Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Numerous compounds present in the ocean are contributing to the development of the biomedical field. Agarose, a polysaccharide derived from marine red algae, plays a vital role in biomedical applications because of its reversible temperature-sensitive gelling behavior, excellent mechanical properties, and high biological activity. Natural agarose hydrogel has a single structural composition that prevents it from adapting to complex biological environments.
- agarose
- modification
- hydrogel
- biomedical application
1. Introduction
Seaweed is an enormous marine flora and a major component of marine biological resources. About 90% of aquatic plant species are seaweed [1]. According to the composition of photosynthetic pigments, seaweed is usually divided into three groups: Chlorophyceae (green algae), Phaeophyceae (brown algae), and Rhodophyceae (red algae) [2]. Red algae, in particular, are the most diverse, containing around 6500 species [3]. The components of red algae, such as polysaccharides, proteins, and minerals, have been studied extensively. Polysaccharides have become the most exciting seaweed component for researchers because of their wide range of food and medicinal functions.
Agarose, a natural polysaccharide obtained from red algae, is a linear polysaccharide consisting of d-galactose and 3,6-anhydrous-l-galactose with many hydroxyl groups in its structural unit [4], which readily form hydrogen bonds with hydrogen atoms in the structure or with those of water. Its molecular weight is generally between 80 kDa ~ and 140 kDa. Agarose can form a controlled hydrogel with stability and hysteresis [5]. As shown in Figure 1, at 90–100 °C, the hydrogen bonds between the structural units of agarose break, and agarose are dispersed into the water as random coils to form a clear solution. When the temperature is lowered to 30–40 °C, the molecular chains of agarose are intertwined by hydrogen bonds, forming a double helix structure that is tightly arranged to form a gel. According to recent research, the global agarose market looks promising for the next five years. The global agarose market was estimated to be USD 83.35 million by 2022 and is expected to reach USD 99.35 million by 2028, growing at a compound annual growth rate of 2.97% in the forecast years [6]. The end user segment of agarose is mainly in the biomedical sector, which includes academic institutions, hospitals, diagnostic centers, pharmaceuticals, and biotechnology. The regional features of this market include Asia Pacific, Europe, North America, the Middle East and Africa, and Latin America [6].
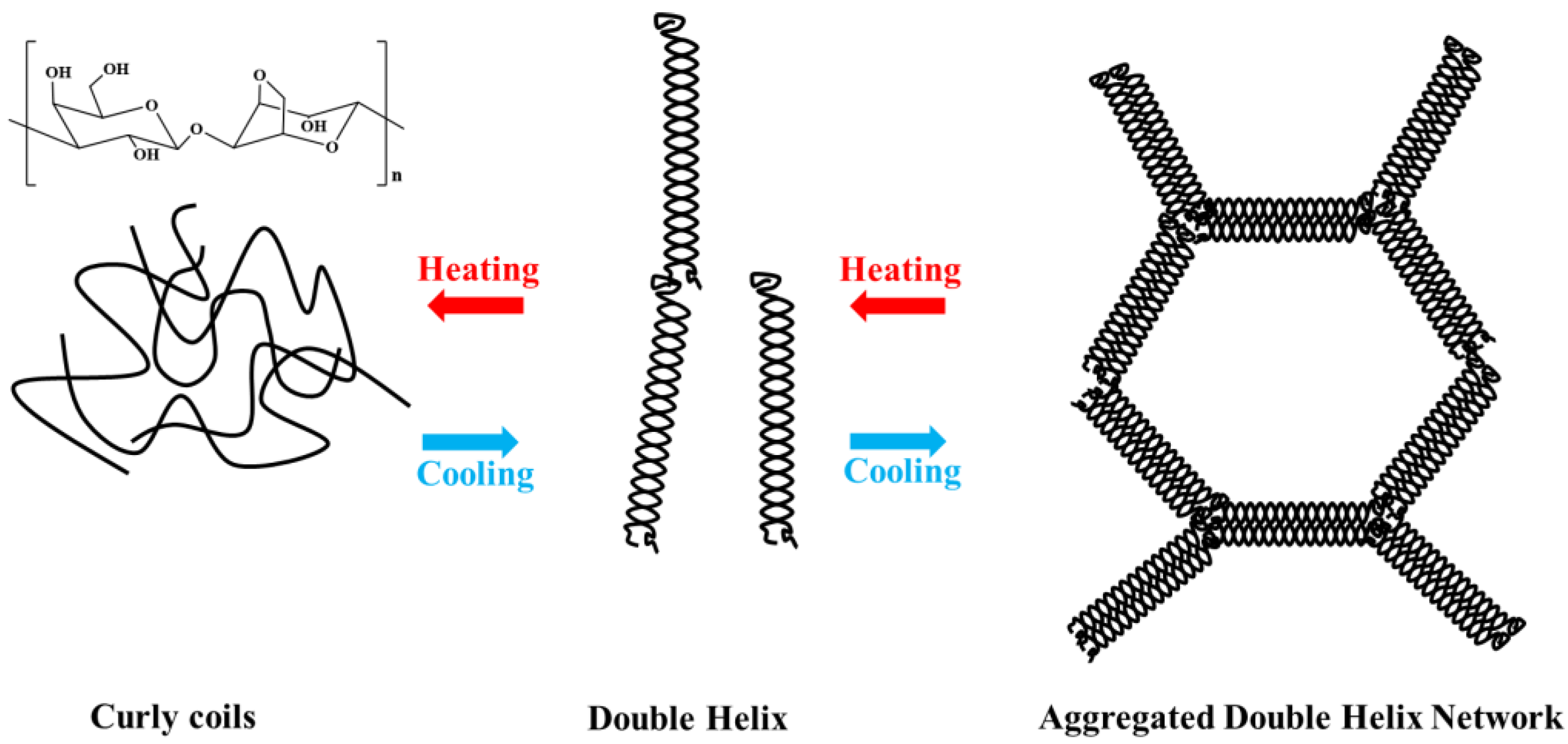
Figure 1. Structure and gelling mechanism of agarose.
As functional biomolecules, bioactive polysaccharides are constantly being developed in the biomedical industry and are attracting considerable interest from scientists [7]. Polysaccharides and their derivatives from natural sources are preferred to synthetic substances in food, household products, and pharmaceuticals because of their biodegradability, non-toxicity, and biocompatibility [8][9][10]. Polysaccharides are now widely used for disease diagnosis, inhibition and treatment, drug delivery, antibacterial and antiviral applications, and tissue engineering [8][11][12][13]. Agarose is a natural source of neutral gel polysaccharide, which plays a vital role in the biomedical industry. It can inhibit the growth of bacteria on the surfaces of medical devices because of its low viscosity as an antimicrobial material [14][15]. The excellent biocompatibility of agarose makes it suitable as a hydrogel for the controlled release of drugs [16][17] and materials for tissue engineering [18][19]. Agarose gel is electrically neutral and rigid; therefore, it is widely used as a filling material for gel electrophoresis and separation media. Agarose can be modified by compounding or grafting with other materials to provide specific adhesion and a pH response [16], making agarose more promising for tissue regeneration and other applications.
2. Biomedical Applications of Agarose and Its Derivatives
As the sol—agarose chains that form a porous network through hydrogen bonding [20]—are gelled, it allows the diffusion of high molecular weight substances. Meanwhile, many biological processes, such as the separation and purification of biological macromolecules, the complexation of proteins, and cell growth and multiplication, depend on buffers. For this reason, the hydrophilicity of the medium is essential for use in biological processes. Agarose is a polyhydroxy hydrocolloid, and the diffusion of biomolecules in the gel is not clearly different from that in an aqueous solution [21]. Synthetic and inorganic biomaterials, such as polyacrylamide gel and porous glass, are limited in separation and purification because of their nonspecific adsorption with some biomacromolecules. In theory, biologically active substances interact weakly with agarose and can remain biologically active in agarose gel [22]. It is possible to functionalize agarose to obtain specific properties that can lead to the derivation of different types of chromatographic separation media, extending the range of separation applications [23]. Figure 2 shows the application of agarose and its derivatives in the biomedical field.
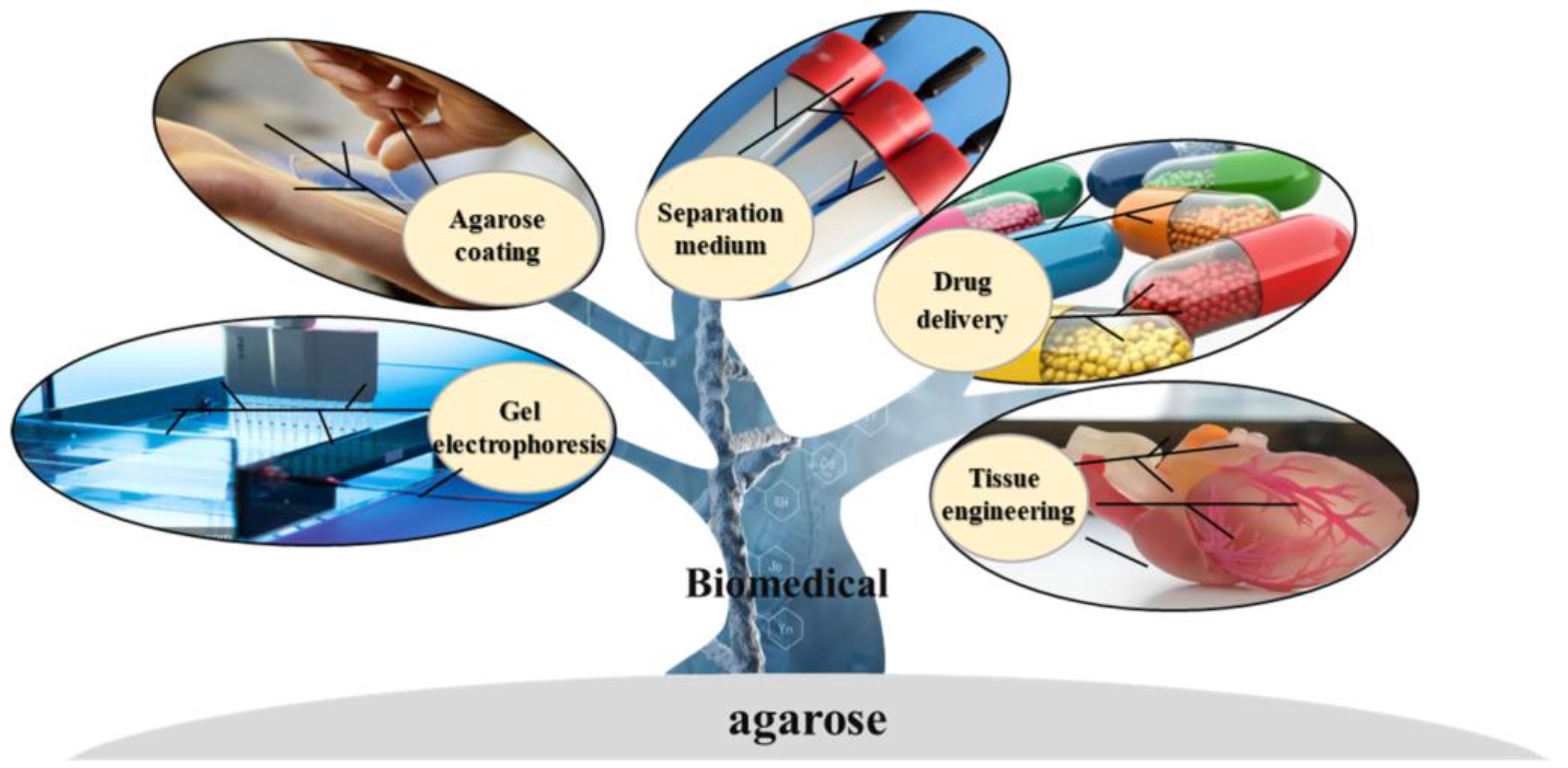
Figure 2. Application of agarose.
2.1. Agarose Gel Electrophoresis
Electrophoresis is increasingly used in several fields, including clinical chemistry, toxicology, pharmacology, and immunology. Electrically charged molecules are driven through the agarose matrix by an electric field. They are separated in the agarose gel matrix according to their size, conformation, shape, and amount of charge they carry [24]. For this reason, agarose gel electrophoresis is widely used to separate and identify biological macromolecules such as nucleic acids, polysaccharides, proteins, and viruses [25][26].
The concentration of agarose gel electrophoresis is usually in the range of 0.5 to 2% (w/w) [27][28]. Agarose gel electrophoresis has the advantage of easy operation, simple equipment, a low sample volume, and high resolution. It serves the dual purpose of a “molecular sieve” and “electrophoresis” and is widely used in the study of nucleic acids [24]. In particular, chemically modified agarose with a low melting point has a higher sieving capacity and is ideal for the electrophoresis of DNA and RNA, allowing the recovery of the natural forms of DNA from the gel [29]. Green et al. [30] separated DNA fragments of different sizes using low-melting agarose and then recovered DNA by organic extraction from molten agarose using phenol-chloroform.
Zhang et al. prepared low melting point agarose through the oxy alkylation of agarose using ethylene oxide, 1,2-epoxypropane, and 1,2-epoxy butane [31]. They found that oxy-alkylated agarose underwent a superior separation performance in DNA gel electrophoresis. Recently, cyclic RNA migration under various conditions, various monoclonal and polyclonal antibodies, and the molecular weight of serum albumin have also been shown to be analyzed by agarose gel electrophoresis. Li et al. [27] characterized monoclonal antibodies developed against short peptide phosphotyrosines for reactive kinase phosphorylation by agarose gel electrophoresis. They optimized electrophoretic conditions by adjusting critical parameters of the electrophoretic process. Tomioka et al. [32] performed the agarose gel electrophoresis of commercially available bovine serum proteins using UltraPure and MetaPhor agarose. They found that the agarose gel electrophoresis obtained with MetaPhor agarose had a more significant molecular sieving effect, with high-resolution size differences and the ability to show more than 4%, oligomeric bands. In addition, agarose gel electrophoresis has been used to separate proteins and their complexes. Sakuma and colleagues [33] effectively extended the scope of agarose gel electrophoresis for biomedical applications by using agarose gel electrophoresis to analyze physically modified, aggregated, or post-translationally modified proteins. Agarose can also be mixed with polyacrylamide and dextran to support gel electrophoresis.
2.2. Agarose Separation Medium
Agarose gel is a hydrophilic medium that is highly compatible with biological macromolecules. Its porous properties make it ideal for separating proteins and nucleic acids [34]. Additionally, agarose can be customized for various applications by incorporating other functional groups as needed [35]. Agarose is a popular chromatographic separation media due to its availability in various forms, such as affinity, ion exchange, and hydrophobic chromatography media [36]. These different forms are based on distinct separation principles, making agarose one of the most widely used polysaccharide-based separation media.
2.2.1. Affinity Chromatography
Affinity chromatography is a technique used to separate and purify biological macromolecules by taking advantage of their specific recognition or reversible binding to corresponding molecules [37]. Agarose is a preferred matrix for affinity chromatography due to its low cost, large pore size, minimal non-specific binding to biological reagents, and stability across a wide range of pH values [38][39]. Affinity chromatography can be classified into four main types based on the interaction systems between biological macromolecules and ligands. These types are affinity chromatography, biomimetic affinity chromatography, immunoaffinity chromatography, and metal ion affinity chromatography.
Biological affinity chromatography is a kind of affinity chromatography that uses highly selective biological specificity to select interacting substances [40]. The enzyme-substrate, enzyme-inhibitor, and hormone-receptor pairs are commonly used. Yi et al. [41] exploited the biospecific choice between enzymes and enzyme inhibitors by using ST/SC (SpyTag/SpyCatcher) chemistry and targeted the immobilization of the green fluorescent protein as a model protein on agarose microspheres. Subsequently, ST-GFP (green fluorescent protein) was replaced by ST-PqsA (a key enzyme in the Pseudomonas aeruginosa quorum sensing pathway), and PqsA inhibitors were isolated by a biospecific selection between PqsA and PqsA inhibitors. Biomimetic affinity chromatography uses molecular interactions to synthesize ligands that mimic specific structures and sites on biological molecules as stationary phases for protein adsorption. Bai et al. [42] isolated and purified formate dehydrogenase (FDH) from bovine cell extracts by immobilizing four triazine reactive dyes on agarose and found that Procion Blue HERB was a suitable dye ligand for FDH, enabling simple, inexpensive, and efficient agarose affinity chromatography. Immunoaffinity chromatography is a separation system in which one of the antigens and antibodies is used as a ligand to affinity adsorb the other side. Hou et al. [43] prepared immunoaffinity chromatography columns to separate avermectin, ivermectin, doramectin, and ivermectin from samples by coupling avermectin polyclonal antibodies to CNBr-activated agarose, followed by an analysis of the residues eluting from the columns by high-performance liquid chromatography-tandem mass spectrometry. Metal ion affinity chromatography uses metal ion complexes to bind to proteins for separation and purification. The polymer, supported by super-porous agarose particles, has a high osmotic pressure, a high tolerance to interference mechanisms, and low back pressure. Zheng et al. [44] constructed complex immobilized metal ion affinity agarose particles to selectively isolate and purify histidine-tagged proteins using super porous agarose particles as supports, flexible copolymer brushes as backbones, and Ni2+-chelated iminodiacetic acid as ligands.
2.2.2. Size Exclusion Chromatography
Size exclusion chromatography (SEC) is a chromatographic technique for the separation of polymer samples based on the correspondence between the pore size of the gel pores and the molecular size of the polymer, with the advantages of the simplicity of the operation and high sample recovery [45]. Because of the 3D network structure of the gel filtration media, biomacromolecules with high molecular weight are blocked on the outside as they pass through the pores of gel particles and elute directly downwards at a rapid rate. By contrast, biomacromolecules with a low molecular weight can enter the interior of the gel particles as they pass through the column and are retained at a slow elution rate. The molecular sieving effect of the gels allows the molecular size of the samples to be screened for different elution times for biomolecules of different molecular weights [46]. Dextran, polyacrylamide, and agarose gel are the most commonly used gel filtration media. In particular, agarose gels have a higher mechanical strength and sieve stability, allowing for higher flow rates, a fuller range of pH conditions, and molecular weights. Size exclusion chromatography is often divided into gel permeation chromatography and gel filtration chromatography, depending on the mobile phase.
Zhao et al. [47] prepared homogeneous agarose microspheres of controlled particle size by an emulsion membrane method, followed by multi-step cross-linking and dextran grafting to obtain high-resolution chromatography media, finely controlling the molecular range with good pressure resistance. Site-specific glycosylation and its associated heterogeneity affect the functional activity of glycoproteins, and studies have shown that aberrant glycosylation can be closely associated with many diseases. Yet, the analysis of intact glycopeptides remains a significant challenge. For this purpose, Zhao et al. [48] developed a method to enrich intact tryptic N-glycopeptides using the excellent properties of the acrylamide–agarose composite gel and the hydrophilic properties of volume exclusion chromatography.
2.2.3. Ion Exchange Chromatography
Ion exchange chromatography (IEC) is a separation method based on the difference in electrostatic interactions between the sample and the stationary phase (ion exchange chromatography medium [49][50]). It has the advantages of wide applicability, high resolution, no effect on the biological activity of the sample, and high resolution, and it is widely used for the separation and purification of biological macromolecules [51] such as proteins [52]. The derivatizability of the hydroxyl groups of agarose allows ligands of different charge types and densities to be introduced into the gel [49]. Depending on the properties of ion-exchange ligands coupled to the agarose surface, IEC can be divided into anion-exchange chromatography media and cation-exchange chromatography. Currently, the most frequently used functional ligands are diethyl aminoethyl [53], carboxymethyl [54], and sulfopropyl [55], which make an agarose-based ion-exchange medium for weak anions, strong anions, weak cations, and strong cations, respectively. Separation can be achieved according to the difference in electrostatic interactions between the separation medium and the molecules because of the difference in charge type, charge number, and charge distribution on the surface of different molecules. In specialty biochemical separation media, the Agarose range of ion exchange media is currently a mainstream product. Anion-exchange chromatography separates and purifies positively charged proteins (lysozyme and cytochrome C) using an agarose-based anion-exchange medium. Silva-Santos et al. [56] used anion-exchange chromatography with HiTrap Q-agarose as the anion-exchange medium to purify ssDNA generated by aPCR from the reaction mixture. Barroca-Ferreira et al. [57] used Q-Sepharose anion-exchange chromatography to efficiently and finely purify prostaglandin 1.0 from K. pastoris small bioreactor lysates in a series of separation and purification steps; this is involved in cellular communication, stimulating cell proliferation and is specific to the cancer microenvironment. Cation exchange chromatography combines an ion exchange stationary phase with a negatively charged moiety coupled to a positively charged cation for separation and purification. Li et al. [58] investigated the effect of chain length and ionic strength on lysozyme adsorption and chromatographic interactions with γ-globulin by developing a cation exchange medium and co-grafting sodium methacrylate onto the commercial agarose gel Sepharose FF. Zhao et al. [36] developed a method by combining pre-crosslinking and surfactant micelle swelling to produce highly crosslinked macroporous agarose microspheres with a homogeneous network structure, low backpressure, and high flow rate, and, based on this, they created a carboxymethyl-coupled cation exchange chromatography media that could effectively separate proteins at high flow rates. Using sulphopropyl-Sepharose cation exchange chromatography, Li et al. [59] isolated and purified a 17.5 kDa bean protein inhibitor with anti-proliferative activity against leukemia and lymphoma cells from canola.
2.2.4. Hydrophobic Interaction Chromatography
Hydrophobic interaction chromatography (HIC) is a method that uses the reversible binding of a hydrophobic target in the mobile phase to the separation media together with a hydrophobic binding partner to achieve separation and purification [60]. HIC is widely used for the isolation and purification of biomolecules, such as proteins and peptides [60][61][62], for the advantages of mild reaction conditions, the high recovery of biomolecules, a low environmental impact, and cost savings [63][64]. They are typically composed of a hydrophobic matrix with a hydrophobic ligand. Currently, HIC media are commonly used with natural polysaccharides such as agarose and cellulose and synthetic polymers such as polystyrene and polyacrylates [65].
Agarose hydrophobic chromatography media consisting of agarose and hydrophobic ligands were introduced by chemical modification. Here, there are two main types of hydrophobic ligands: a hydrocarbon group with different carbon chain lengths, CH3(CH2)n-X, where X can be NH2, COOH, or OH, and hydrophobicity increases with the value of n; the second is a butyl or phenyl group with general hydrophobicity [66][67]. Its separation is based solely on the strength of the hydrophobic interaction, as the absence of groups such as NH2, COOH, or OH eliminates the interference of hydrogen bonding or charge interactions.
Superporous agarose beads are frequently employed as a foundation for hydrophobic chromatographic media. Gustavsson et al. [61] conducted a study in which they separated ribonuclease A, lysozyme, and bovine serum albumin using hydrophobic agarose chromatographic media prepared from phenyl-derived super porous agarose beads. The results showed that these proteins could be effectively separated and purified. The use of the chromatographic method resulted in higher flow rates compared to the use of homogeneous agarose beads as support. Hydrophobic chromatography was found to provide even higher flow rates. Lipases play a crucial role in various fields, such as oleochemistry, organic chemistry, biofuels, and pharmaceuticals, due to their ability to catalyze the hydrolysis of triacylglycerols into glycerol and free fatty acids. Mehta et al. [68] purified lipases from Aspergillus fumigatus through the use of octyl-Sepharose column chromatography for this purpose. The activation of angiogenesis by an essential fibroblast growth factor (bFGF) and vascular endothelial growth factor A (VEGFA) could promote tumorigenesis. However, the use of peptidomes containing bFGF/VEGFA can effectively block the activation of these growth factors, making them a promising option for the development of therapeutic tumor vaccines. Holková et al. [69] employed phenyl agarose CL-4B hydrophobic chromatography to isolate and purify lipoxygenases from poppy cultures. These enzymes play a crucial role in regulating growth, development, and resistance to biotic and abiotic stresses.
2.3. Agarose Coating
Infections caused by various bacterial biofilms have become one of the most common public health problems in clinical settings [70]. Bacterial proliferation and perithecia formation on medical devices pose a serious health challenge to patients [14]. As a result, the provision of anti-fouling and anti-bacterial capabilities for medical devices has become a hot research topic [71]. One of the most effective ways of doing this is to form coatings of a certain thickness and adhesion on the surface of the equipment. Agarose and its derivatives have been widely used as substrates for functional hydrogel coatings due to their excellent lubricity, biocompatibility, electrical conductivity, and mechanical properties [70][72].
Agarose shows no excellent anti-fouling or anti-bacterial properties. Early research has shown that agarose gel could resist the attachment of A. aeruginosa at a pH below 8.0, but when the pH increased beyond 8.2, the bacteria aggregated and was deposited on the agarose surface. Therefore, the majority of research has been carried out to produce novel coatings by combining agarose with other substances, commonly combining materials with antimicrobial properties and agarose [73]. Eric et al. [74] developed a novel coating by incorporating Cu/bioactive glass into an agarose matrix and showed that released Cu improved anti-adhesive properties and slowed biofilm formation on the biomaterial surface. Li et al. [75] combined ZnO nanoparticles and Ag nanoparticles on silk glue–agarose composites, which showed excellent antibacterial activity against Gram-positive and Gram-negative bacteria and great potential as a novel antibacterial biomaterial. Rather than simply compounding the agarose, chemical reactions such as crosslinking and grafting bound the agarose to different molecules of different sizes. This method improved the antibacterial and contamination resistance of agarose while maintaining superior gelling properties. Li and colleagues [71] introduced acrylate groups onto agarose molecules to form a covalent, crosslinked agarose coating on the surface of a medical silicone diaphragm that reduced the formation of bacteria by more than two orders of magnitude. He grafted and cross-linked agarose and quaternary ammonium chitosan using thiol-alcohol chemistry as an antimicrobial coating. This crosslinked coating effectively inhibited the biofilm formation of Gram-negative and Gram-positive bacteria. Interestingly, after 30 days of repeated wiping with ethanol, autoclave, or lysozyme solutions, the layer retained its antimicrobial activity [76].
2.4. Drug Delivery
A wide variety of drug carriers have been developed and are in use. Still, drug delivery has been on a path of innovation due to multidimensional challenges from materials to cost [77]. Carbohydrate polymers are one of the most important materials for the design of efficient drug carriers and have great potential to meet a wide range of needs [78][79]. Common carbohydrate polymers such as chitosan, starch, and cellulose have been widely used in drug delivery systems but still have considerable drawbacks. For example, natural chitosan is only soluble in dilute acidic aqueous media and loses its mechanical properties [80]; starch has poor mechanical properties and high-water solubility [81]; cellulose is insoluble in organic and aqueous solvents and has poor antibacterial properties [82]. All of these are unavoidable shortcomings. Agarose, with its non-toxicity, gelatinization, and gel structure with suitable viscoelasticity and thermal reversibility, is a common substrate for constructing particles, microcapsules [83], and microspheres and is excellent for controlled drug release. Drug release systems can be built using the biocompatibility and haemocompatibility of agarose [84]. For example, active ingredients can be encapsulated in agarose hydrogel nanoparticles and prepared by the emulsion template method [85]. Agarose can also be mixed with various polysaccharides (e.g., chitosan, cyclodextrin, and konjac glucomannan), proteins, and magnetic nanoparticles to form complexes with enhanced physicochemical properties for efficient drug delivery [84]. In general, agarose hydrogel drug delivery systems are dominated by mass diffusion release (Fickian diffusion) in agarose hydrogels, where the diffusion time depends on the network structure and the size of the particles [86]. In targeted drug delivery systems, agarose gels can be adapted in terms of their pore size, structure, and function by adjusting the agarose concentration [87], mixing with other compounds, and making chemical modifications to create an interesting and versatile drug delivery system [86].
In 1994, Haglund et al. [88] loaded Ibuprofen and Indomethacin onto agarose beads and studied their release mechanisms. To reduce the severe side effects associated with high doses of cyclophosphamide as a premedication in oncological treatment, Sakai et al. [89] encapsulated cells that were genetically modified to express cytochrome P450 2B1 enzymes in sub-sieve-sized agarose capsules before placing the cell-encapsulated microcapsules in arteries near the tumor via a microcatheter and activating them locally. The side effects were effectively reduced without reducing the dose. The subcapsules of agarose were smaller in diameter than standard agarose, reducing surgical trauma while also reducing the occlusion of the dripping vessel.
Apart from agarose pellets for drug delivery, agarose hydrogels are widely used in drug delivery systems due to their biocompatibility and solute permeability [90]. However, natural agarose has a high mechanical strength and solidification temperature [31], making it difficult to load thermosensitive drugs due to their inactivation during natural agarose gelation. As mentioned above, Kim et al. [31] prepared low gel temperature agarose by introducing β-cyclodextrin into ethylenediamine-modified agarose and found that this gel could be used for the sustained release of the thermosensitive drug doxorubicin because of its low mechanical strength and solidification temperature. In addition, hydrogel complexes of agarose with polysaccharides and proteins are often used as carriers for drug delivery systems to compensate for the shortcomings of natural agarose hydrogel in drug delivery. Rossi et al. [91] used agarose in a synthetic hydrogel with a Carbomer 974P macromolecule monomer as a spatially blocked and molecularly structured drug and mimetic molecule sodium fluorescein as a drug delivery vehicle.
This entry is adapted from the peer-reviewed paper 10.3390/md21050299
References
- Ramkumar, V.S.; Prakash, S.; Ramasubburayan, R.; Pugazhendhi, A.; Gopalakrishnan, K.; Kannapiran, E.; Rajendran, R.B. Seaweeds: A resource for marine bionanotechnology. Enzyme Microb. Technol. 2016, 95, 45–57.
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342.
- Nan, F.R.; Feng, J.; Lv, J.P.; Liu, Q.; Fang, K.P.; Gong, C.Y.; Xie, S.L. Origin and evolutionary history of freshwater Rhodophyta: Further insights based on phylogenomic evidence. Sci. Rep. 2017, 7, 2934.
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159.
- Lahaye, M. Developments on gelling algal galactans, their structure and physico-chemistry. J. Appl. Phycol. 2001, 13, 173–184.
- MarketWatch Global Agarose Market Analysis and Business Growth Outlook . Available online: https://www.marketwatch.com/ (accessed on 12 April 2023).
- Lee, Y.E.; Kim, H.; Seo, C.; Park, T.; Lee, K.B.; Yoo, S.Y.; Hong, S.C.; Kim, J.T.; Lee, J. Marine polysaccharides: Therapeutic efficacy and biomedical applications. Arch Pharm. Res. 2017, 40, 1006–1020.
- Sharma, A.; Kaur, I.; Dheer, D.; Nagpal, M.; Kumar, P.; Venkatesh, D.N.; Puri, V.; Singh, I. A propitious role of marine sourced polysaccharides: Drug delivery and biomedical applications. Carbohydr. Polym. 2023, 308, 120448.
- Xiao, Q.; Ma, M.Z.; Chen, J.; Zhang, Y.H.; Chen, F.Q.; Weng, H.F.; Xiao, A.F. Preparation of macroporous rigid agarose microspheres by pre-crosslinking with cyclic anhydride. Int. J. Biol. Macromol. 2022, 222, 41–54.
- Song, R.; Murphy, M.; Li, C.S.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145.
- Gaharwar, A.K. Engineered Biomaterials for in Situ Tissue Regeneration. Tissue Eng. Part A 2022, 28, S590.
- Ciancia, M.; Fernandez, P.V.; Leliaert, F. Diversity of Sulfated Polysaccharides From Cell Walls of Coenocytic Green Algae and Their Structural Relationships in View of Green Algal Evolution. Front. Plant Sci. 2020, 11, 554585.
- Lopez-Heredia, M.A.; Lapa, A.; Mendes, A.C.; Balcaen, L.; Samal, S.K.; Chai, F.; Van der Voort, P.; Stevens, C.V.; Parakhonskiy, B.V.; Chronakis, I.S.; et al. Bioinspired, biomimetic, double-enzymatic mineralization of hydrogels for bone regeneration with calcium carbonate. Mater. Lett. 2017, 190, 13–16.
- Wu, F.; Xu, T.T.; Zhao, G.Y.; Meng, S.S.; Wan, M.M.; Chi, B.; Mao, C.; Shen, J. Mesoporous Silica Nanoparticles-Encapsulated Agarose and Heparin as Anticoagulant and Resisting Bacterial Adhesion Coating for Biomedical Silicone. Langmuir 2017, 33, 5245–5252.
- Chu, W.T.; Ma, Y.H.; Zhang, Y.N.; Cao, X.J.; Shi, Z.Y.; Liu, Y.; Ding, X.J. Significantly improved antifouling capability of silicone rubber surfaces by covalently bonded acrylated agarose towards biomedical applications. Colloids Surf. B-Biointerfaces 2023, 222, 112979.
- Hu, Y.; Kim, Y.; Hong, I.; Kim, M.; Jung, S. Fabrication of Flexible pH-Responsive Agarose/Succinoglycan Hydrogels for Controlled Drug Release. Polymers 2021, 13, 2049.
- Apte, G.; Lindenbauer, A.; Schemberg, J.; Rothe, H.; Nguyen, T.H. Controlling Surface-Induced Platelet Activation by Agarose and Gelatin-Based Hydrogel Films. ACS Omega 2021, 6, 10963–10974.
- Krommelbein, C.; Xie, X.F.; Seifert, J.; Konieczny, R.; Friebe, S.; Kas, J.; Riedel, S.; Mayr, S.G. Electron beam treated injectable agarose/alginate beads prepared by electrospraying. Carbohydr. Polym. 2022, 298, 120024.
- Kinoshita, K.; Iwase, M.; Yamada, M.; Yajima, Y.; Seki, M. Fabrication of multilayered vascular tissues using microfluidic agarose hydrogel platforms. Biotechnol. J. 2016, 11, 1415–1423.
- Xiao, Q.; Weng, H.F.; Chen, G.; Xiao, A.F. Preparation and characterization of octenyl succinic anhydride modified agarose derivative. Food Chem. 2019, 279, 30–39.
- Zhang, L.Y.; Xiao, Q.; Xiao, Z.C.; Zhang, Y.H.; Weng, H.F.; Chen, F.Q.; Xiao, A.F. Hydrophobic modified agar: Structural characterization and application in encapsulation and release of curcumin. Carbohydr. Polym. 2023, 308, 120644.
- Zhao, L.S.; Li, S.S.; Liang, C.; Qiao, L.Z.; Du, K.F. High-strength and low-crystallinity cellulose/agarose composite microspheres: Fabrication, characterization and protein adsorption. Biochem. Eng. J. 2021, 166, 107826.
- Zhao, L.; Huang, Y.D.; Zhu, K.; Miao, Z.; Zhao, J.Z.; Che, X.J.; Hao, D.X.; Zhang, R.Y.; Ma, G.H. Manipulation of pore structure during manufacture of agarose microspheres for bioseparation. Eng. Life Sci. 2020, 20, 504–513.
- Tantray, J.A.; Mansoor, S.; Wani, R.F.C.; Nissa, N.U. (Eds.) Chapter 24—Agarose gel electrophoresis. In Basic Life Science Methods; Academic Press: Cambridge, MA, USA, 2023; pp. 103–106.
- Li, C.; Arakawa, T. Agarose native gel electrophoresis of proteins. Int. J. Biol. Macromol. 2019, 140, 668–671.
- Tomioka, Y.; Arakawa, T.; Akuta, T.; Nakagawa, M.; Ishibashi, M. Analysis of proteins by agarose native gel electrophoresis in the presence of solvent additives. Int. J. Biol. Macromol. 2022, 198, 26–36.
- Li, C.; Akuta, T.; Nakagawa, M.; Sato, T.; Shibata, T.; Maruyama, T.; Okumura, C.J.; Kurosawa, Y.; Arakawa, T. Agarose native gel electrophoresis for characterization of antibodies. Int. J. Biol. Macromol. 2020, 151, 885–890.
- Abe, B.T.; Wesselhoeft, R.A.; Chen, R.; Anderson, D.G.; Chang, H.Y. Circular RNA migration in agarose gel electrophoresis. Mol. Cell 2022, 82, 1768–1777.e3.
- Song, N.L.; He, X.; Zhao, Q.R.; Yan, T.D.; Wen, L. Cloning and expression of the tumstatin active peptides-T-7 and its derivant-T-7-NGR. Clin. Exp. Med. 2009, 9, 165–171.
- Green, M.R.; Sambrook, J. Recovery of DNA from Low-Melting-Temperature Agarose Gels: Organic Extraction. Cold Spring Harb. Protoc. 2020, 2020, 100461.
- Zhang, N.; Wang, J.L.; Ye, J.; Zhao, P.; Xiao, M.T. Oxyalkylation modification as a promising method for preparing low-melting-point agarose. Int. J. Biol. Macromol. 2018, 117, 696–703.
- Tomioka, Y.; Nakagawa, M.; Sakuma, C.; Nagatoishi, S.; Tsumoto, K.; Arakawa, T.; Akuta, T. Ladder observation of bovine serum albumin by high resolution agarose native gel electrophoresis. Int. J. Biol. Macromol. 2022, 215, 512–520.
- Sakuma, C.; Tomioka, Y.; Li, C.; Shibata, T.; Nakagawa, M.; Kurosawa, Y.; Arakawa, T.; Akuta, T. Analysis of protein denaturation, aggregation and post-translational modification by agarose native gel electrophoresis. Int. J. Biol. Macromol. 2021, 172, 589–596.
- Lira, R.B.; Steinkuhler, J.; Knorr, R.L.; Dimova, R.; Riske, K.A. Posing for a picture: Vesicle immobilization in agarose gel. Sci. Rep. 2016, 6, 25254.
- Pourmadadi, M.; Yazdian, F.; Koulivand, A.; Rahmani, E. Green synthesized polyvinylpyrrolidone/titanium dioxide hydrogel nanocomposite modified with agarose macromolecules for sustained and pH-responsive release of anticancer drug. Int. J. Biol. Macromol. 2023, 240, 124345.
- Zhao, X.; Huang, L.; Wu, J.; Huang, Y.D.; Zhao, L.; Wu, N.; Zhou, W.Q.; Hao, D.X.; Ma, G.H.; Su, Z.G. Fabrication of rigid and macroporous agarose microspheres by pre-cross-linking and surfactant micelles swelling method. Colloids Surf. B-Biointerfaces 2019, 182, 110377.
- Iftekhar, S.; Ovbude, S.T.; Hage, D.S. Kinetic Analysis by Affinity Chromatography. Front. Chem. 2019, 7, 673.
- Behar, G.; Renodon-Corniere, A.; Mouratou, B.; Pecorari, F. Affitins as robust tailored reagents for affinity chromatography purification of antibodies and non-immunoglobulin proteins. J. Chromatogr. A 2016, 1441, 44–51.
- Yin, J.L.; Zheng, H.W.; Lin, H.; Sui, J.X.; Wang, B.C.; Pavase, T.R.; Cao, L.M. Boronic acid-functionalized agarose affinity chromatography for isolation of tropomyosin in fishes. J. Sci. Food Agric. 2019, 99, 6490–6499.
- Fang, Y.M.; Lin, D.Q.; Yao, S.J. Review on biomimetic affinity chromatography with short peptide ligands and its application to protein purification. J. Chromatogr. A 2018, 1571, 1–15.
- Yi, Y.; Shi, K.F.; Ding, S.W.; Hu, J.M.; Zhang, C.; Mei, J.F.; Ying, G.Q. A general strategy for protein affinity-ligand oriented-immobilization and screening for bioactive compounds. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2023, 1218, 123591.
- Bai, Y.L.; Yang, S.T. Production and separation of formate dehydrogenase from Candida boidinii. Enzyme Microb. Technol. 2007, 40, 940–946.
- Hou, X.L.; Li, X.W.; Ding, S.Y.; He, J.H.; Jiang, H.Y.; Shen, J.Z. Simultaneous analysis of avermectins in bovine tissues by LC-MS-MS with immunoaffinity chromatography cleanup. Chromatographia 2006, 63, 543–550.
- Zheng, H.W.; Wang, C.Y.; Pavase, T.R.; Xue, C.H. Fabrication of copolymer brushes grafted superporous agarose gels: Towards the ultimate ideal particles for efficient affinity chromatography. Colloids Surf. B-Biointerfaces 2022, 217, 112705.
- Barth, H.G. Size Exclusion Chromatography: A Teaching Aid for Physical Chemistry. J. Chem. Educ. 2018, 95, 1125–1131.
- Zhao, L.; Huang, L.; Huang, Y.D.; Zhu, K.; Che, X.J.; Du, Y.X.; Gao, J.W.; Hao, D.X.; Zhang, R.Y.; Wang, Q.B.; et al. Preparation and structural regulation of macroporous agarose microspheres for highly efficient adsorption of giant biomolecules. Colloid. Polym. Sci. 2022, 300, 691–705.
- Zhao, L.; Che, X.J.; Huang, Y.D.; Zhu, K.; Du, Y.X.; Gao, J.W.; Zhang, R.Y.; Zhang, Y.Q.; Ma, G.H. Regulation on both pore structure and pressure-resistant property of uniform agarose microspheres for high-resolution chromatography. J. Chromatogr. A 2022, 1681, 463461.
- Zhao, T.; Zhang, C.; Ma, W.D.; Xiong, Y.; Yao, J.; Yan, G.Q.; Chen, G.; Lu, H.J. A practical approach to enrich intact tryptic N-glycopeptides through size exclusion chromatography and hydrophilicity (SELIC) using an acrylamide-agarose composite gel system. Anal. Chim. Acta 2019, 1058, 107–116.
- Stone, M.C.; Tao, Y.Y.; Carta, G. Protein adsorption and transport in agarose and dextran-grafted agarose media for ion exchange chromatography: Effect of ionic strength and protein characteristics. J. Chromatogr. A 2009, 1216, 4465–4474.
- Zhang, Z.R.; Zhou, S.Y.; Han, L.J.; Zhang, Q.Y.; Pritts, W.A. Impact of linker-drug on ion exchange chromatography separation of antibody-drug conjugates. Mabs 2019, 11, 1113–1121.
- Apolinar-Valiente, R.; Williams, P.; Nigen, M.; Tamayo, V.M.; Doco, T.; Sanchez, C. Fractionation of Acacia seyal gum by ion exchange chromatography. Food Hydrocoll. 2020, 98, 105283.
- Kristl, A.; Luksic, M.; Pompe, M.; Podgornik, A. Effect of Pressure Increase on Macromolecules’ Adsorption in Ion Exchange Chromatography. Anal. Chem. 2020, 92, 4527–4534.
- Oksanen, H.M.; Domanska, A.; Bamford, D.H. Monolithic ion exchange chromatographic methods for virus purification. Virology 2012, 434, 271–277.
- Cao, Y.L.; Ding, Y.Y.; Zhang, L.P.; Shi, G.; Sang, X.X.; Ni, C.H. Preparation of surface-modified, micrometer-sized carboxymethyl chitosan drug-loaded microspheres. J. Appl. Polym. Sci. 2018, 135, 45731.
- Ljunglof, A.; Lacki, K.M.; Mueller, J.; Harinarayan, C.; van Reis, R.; Fahrner, R.; Van Alstine, J.M. Ion exchange chromatography of antibody fragments. Biotechnol. Bioeng. 2007, 96, 515–524.
- Silva-Santos, A.R.; Paulo, P.M.R.; Prazeres, D.M.F. Scalable purification of single stranded DNA scaffolds for biomanufacturing DNA-origami nanostructures: Exploring anion-exchange and multimodal chromatography. Sep. Purif. Technol. 2022, 298, 121623.
- Barroca-Ferreira, J.; Goncalves, A.M.; Santos, M.F.A.; Santos-Silva, T.; Maia, C.J.; Passarinha, L.A. A chromatographic network for the purification of detergent-solubilized six-transmembrane epithelial antigen of the prostate 1 from Komagataella pastoris mini-bioreactor lysates. J. Chromatogr. A 2022, 1685, 463576.
- Li, X.; Liu, Y.; Sun, Y. Development of poly(methacrylate)-grafted Sepharose FF for cation-exchange chromatography of proteins. J. Chromatogr. A 2020, 1634, 461669.
- Li, M.; Liu, Q.; Cui, Y.; Li, D.; Wang, H.; Ng, T.B. Isolation and Characterization of a Phaseolus vulgaris Trypsin Inhibitor with Antiproliferative Activity on Leukemia and Lymphoma Cells. Molecules 2017, 22, 187.
- Lienqueo, M.E.; Salazar, O.; Henriquez, K.; Calado, C.R.C.; Fonseca, L.P.; Cabral, J.M.S. Prediction of retention time of cutinases tagged with hydrophobic peptides in hydrophobic interaction chromatography. J. Chromatogr. A 2007, 1154, 460–463.
- Gustavsson, P.E.; Axelsson, A.; Larsson, P.O. Superporous agarose beads as a hydrophobic interaction chromatography support. J. Chromatogr. A 1999, 830, 275–284.
- Hall, T.; Kelly, G.M.; Emery, W.R. Use of mobile phase additives for the elution of bispecific and monoclonal antibodies from phenyl based hydrophobic interaction chromatography resins. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2018, 1096, 20–30.
- Rodler, A.; Ueberbacher, R.; Beyer, B.; Jungbauer, A. Calorimetry for studying the adsorption of proteins in hydrophobic interaction chromatography. Prep. Biochem. Biotechnol. 2019, 49, 1–20.
- Fekete, S.; Murisier, A.; Verscheure, L.; Sandra, K.; Guillarme, D. Hydrophobic Interaction Chromatography (HIC) for the Characterization of Therapeutic Monoclonal Antibodies and Related Products, Part 2: Practical Considerations. LC GC Eur. 2021, 34, 139–148.
- Wang, L.H.; Fu, Q.X.; Yu, J.Y.; Liu, L.F.; Ding, B. Nanoparticle-doped polystyrene/polyacrylonitrile nanofiber membrane with hierarchical structure as promising protein hydrophobic interaction chromatography media. Compos. Commun. 2019, 16, 33–40.
- Ren, K.; Li, Y.; Shi, F.; Wang, X.Y. Separation of lipopolysaccharides containing different fatty acid chains using hydrophobic interaction chromatography. Anal. Methods 2012, 4, 838–843.
- Brandts, P.M.; Middelkoop, C.M.; Gelsema, W.J.; Deligny, C.L. Hydrophobic Interaction Chromatography of Simple Compounds on Alkyl-Agaroses with Different Alkyl Chain Lengths and Chain Densities—Mechanism and Thermodynamics. J. Chromatogr. 1986, 356, 247–259.
- Mehta, A.; Grover, C.; Gupta, R. Purification of lipase from Aspergillus fumigatus using Octyl Sepharose column chromatography and its characterization. J. Basic Microbiol. 2018, 58, 857–866.
- Holkova, I.; Rauova, D.; Mergova, M.; Bezakova, L.; Mikus, P. Purification and Product Characterization of Lipoxygenase from Opium Poppy Cultures (Papaver somniferum L.). Molecules 2019, 24, 4268.
- Ghosh, S.; Saraswathi, A.; Indi, S.S.; Hoti, S.L.; Vasan, H.N. , Structure in Agarose Matrix as Hybrid: Synthesis, Characterization, and Antimicrobial Activity. Langmuir 2012, 28, 8550–8561.
- Li, M.; Neoh, K.G.; Kang, E.T.; Lau, T.; Chiong, E. Surface Modifi cation of Silicone with Covalently Immobilized and Crosslinked Agarose for Potential Application in the Inhibition of Infection and Omental Wrapping. Adv. Funct. Mater. 2014, 24, 1631–1643.
- Chen, X.Y.; Li, H.J.; Qiao, X.N.; Jiang, T.Z.; Fu, X.; He, Y.; Zhao, X. Agarose oligosaccharide- silver nanoparticle- antimicrobial peptide-composite for wound dressing. Carbohydr. Polym. 2021, 269, 118258.
- Stickler, D.J.; Lear, J.C.; Morris, N.S.; Macleod, S.M.; Downer, A.; Cadd, D.H.; Feast, W.J. Observations on the adherence of Proteus mirabilis onto polymer surfaces. J. Appl. Microbiol. 2006, 100, 1028–1033.
- Wers, E.; Lefeuvre, B. New hybrid agarose/Cu-Bioglass® biomaterials for antibacterial coatings. Korean J. Chem. Eng. 2017, 34, 2241–2247.
- Li, W.T.; Huang, Z.X.; Cai, R.; Yang, W.; He, H.W.; Wang, Y.J. Rational Design of Ag/ZnO Hybrid Nanoparticles on Sericin/Agarose Composite Film for Enhanced Antimicrobial Applications. Int. J. Mol. Sci. 2021, 22, 105.
- Li, M.; Mitra, D.; Kang, E.T.; Lau, T.; Chiong, E.; Neoh, K.G. Thiol-ol Chemistry for Grafting of Natural Polymers to Form Highly Stable and Efficacious Antibacterial Coatings. ACS Appl. Mater. Interfaces 2017, 9, 1847–1857.
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536.
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53.
- Demchenko, D.V.; Pozharitskaya, O.N.; Shikov, A.N.; Flisyuk, E.V.; Rusak, A.V.; Makarov, V.G. Rheological Study of Agar Hydrogels for Soft Capsule Shells. Pharm. Chem. J. 2014, 47, 556–558.
- Felfel, R.M.; Gideon-Adeniyi, M.J.; Hossain, K.M.Z.; Roberts, G.A.F.; Grant, D.M. Structural, mechanical and swelling characteristics of 3D scaffolds from chitosan-agarose blends. Carbohyd. Polym. 2019, 204, 59–67.
- Ribba, L.; Garcia, N.L.; D’Accorso, N.; Goyanes, S. Chapter 3—Disadvantages of Starch-Based Materials, Feasible Alternatives in Order to Overcome These Limitations. In Starch-Based Materials in Food Packaging; Villar, M.A., Barbosa, S.E., García, M.A., Castillo, L.A., López, O.V., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 37–76.
- Devi, L.S.; Das, A.J.; Das, A.B. Characterization of high amylose starch-microcrystalline cellulose based floatable gel for enhanced gastrointestinal retention and drug delivery. Carbohydr. Polym. Technol. Appl. 2022, 3, 100185.
- Shikov, A.; Pozharitskaya, O.; Makarov, V.; Makarova, M. New Technology for Preparation of Herbal Extracts and Soft Halal Capsules on its Base. Am. -Eurasian J. Sustain. Agric. 2009, 3, 130–134.
- Awadhiya, A.; Tyeb, S.; Rathore, K.; Verma, V. Agarose bioplastic-based drug delivery system for surgical and wound dressings. Eng. Life Sci. 2017, 17, 204–214.
- Zhang, B.; Yang, T.Y.; Wang, Q.B.; Zhang, G.F.; Huo, J.S.; Huang, J.; Wang, L.Y. Fabrication of uniform alginate-agarose microcapsules loading FeSO4 using water-oil-water-oil multiple emulsions system combined with premix membrane emulsification technique. Colloids Surf. A-Physicochem. Eng. Asp. 2016, 498, 128–138.
- Yazdi, M.K.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; et al. Agarose-based biomaterials for advanced drug delivery. J. Control Release 2020, 326, 523–543.
- Pozharitskaya, O.; Shikov, A.; Demchenko, D.; Flisyuk, E.; Makarov, V. Effect of Plasticizers on Moisture Absorption and Mechanical Properties of Agar Films. Farmatsiya 2017, 66, 18–23.
- Haglund, B.O.; Upadrashta, S.M.; Neau, S.H.; Cutrera, M.A. Dissolution Controlled Drug-Release from Agarose Beads. Drug Dev. Ind. Pharm. 1994, 20, 947–959.
- Sakai, S.; Kawabata, K.; Tanaka, S.; Harimoto, N.; Hashimoto, I.; Mu, C.J.; Salmons, B.; Ijima, H.; Kawakami, K. Subsieve-size agarose capsules enclosing ifosfamide-activating cells: A strategy toward chemotherapeutic targeting to tumors. Mol. Cancer Ther. 2005, 4, 1786–1790.
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011.
- Rossi, F.; Santoro, M.; Casalini, T.; Veglianese, P.; Masi, M.; Perale, G. Characterization and Degradation Behavior of Agar-Carbomer Based Hydrogels for Drug Delivery Applications: Solute Effect. Int. J. Mol. Sci. 2011, 12, 3394–3408.
This entry is offline, you can click here to edit this entry!
