There is an enormous body of literature that has identified the intervertebral disc as a potent pain generator. However, with regard to lumbar degenerative disc disease, the specific diagnostic criteria lack clarity and fail to capture the primary components which include axial midline low back pain with or without non-radicular/non-sciatic referred leg pain in a sclerotomal distribution. In fact, there is no specific ICD-10-CM diagnostic code to classify and define discogenic pain as a unique source of pain distinct from other recognized sources of chronic low back pain including facetogenic, neurocompressive including herniation and/or stenosis, sacroiliac, vertebrogenic, and psychogenic. All of these other sources have well-defined ICD-10-CM codes. Corresponding codes for discogenic pain remain absent from the diagnostic coding vernacular. The International Society for the Advancement of Spine Surgery (ISASS) has proposed a modernization of ICD-10-CM codes to specifically define pain associated with lumbar and lumbosacral degenerative disc disease. The proposed codes would also allow the pain to be characterized by location: lumbar region only, leg only, or both. Successful implementation of these codes would benefit both physicians and payers in distinguishing, tracking, and improving algorithms and treatments for discogenic pain associated with intervertebral disc degeneration.
- lumbar
- discogenic
- pain
- referred
- diagnosis
- reimbursement
1. Introduction
2. Pathoetiology of Discogenic Pain
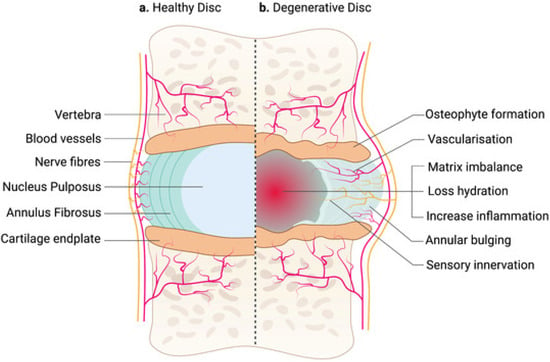
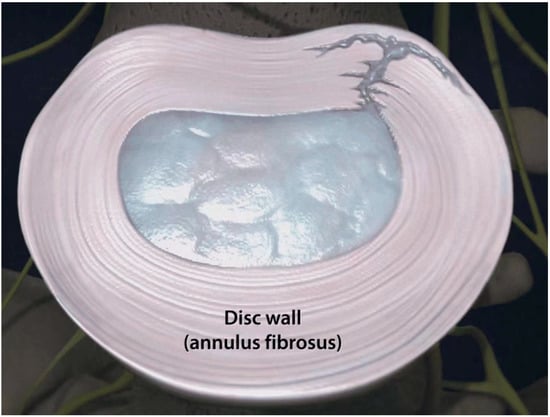
3. Clinical Presentation
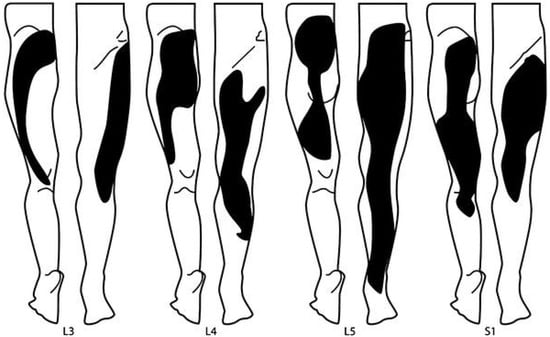
4. Diagnostic Criteria
5. Refining the Definition of Discogenic Pain: A Proposal
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Alini, M.; Diwan, A.D.; Erwin, W.M.; Little, C.B.; Melrose, J. An update on animal models of intervertebral disc degeneration and low back pain: Exploring the potential of artificial intelligence to improve research analysis and development of prospective therapeutics. JOR Spine 2023, 6, e1230. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N. The Lumbar Disc and Low Back Pain. Neurosurg. Clin. N. Am. 1991, 2, 791–806. [Google Scholar] [CrossRef]
- Bogduk, N.; Aprill, C.; Derby, R. Lumbar Discogenic Pain: State-of-the-Art Review. Pain Med. 2013, 14, 813–836. [Google Scholar] [CrossRef] [PubMed]
- Diwan, A.D.; Melrose, J. Intervertebral disc degeneration and how it leads to low back pain. JOR Spine 2022, 6, e1231. [Google Scholar] [CrossRef] [PubMed]
- Fardon, D.F.; Milette, P.C. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine 2001, 26, E93–E113. [Google Scholar] [CrossRef]
- Fine, N.; Lively, S.; Séguin, C.A.; Perruccio, A.V.; Kapoor, M.; Rampersaud, R. Intervertebral disc degeneration and osteoarthritis: A common molecular disease spectrum. Nat. Rev. Rheumatol. 2023, 19, 136–152. [Google Scholar] [CrossRef]
- Fujii, K.; Yamazaki, M.; Kang, J.D.; Risbud, M.V.; Cho, S.K.; A Qureshi, S.; Hecht, A.C.; Iatridis, J.C. Discogenic Back Pain: Literature Review of Definition, Diagnosis, and Treatment. JBMR Plus 2019, 3, e10180. [Google Scholar] [CrossRef] [PubMed]
- García-Cosamalón, J.; Del Valle, M.E.; Calavia, M.G.; García-Suárez, O.; López-Muñiz, A.; Otero, J.; Vega, J.A. Intervertebral disc, sensory nerves and neurotrophins: Who is who in discogenic pain? J. Anat. 2010, 217, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hadjipavlou, A.G.; Tzermiadianos, M.N.; Bogduk, N.; Zindrick, M.R. The pathophysiology of disc degeneration: A critical review. J. Bone Joint Surg. Br. 2008, 90, 1261–1270. [Google Scholar] [CrossRef]
- Haefeli, M.; Kalberer, F.; Saegesser, D.; Nerlich, A.G.; Boos, N.; Paesold, G. The Course of Macroscopic Degeneration in the Human Lumbar Intervertebral Disc. Spine 2006, 31, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Han, C.S.; Hancock, M.J.; Sharma, S.; Sharma, S.; Harris, I.A.; Cohen, S.P.; Magnussen, J.; Maher, C.G.; Traeger, A.C. Low back pain of disc, sacroiliac joint, or facet joint origin: A diagnostic accuracy systematic review. Eclinicalmedicine 2023, 59, 101960. [Google Scholar] [CrossRef]
- Hurri, H.; Karppinen, J. Discogenic pain. Pain 2004, 112, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Kallewaard, J.W.; Terheggen, M.A.; Groen, G.J.; Sluijter, M.E.; Derby, R.; Kapural, L.; Mekhail, N.; van Kleef, M. 15. Discogenic low back pain. Pain Pract. 2010, 10, 560–579. [Google Scholar] [CrossRef] [PubMed]
- Kushchayev, S.V.; Glushko, T.; Jarraya, M.; Schuleri, K.H.; Preul, M.C.; Brooks, M.L.; Teytelboym, O.M. ABCs of the degenerative spine. Insights Imaging 2018, 9, 253–274. [Google Scholar] [CrossRef]
- Malik, K.M.; Cohen, S.P.; Walega, D.R.; Benzon, H.T. Diagnostic criteria and treatment of discogenic pain: A systematic review of recent clinical literature. Spine J. 2013, 13, 1675–1689. [Google Scholar] [CrossRef]
- Manchikanti, L.; Singh, V.; Pampati, V.; Damron, K.S.; Barnhill, R.C.; Beyer, C.; A Cash, K. Evaluation of the relative contributions of various structures in chronic low back pain. Pain Physician 2001, 4, 308–316. [Google Scholar] [CrossRef]
- Mohd Isa, I.L.; Teoh, S.L.; Mohd Nor, N.H.; Mokhtar, S.A. Discogenic Low Back Pain: Anatomy, Pathophysiology and Treatments of Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2002, 24, 208. [Google Scholar] [CrossRef]
- Peng, B.; Hao, J.; Hou, S.; Wu, W.; Jiang, D.; Fu, X.; Yang, Y. Possible Pathogenesis of Painful Intervertebral Disc Degeneration. Spine 2006, 31, 560–566. [Google Scholar] [CrossRef]
- Peng, B.; Wu, W.; Hou, S.; Li, P.; Zhang, C.; Yang, Y. The pathogenesis of discogenic low back pain. J. Bone Joint Surg. Br. 2005, 87, 62–67. [Google Scholar] [CrossRef]
- Ruffilli, A.; Viroli, G.; Neri, S.; Traversari, M.; Barile, F.; Manzetti, M.; Assirelli, E.; Ialuna, M.; Vita, F.; Faldini, C. Mechanobiology of the Human Intervertebral Disc: Systematic Review of the Literature and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 2728. [Google Scholar] [CrossRef] [PubMed]
- Vernon-Roberts, B.; Moore, R.J.; Fraser, R.D. The Natural History of Age-related Disc Degeneration. The pathology and sequelae of tears. Spine 2007, 32, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Kuslich, S.D.; Ulstrom, C.L.; Michael, C.J. The tissue origin of low back pain and sciatica: A report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop. Clin. N. Am. 1991, 22, 181–187. [Google Scholar] [CrossRef]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Zehra, U.; Bow, C.; Lotz, J.C.; Williams, F.M.K.; Rajasekaran, S.; Karppinen, J.; Luk, K.D.K.; Battiê, M.C.; Samartzis, D. Structural vertebral endplate nomenclature and etiology: A study by the ISSLS Spinal Phenotype Focus Group. Eur. Spine J. 2018, 27, 2–12. [Google Scholar] [CrossRef]
- Rode, D. Coding standards remain AHIMA priority in 2002. J. AHIMA 2002, 73, 18. [Google Scholar]
- Deyo, R.A.; Cherkin, D.; Conrad, D.; Volinn, E. Cost, controversy, crisis: Low back pain and the health of the public. Annu. Rev. Public Health 1991, 12, 141–156. [Google Scholar] [CrossRef]
- Brodke, D.S.; Ritter, S.M. Nonoperative management of low back pain and lumbar disc degeneration. J. Bone Joint Surg. Am. 2004, 86-A, 1810–1818. [Google Scholar] [CrossRef]
- Guzman, J.; Esmail, R.; Karjalainen, K.; Malmivaara, A.; Irvin, E.; Bombardier, C. Multidisciplinary rehabilitation for chronic low back pain: Systematic review. BMJ 2001, 322, 1511–1516. [Google Scholar] [CrossRef]
- Carey, T.S.; Garrett, J.M.; Jackman, A.M. Beyond the good prognosis. Examination of an inception cohort of patients with chronic low back pain. Spine 2000, 25, 115–120. [Google Scholar] [CrossRef]
- Nachemson, A.L. Newest knowledge of low back pain. A critical look. Clin. Orthop. 1992, 279, 8–20. [Google Scholar] [CrossRef]
- Christenson, P.C. The radiologic study of the normal spine: Cervical, thoracic, lumbar, and sacral. Radiol. Clin. N. Am. 1977, 15, 133–154. [Google Scholar] [CrossRef] [PubMed]
- Thurston, A.J.; Harris, J.D. Normal Kinematics of the Lumbar Spine and Pelvis. Spine 1983, 8, 199–205. [Google Scholar] [CrossRef] [PubMed]
- White, A.A., 3rd; Panjabi, M.M. The Basic Kinematics of the Human Spine. A review of past and current knowledge. Spine 1978, 3, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.B.; Caterson, B.; Eisenstein, S.M.; Hynds, D.; Snow, D.M.; Roberts, S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002, 46, 2658–2664. [Google Scholar] [CrossRef]
- Johnson, W.E.B.; Caterson, B.; Eisenstein, S.M.; Roberts, S. Human Intervertebral Disc Aggrecan Inhibits Endothelial Cell Adhesion and Cell Migration In Vitro. Spine 2005, 30, 1139–1147. [Google Scholar] [CrossRef]
- Kepler, C.K.; Ponnappan, R.K.; Tannoury, C.A.; Risbud, M.V.; Anderson, D.G. The molecular basis of intervertebral disc degeneration. Spine J. 2013, 13, 318–330. [Google Scholar] [CrossRef]
- Boos, N.; Weissbach, S.; Rohrbach, H.; Weiler, C.; Spratt, K.F.; Nerlich, A.G. Classification of Age-Related Changes in Lumbar Intervertebral Discs. Spine 2002, 27, 2631–2644. [Google Scholar] [CrossRef]
- Buckwalter, J.A. Aging and Degeneration of the Human Intervertebral Disc. Spine 1995, 20, 1307–1314. [Google Scholar] [CrossRef]
- Miller, J.A.; Schmatz, C.; Schultz, A.B. Lumbar disc degeneration: Correlation with age, sex, and spine level in 600 autopsy specimens. Spine 1988, 13, 173–178. [Google Scholar] [CrossRef]
- Vergroesen, P.-P.; Kingma, I.; Emanuel, K.; Hoogendoorn, R.; Welting, T.; van Royen, B.; van Dieën, J.; Smit, T. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthr. Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Evans, H.; Trivedi, J.; Menage, J. Histology and pathology of the human intervertebral disc. J. Bone Joint Surg. Am. 2006, 88 (Suppl. 2), 10–14. [Google Scholar] [PubMed]
- Adams, M.; Freeman, B.J.C.; Morrison, H.P.; Nelson, I.W.; Dolan, P. Mechanical Initiation of Intervertebral Disc Degeneration. Spine 2000, 25, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Videman, T.; Nurminen, M. The Occurrence of Anular Tears and Their Relation to Lifetime Back Pain History: A Cadaveric Study Using Barium Sulfate Discography. Spine 2004, 29, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.-J.; Cui, H.; Pan, H.; MC Cheung, K.; Cao, X.; Iatridis, J.C.; Zheng, Z. Painful intervertebral disc degeneration and inflammation: From laboratory evidence to clinical interventions. Bone Res. 2021, 9, 7. [Google Scholar] [CrossRef]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Thromb. Haemost. 2003, 5, 120–130. [Google Scholar] [CrossRef]
- Vo, N.V.; Hartman, R.A.; Patil, P.R.; Risbud, M.V.; Kletsas, D.; Iatridis, J.C.; Hoyland, J.A.; Le Maitre, C.L.; Sowa, G.A.; Kang, J.D. Molecular mechanisms of biological aging in intervertebral discs. J. Orthop. Res.2016, 34, 1289–1306. [Google Scholar] [CrossRef]
- Vernon-Roberts, B.; Fazzalari, N.L.; Manthey, B.A. Pathogenesis of Tears of the Anulus Investigated by Multiple-Level Transaxial Analysis of the T12-L1 Disc. Spine 1997, 22, 2641–2646. [Google Scholar] [CrossRef]
- Lotz, J.C.; Ulrich, J.A. Innervation, Inflammation, and Hypermobility May Characterize Pathologic Disc Degeneration: Review of Animal Model Data. J. Bone Jt. Surg. 2006, 88, 76–82. [Google Scholar] [CrossRef]
- Tenny, S.; Gillis, C.C. Annular Disc Tear; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Osti, O.L.; Vernon-Roberts, B.; Fraser, R.D. 1990 Volvo Award in Experimental Studies: Anulus Tears and Intervertebral Disc Degeneration. An experimental study using an animal model. Spine 1990, 15, 762–767. [Google Scholar] [CrossRef]
- Osti, O.L.; Vernon-Roberts, B.; Moore, R.; Fraser, R.D. Annular tears and disc degeneration in the lumbar spine. A post-mortem study of 135 discs. J. Bone Jt. Surg. 1992, 74, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, J.A.; Liebenberg, E.C.; Thuillier, D.U.; Lotz, J.C. Repeated Disc Injury Causes Persistent Inflammation. Spine 2007, 32, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Tessier, S.; Silagi, E.S.; Kyada, R.; Yousefi, F.; Pleshko, N.; Shapiro, I.M.; Risbud, M.V. A novel mouse model of intervertebral disc degeneration shows altered cell fate and matrix homeostasis. Matrix Biol.2018, 70, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Iatridis, J.C.; Michalek, A.J.; Purmessur, D.; Korecki, C.L. Localized Intervertebral Disc Injury Leads to Organ Level Changes in Structure, Cellularity, and Biosynthesis. Cell. Mol. Bioeng. 2009, 2, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, M.; Al-Abbasi, M.; Harding, I.; Pollintine, P.; Dolan, P.; Tarlton, J.; Adams, M.A. Annulus Fissures Are Mechanically and Chemically Conducive to the Ingrowth of Nerves and Blood Vessels. Spine 2012, 37, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N. Degenerative Joint Disease of the Spine. Radiol. Clin. N. Am. 2012, 50, 613–628. [Google Scholar] [CrossRef]
- Peng, B.; Hou, S.; Wu, W.; Zhang, C.; Yang, Y. The pathogenesis and clinical significance of a high-intensity zone (HIZ) of lumbar intervertebral disc on MR imaging in the patient with discogenic low back pain. Eur. Spine J. 2006, 15, 583–587. [Google Scholar] [CrossRef]
- Griffith, J.F.; Wang, Y.-X.J.; Antonio, G.E.; Choi, K.C.; Yu, A.; Ahuja, A.T.; Leung, P.C. Modified Pfirrmann Grading System for Lumbar Intervertebral Disc Degeneration. Spine 2007, 32, E708–E712. [Google Scholar] [CrossRef]
- Simpson, E.K.; Parkinson, I.H.; Manthey, B.; Fazzalari, N.L. Intervertebral Disc Disorganization Is Related to Trabecular Bone Architecture in the Lumbar Spine. J. Bone Miner. Res. 2001, 16, 681–687. [Google Scholar] [CrossRef]
- Coppes, M.H.; Marani, E.; Thomeer, R.T.; Groen, G.J. Innervation of "Painful" Lumbar Discs. Spine 1997, 22, 2342–2349. [Google Scholar] [CrossRef]
- Freemont, A.; Peacock, T.; Goupille, P.; Hoyland, J.; O’Brien, J.; Jayson, M. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet 1997, 350, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.E.; Evans, H.; Menage, J.; Eisenstein, S.M.; El Haj, A.; Roberts, S. Immunohistochemical Detection of Schwann Cells in Innervated and Vascularized Human Intervertebral Discs. Spine 2001, 26, 2550–2557. [Google Scholar] [CrossRef] [PubMed]
- Freemont, A.J.; Watkins, A.; Le Maitre, C.; Baird, P.; Jeziorska, M.; Knight, M.T.N.; Ross, E.R.S.; O’Brien, J.P.; Hoyland, J.A. Nerve growth factor expression and innervation of the painful intervertebral disc. J. Pathol.2002, 197, 286–292. [Google Scholar] [CrossRef]
- Fields, A.J.; Liebenberg, E.C.; Lotz, J.C. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J. 2014, 14, 513–521. [Google Scholar] [CrossRef]
- Fournier, D.E.; Kiser, P.K.; Shoemaker, J.K.; Battié, M.C.; Séguin, C.A. Vascularization of the human intervertebral disc: A scoping review. JOR Spine 2020, 3, e1123. [Google Scholar] [CrossRef]
- Groh, A.M.R.; E Fournier, D.; Battié, M.C.; Séguin, C.A. Innervation of the Human Intervertebral Disc: A Scoping Review. Pain. Med. 2021, 22, 1281–1304. [Google Scholar] [CrossRef] [PubMed]
- Inoue, G.; Ohtori, S.; Aoki, Y.; Ozawa, T.; Doya, H.; Saito, T.; Ito, T.; Akazawa, T.; Moriya, H.; Takahashi, K. Exposure of the Nucleus Pulposus to the Outside of the Anulus Fibrosus Induces Nerve Injury and Regeneration of the Afferent Fibers Innervating the Lumbar Intervertebral Discs in Rats. Spine 2006, 31, 1433–1438. [Google Scholar] [CrossRef]
- Moneta, G.B.; Videman, T.; Kaivanto, K.; Aprill, C.; Spivey, M.; Vanharanta, H.; Sachs, B.L.; Guyer, R.D.; Hochschuler, S.H.; Raschbaum, R.F.; et al. Reported Pain During Lumbar Discography As a Function of Anular Ruptures and Disc Degeneration. Spine 1994, 19, 1968–1974. [Google Scholar] [CrossRef]
- Shahraki, N.M.; Fatemi, A.; Agarwal, A.; Goel, V.K. Prediction of clinically relevant initiation and progression of tears within annulus fibrosus. J. Orthop. Res. 2016, 35, 113–122. [Google Scholar] [CrossRef]
- Weiler, C.; Nerlich, A.G.; Bachmeier, B.; Boos, N. Expression and Distribution of Tumor Necrosis Factor Alpha in Human Lumbar Intervertebral Discs: A Study in Surgical Specimen and Autopsy Controls. Spine2005, 30, 44–53. [Google Scholar] [CrossRef]
- Burke, J.G.; Watson, R.W.G.; McCormack, D.; Dowling, F.E.; Walsh, M.G.; Fitzpatrick, J.M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J. Bone Jt. Surg. 2002, 84, 196–201. [Google Scholar] [CrossRef]
- Brisby, H. Pathology and possible mechanisms of nervous system response to disc degeneration. J. Bone Joint Surg. Am. 2006, 88 (Suppl. 2), 68–71. [Google Scholar] [PubMed]
- Simon, J.; McAuliffe, M.; Shamim, F.; Vuong, N.; Tahaei, A. Discogenic Low Back Pain. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 305–317. [Google Scholar] [CrossRef]
- Schwarzer, A.C.; Aprill, C.N.; Derby, R.; Fortin, J.; Kine, G.; Bogduk, N. The Prevalence and Clinical Features of Internal Disc Disruption in Patients With Chronic Low Back Pain. Spine 1995, 20, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Rhyne, A.L.; Smith, S.E.; Wood, K.E.; Darden, B.V. Outcome of Unoperated Discogram-Positive Low Back Pain. Spine 1995, 20, 1997–1999. [Google Scholar] [CrossRef]
- Von Korff, M. Studying the Natural History of Back Pain. Spine 1994, 19, 2041S–2046S. [Google Scholar] [CrossRef]
- Turk, D.C. Understanding pain sufferers: The role of cognitive processes. Spine J. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Boswell, M.V.; Trescot, A.M.; Datta, S.; Schultz, D.M.; Hansen, H.C.; Abdi, S.; Sehgal, N.; Shah, R.V.; Singh, V.; Benyamin, R.M.; et al. Interventional techniques: Evidence-based practice guidelines in the management of chronic spinal pain. Pain. Physician 2007, 10, 7–111. [Google Scholar]
- Department of Clinical Epidemiology and Biostatistics, Mcmaster University. How to read clinical journals:, V.I.I. To understand an economic evaluation (part B). Can. Med. Assoc. J. 1984, 130, 1542–1549. [Google Scholar]
- Bogduk, N. On the definitions and physiology of back pain, referred pain, and radicular pain. Pain 2009, 147, 17–19. [Google Scholar] [CrossRef]
- DePalma, M.J.; Ketchum, J.M.; Trussell, B.S.; Saullo, T.R.; Slipman, C.W. Does the Location of Low Back Pain Predict Its Source? PMR 2011, 3, 33–39. [Google Scholar] [CrossRef] [PubMed]
- O’neill, C.W.; Kurgansky, M.E.; Derby, R.; Ryan, D.P. Disc Stimulation and Patterns of Referred Pain. Spine2002, 27, 2776–2781. [Google Scholar] [CrossRef] [PubMed]
- Stynes, S.; Konstantinou, K.; Dunn, K.M. Classification of patients with low back-related leg pain: A systematic review. BMC Musculoskelet. Disord. 2016, 17, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Stynes, S.; Konstantinou, K.; Dunn, K.M.; Lewis, M.; Hay, E.M. Reliability among clinicians diagnosing low back-related leg pain. Eur. Spine J. 2015, 25, 2734–2740. [Google Scholar] [CrossRef]
- Guyer, R.D.; Ohnmeiss, D.D. Lumbar discography. Spine J. 2003, 3 (Suppl. 3), 11S–27S. [Google Scholar] [CrossRef]
- Fritz, J.; Niemeyer, T.; Clasen, S.; Wiskirchen, J.; Tepe, G.; Kastler, B.; Nägele, T.; König, C.W.; Claussen, C.D.; Pereira, P.L. Management of Chronic Low Back Pain: Rationales, Principles, and Targets of Imaging-guided Spinal Injections. RadioGraphics 2007, 27, 1751–1771. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N. (Ed.) Lumbar disc stimulation (Provocation Discography). In Practice Guidelines for Spinal Diagnostic and Treatment Procedures; International Spine Intervention Society: San Francisco, CA, USA, 2004; pp. 20–46. [Google Scholar]
- Shah, R.V.; Everett, C.R.; McKenzie-Brown, A.; Sehgal, N. Discography as a diagnostic test for spinal pain: A systematic and narrative review. Pain. Physician 2005, 8, 187–209. [Google Scholar] [CrossRef]
- Derby, R.; Kim, B.J.; Lee, S.H.; Chen, Y.; Seo, K.S.; Aprill, C. Comparison of discographic findings in asymptomatic subject discs and the negative discs of chronic LBP patients: Can discography distinguish asymptomatic discs among morphologically abnormal discs? Spine J. 2005, 5, 389–394. [Google Scholar] [CrossRef]
- Derby, R.; Lee, S.-H.; Kim, B.-J.; Chen, Y.; Aprill, C.; Bogduk, N. Pressure-Controlled Lumbar Discography in Volunteers Without Low Back Symptoms. Pain Med. 2005, 6, 213–221. [Google Scholar] [CrossRef]
- Wolfer, L.R.; Derby, R.; Lee, J.-E.; Lee, S.-H. Systematic review of lumbar provocation discography in asymptomatic subjects with a meta-analysis of false-positive rates. Pain Physician 2008, 11, 513–538. [Google Scholar] [CrossRef]
- Alamin, T.F.; Kim, M.J.; Agarwal, V. Provocative lumbar discography versus functional anesthetic discography: A comparison of the results of two different diagnostic techniques in 52 patients with chronic low back pain. Spine J. 2011, 11, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Vanharanta, H.; Sachs, B.L.; Spivey, M.A.; Guyer, R.D.; Hochschuler, S.H.; Rashbaum, R.F.; Johnson, R.G.; Ohnmeiss, D.; Mooney, V. The Relationship of Pain Provocation to Lumbar Disc Deterioration as Seen by CT/Discography. Spine 1987, 12, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Gornet, M.F.; Eastlack, R.K.; Peacock, J.; Schranck, F.W.; Lotz, J.C. Magnetic resonance spectroscopy (MRS) identification of chemically painful lumbar discs leads to improved 6-, 12-, and 24-month outcomes for discogenic low back pain surgeries. Eur. Spine J. 2023; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
