators B 2020, 10, 127695.
- Korotcenkov, G.; Cho, B.K. Metal oxide composites in conductometric gas sensors: Achievements and challenges. Sens. Actuators B 2017, 244, 182–210.
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nanoheterostructures: A review. Sens. Actuators B 2019, 286, 624–640.
- Kim, J.-H.; Wu, P.; Kim, H.W.; Kim, S.S. Highly selective sensing of CO, C6H6, and C7H8 gases by catalytic functionalization with metal nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 7173–7183.
- Gromov, V.F.; Gerasimov, G.N.; Belysheva, T.V.; Ikim, M.I.; Spiridonova, E.Y.; Grekhov, M.M.; Ali-zade, R.A.; Trakhtenberg, L.I. Sensor properties of nanostructured systems based on indium oxide with Co3O4 or ZrO2 additives. Russ. J. Phys. Chem. B 2018, 12, 129–134.
- Nowotny, J.; Sloma, M.; Weppner, W. Surface reactivity of yttria-doped zirconia with oxygen. Solid State Ionics 1989, 32–33, 709–713.
- Zakrzewska, K.; Radecka, M. TiO2-SnO2 composites and solid solutions for chemical nanosensors. Procedia Eng. 2012, 47, 1077–1083.
- Korotcenkov, G.; Han, S.H.; Cho, B.K. Metal Oxide Nanocomposites: Advantages and shortcomings for application in conductometric gas sensors. Mater. Sci. Forum 2016, 872, 223–229.
- Xu, H.; Ju, D.; Li, W.; Zhang, J.; Wang, J.; Cao, B. Superior triethylamine-sensing properties based on TiO2/SnO2 n-n heterojunction nanosheets directly grown on ceramic tubes. Sens. Actuators B 2016, 228, 634–642.
- Zhang, K.; Shen, Y.; Li, Y.; Zhang, W. Synthesis of 2O3 heterojunction with unique hexagonal three-dimensional structure for ultra sensitive ethanol detection. Mater. Sci. Semicond. Process. 2022, 143, 106523.
- Li, R.; Zhou, Y.; Sun, M.; Gong, Z.; Guo, Y.; Wu, F.; Li, W.; Ding, W. Influence of charge carriers concentration and mobility on the gas sensing behavior of tin dioxide thin films. Coatings 2019, 9, 591–597.
- Park, S.; Sun, G.-J.; Kheel, H.; Lee, W.I.; Lee, S.; Choi, S.-B.; Lee, C. Synergistic effects of codecoration of oxide nanoparticles on the gas sensing performance of In2O3 nanorods. Sens. Actuators B 2016, 227, 591–599.
- Wei, X.; Xie, T.; Peng, L.; Fu, W.; Chen, J.; Gao, Q.; Hong, G.; Wang, D. Effect of heterojunction on the behavior of photogenerated charges in Fe3O4@Fe2O3 nanoparticle photocatalysts. J. Phys. Chem. C 2011, 115, 8637–8642.
- Feste, P.D.; Crisci, M.; Barbon, F.; Tajoli, F.; Salerno, M.; Drago, F.; Prato, M.; Gross, S.; Gatt, T.; Lamberti, F. Work function tuning in hydrothermally synthesized vanadium-doped MoO3 and Co3O4 mesostructures for energy conversion devices. Appl. Sci. 2021, 11, 2016.
- Sun, Y.; Zhao, Z.; Suematsu, K.; Zhang, W.; Zhang, W.; Zhuiykov, S.; Shimanoe, K.; Hu, J. MOF-derived Au-NiO/In2O3 for selective and fast detection of toluene at ppb-level in high humid environments. Sens. Actuators B 2022, 360, 13163.
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness affects the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B 2018, 256, 270–294.
- Wang, S.; Kang, Y.; Wang, L.; Zhang, H.; Wang, Y. Organic/inorganic hybrid sensors: A review. Sens. Actuators B 2013, 182, 467–482.
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Neri, G. Synthesis, characterization and gas sensing properties of αFe2O3 core-shell nanocomposited. Nanomaterials 2015, 17, 737–749.
- Karnati, P.; Akbar, S.; Morris, P.A. Conduction mechanisms in one dimensional core-shell nanostructures for gas sensing; A review. Sens. Actuators B 2019, 295, 127–143.
- Majhi, P.; Rai, Y.-T. Facile approach to synthesize Au-ZnO core shell nanoparticles and their application for highly sensitive and selective gas sensors. ACS Appl. Mater. Interfaces 2015, 7, 9462–9468.
- Choi, S.-W.; Katoch, A.; Sun, G.-J.; Kim, J.-H.; Kim, S.-H.; Kim, S.-S. Dual functional sensing mechanism in SnO2-ZnO core-shell nanowires. ACS Appl. Mater. Interfaces 2014, 6, 8281.
- Lee, D.-J.; Kim, K.-J.; Kim, S.-H.; Kwon, J.-Y.; Xu, J.; Kim, K.-B. Atomic layer deposition of Ti-doped ZnO films with enhanced electron mobility. J. Mater. Chem. C 2013, 1, 4761.
- Katoch, A.; Choi, S.-W.; Sun, G.-J.; Kim, S.-S. An approach to detecting a reducing gas by radial modulation of electron-depleted shells in core-shell nanofibers. J. Mater. Chem. A 2013, 1, 13588–13596.
- Diao, K.; Xiao, J.; Zheng, Z.; Cui, X. Enhanced sensing performance and mechanism of CuO nanoparticle-loaded. Appl. Surf. Sci. 2018, 459, 630–638.
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceram. 2010, 25, 11–19.
- Park, S.; Ko, H.; Kim, S.; Lee, C. Role of the interfaces in multiple networked one-dimensional core-shell nanostructured gas sensors. ACS Appl. Mater. Interfaces 2014, 6, 9595–9600.
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Extremely sensitive and selective sub-ppm CO detection by the synergistic effect of Au nanoparticles and core-shell nanowires. Sens. Actuators B 2017, 249, 177–188.
- Qin, Y.; Wen, Z.; Zhang, T. Facile realization of Ag functionalized W18O49@PPy core-shell nanorods for multieffect modulation on gas sensing response. J. Mater. Sci. Mater. Electron. 2019, 30, 15031–15041.
- Kim, J.-H.; Kim, H.W.; Kim, S.S. Ultra-sensitive benzene detection by a novel approach: Core-shell nanowires combined with Pd-functionalization. Sens. Actuators B 2017, 239, 578–585.
- Kim, J.-H.; Kim, S.S. Realization of ppb-scale toluene-sensing abilities with Pt-functionalized SnO2-ZnO core-shell nanowires. ACS Appl. Mater. Interfaces 2015, 7, 17199–17208.
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Variation of shell thickness in ZnO-SnO2 core-shell nanowires for optimizing sensing behaviors to CO, C6H6, and C7H8 gases. Sens. Actuators B 2020, 302, 127150.
- Park, S.; Lee, S.; Kim, H.W.; Lee, C. Hydrogen sensing properties of multiple networked Nb2O5/ZnO core-shell nanorod sensors. Sens. Actuators B 2014, 202, 840–845.
- Singh, N.; Ponzoni, A.; Gupta, R.K.; Lee, P.S.; Comini, E. Synthesis of In2O3-ZnO core-shell nanowires and their application in gas sensing. Sens. Actuators B 2011, 160, 1346–1351.
- Hwang, I.-S.; Kim, S.-J.; Choi, J.-K.; Choi, J.; Ji, H.; Kim, G.-T.; Cao, G.; Lee, J.-H. Synthesis and gas sensing characteristics of highly crystalline ZnO-SnO2 core-shell nanowires. Sens. Actuators B 2010, 148, 595–600.
This entry is offline, you can click here to edit this entry!
