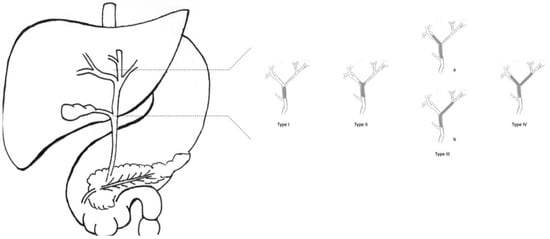
As CCAs arise from cholangiocytes, they can spread across any tract of the biliary epithelium. Therefore, it is possible to distinguish three subtypes of CCA, depending on their anatomical localisation (Figure 1). About 10% of CCAs are intrahepatic, affecting bile ducts proximal to the second-order biliary branches. About 40% of CCAs are perihilar, involving bile ducts distal to second-order biliary branches and proximal to cystic duct implant. The remaining 50% of CCAs are extrahepatic, located distal to the cystic duct implant, up to Vater’s Ampulla.
2. Results of Surgical Resection in pCCA
Complete surgical resection with all gross tumour eradication is the only modality to cure any pCCA. As a rule of thumb, the more distal the malignancy, the more amenable it is to surgical treatment [
5]. The surgical strategy for pCCA is en bloc bile duct and gallbladder resection, maintaining a 5–10 mm margin from neoplastic tissue, with locoregional lymphadenectomy. A hepaticojejunostomy with Roux-en-Y reconstruction is required. Furthermore, radical surgical treatment of pCCA implies a hepatic resection, either extended right or left hepatectomy with caudate [
14]. When hepatic resection is needed, the surgeon must consider the volume of functioning liver remnant (FLR) to avoid postoperative liver failure. There is no agreed cutoff around the minimum percentage of FLR, but most centres consider 25–30% a safe value in normal liver and 40–50% if cirrhotic. However, it is possible to perform either a portal vein embolisation of the to-be-resected side or the ALPPS procedure (associating liver partition and portal vein ligation for staged hepatectomy) to induce remnant liver hypertrophy. Widespread consensus currently revolves around portal vein embolisation as a standard approach, with ALPPS still feasible in particular cases [
15].
About 30% of pCCAs are not eligible for surgery at diagnosis, most of them because they are unfit for surgery or not a resectable disease. An additional 30% prove not surgically treatable following laparotomy/laparoscopic exploration because of metastatic or locally advanced disease [
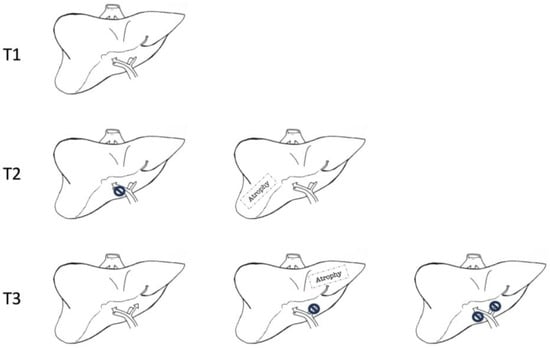
16]. The feasibility of surgical treatment in pCCA depends neither on AJCCs nor Bismuth–Corlette classification. Neither provides information about the local radial spread of cancer, hepatic lobar atrophy, or portal vein invasion. That is why the Blumgart T-staging system has been developed and validated as a tool to assess the resectability of pCCA (
Figure 2) [
17].
Figure 2. Blumgart T-staging system for hilar cholangiocarcinoma. T1 corresponds to a tumour involving biliary confluence with unilateral extension to second-order biliary radicles. T2 corresponds to a tumour involving biliary confluence with unilateral extension to second-order biliary radicles and ipsilateral portal vein involvement or ipsilateral hepatic atrophy. T3 corresponds to tumours involving biliary confluence with bilateral extension to second-order biliary radicles, unilateral extension to second-order biliary radicles with contralateral portal vein involvement, unilateral extension to second-order biliary radicles with contralateral hepatic lobar atrophy, or main or bilateral portal venous involvement.
According to the T-staging system, criteria for non-resectable pCCA are cirrhosis, patient’s unfitness for surgery; bilateral disease extension proximal to II grade biliary ducts; portal vein occlusion proximal to its bifurcation; hepatic lobar atrophy with contralateral portal vein occlusion or extension of contralateral disease distal to II grade ducts; unilobar T with contralateral portal vein occlusion; extralocoregional nodal metastases (N2) at histology; and pulmonary, peritoneal or hepatic metastases [
6].
The R0-resection rate for pCCA ranges from 50% to 90%, and only a minimal improvement in life expectancy has been registered in such cases. The overall median survival of patients undergoing surgery is 42 months for R0 resections and 21 months for R1 resections [
18]. Nevertheless, the 5-year survival rate is significantly different only if R0 resections (up to 60% survival) are compared with non-operated patients, even if it depends on the lymph node status (5-year survival of up to 55% in case of negative lymph nodes vs. 20% in case of positive lymph nodes). There is no difference in 5-year survival rates between patients treated with R1 resections and non-surgically treated patients (less than 10% survival at five years). In other words, the surgical margins, such as lymph node involvement, are an important prognostic factor [
19].
3. Liver Transplantation in pCCA
About one-third of pCCAs are considered unresectable at diagnosis, and another one-third are excluded at the time of exploration because of extensive disease. When resective surgery is deemed unfeasible, liver transplantation (LT) can be an effective alternative [
37]. LT allows the treatment of locally advanced disease with complete eradication of the primary tumour and avoids a hypothetical postoperative liver failure due to insufficient remnant liver volume or the impossibility of performing a biliary reconstruction in tumours extended to both right and left ducts or to the secondary order bile ducts [
5]. Early experiences in LT in patients with pCCA had a poor outcome, with a 5-year survival rate of ~20%, not significantly different from R1 resection and non-operative management. The main problem identified was an elevated rate of disease recurrence, most commonly found in the allograft or lungs [
38].
Therefore, neoadjuvant protocols have been developed to downstage the disease, prevent recurrences and be a bridge strategy to LT. Moreover, neoadjuvant treatment can help identify patients who may not respond to treatment, thereby avoiding futile surgical intervention. Since 2008, the United Network for Organ Sharing (UNOS) has admitted the listing of patients diagnosed with unresectable pCCA as long as a neoadjuvant CRT protocol precedes LT in demonstrated N0-M0 diseases [
39].
The first protocol paving the way to LT in pCCA was developed in 1993 by the University of Nebraska and subsequently implemented by the Mayo Clinic [
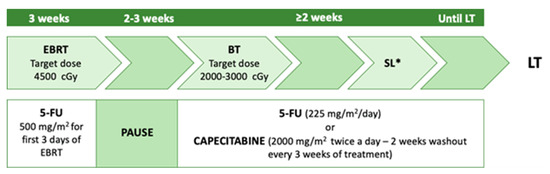
40]. The neoadjuvant therapy described in this study consists of a combination of external-beam radiation, brachytherapy with 5-FU radiosensitising during RT, and subsequent capecitabine administration until LT (
Figure 3) [
41]. Enrollment criteria adopted were the non-resectability of the tumour, a maximum lesion diameter of 3 cm (several studies showed better prognosis when the tumour size was <3 cm) [
42], absence of intrahepatic and extrahepatic metastases, and patients had to be medically fit for both CRT and LT. The exclusion criteria were intrahepatic cholangiocarcinoma, uncontrolled infection, prior radiation or chemotherapy, prior biliary resection or attempted resection, intrahepatic metastases, evidence of extrahepatic disease, history of other malignancy within five years, and transperitoneal biopsy (including percutaneous and EUS-guided FNA).
Figure 3. Mayo Clinic neoadjuvant protocol. EBRT: external beam radiotherapy; BT: brachytherapy; SL: staging laparotomy; LT: a liver transplant. * with biopsies of at least common hepatic artery and distal bile duct lymph nodes.
The overall survival rates after LT at 1 and 3 years were 91% and 81%, respectively [
19]. Five-year survival after transplantation and neoadjuvant was 73%, but 79% for patients with underlying PSC, and 63% for those with de novo CCA [
43].
Multicenter case series reported a 5-year disease-free survival rate of ~65%. However, more than two-thirds of the patients had PSC as an underlying disease, compared to about 5% in other pCCA cohorts [
44]. Some critical points of Mayo Clinic pre-LT protocol were the staging surgery, which involves, 2–6 weeks after initiation of radiotherapy, a complete abdominal exploration with biopsy of any lymph nodes or nodules suspected of tumour, the examination of the tumour, and routine biopsy of regional lymph nodes (along the distal common bile duct, hepatic artery, and in the celiac and peripancreatic area) [
45]. Another criticality was the selection of patients with early-stage pCCA arising in the setting of PSC with negative cytology, negative FISH, and no residual tumour in the specimen after transplantation, putting into question whether the patient ever had cancer. On the other hand, those patients were under close surveillance and had a cancer diagnosis very early, and PSC patients seemed to have the most favourable outcomes [
46]. Nevertheless, several centres performing LT for pCCA adopted the Mayo Clinic protocol as the standard of care whenever complete surgical resection was impossible [
11,
35].
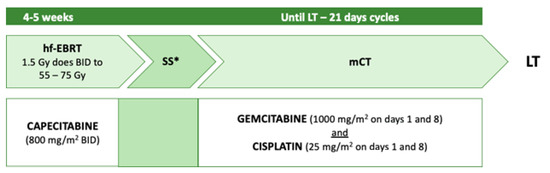
Toronto General Hospital and Princess Margaret Cancer Centre have developed another neoadjuvant regimen [
47]. The protocol was modelled on the Mayo Clinic protocol, aiming to implement the radiosensitising phase and the maintenance chemotherapy, while reducing biliary toxicity stemming from brachytherapy (
Figure 4).
Figure 4. Toronto neoadjuvant protocol. Hf-EBRT: hyperfractionated external beam radiotherapy; mCT: maintenance chemotherapy; SS: staging surgery (either laparotomy or laparoscopic); LT: liver transplant; BID: twice a day. * with biopsies of hepatic artery and hepatoduodenal lymph nodes.
The exclusion criteria in this study were inability to consent, prior attempted resection within the past 12 months, prior upper abdominal radiation therapy, a trans-peritoneal biopsy of the primary tumour within the past 12 months, prior malignancy diagnosed in the last five years, uncontrolled infection, inability or unwillingness to complete the protocol due to comorbid conditions, or ECOG ≥ 3 at an initial consultation. Criteria for exclusion from the protocol were evidence of metastatic disease on follow-up imaging, surgical staging positive for malignancy, or disease progression on neoadjuvant treatment. During neoadjuvant treatment, patients were reviewed weekly, and followed up one month after surgical staging and three months after transplant. Repeat CT chest, abdomen, and pelvis, and CA19-9 testing was performed three months following the protocol’s enrollment until transplant. The median total duration from the first consultation and start of neoadjuvant chemotherapy until LT was 48.6 (40.7–77.9) weeks and 37.0 (30.0–63.7) weeks, respectively. There were no dropouts due to toxicity, although 61% dropped out due to metastatic disease development and medical comorbidity exacerbation. The OS after LT at 1 and 2 years was 83.3% and 55.6%, respectively [
31]. However, due to the Toronto protocol’s recent validation, a 5-year follow-up is not available at present, nor a comparison with the Mayo Clinic experience.
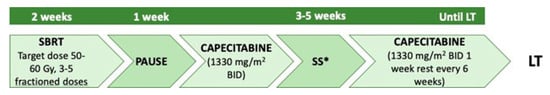
In a pilot study in 2014, the University of Michigan designed a neoadjuvant protocol based on stereotactic body radiation therapy (SBRT) [
48]. According to the authors, this approach can minimise toxicity on adjacent healthy structures by concentrating radiation doses on the local disease. The inclusion criteria were the same as the Mayo group. After two weeks of SBRT and one week of rest, patients were initiated to the capecitabine schedule and underwent a staging operation. Maintenance chemotherapy was provided until LT (
Figure 5). In the reported single-institution experience, histological proven tumour response was detectable in 66% of neoadjuvant therapy patients. A single-centre experience using brachytherapy, external beam radiotherapy and 5-fluorouracil (5-Fu) showed that, in selected patients with unresectable pCCA, long-term survival was 94% and 61% at 1 and 4 years, respectively, with comparable results to other series. However, in-hospital mortality was 20% [
49,
50].
Figure 5. University of Michigan neoadjuvant protocol. SBRT: stereotactic body radiotherapy; SS: staging surgery; LT: liver transplant; BID: twice a day. * with lymph nodes biopsies.