Alkali-activated binders (AABs) are prepared from solid low- or high-calcium aluminosilicate precursors in a strongly alkaline environment, thus generating hardened binders with cement-like properties. In the contemporary literature, the issue of the necessity of distinguishing between AABs and geopolymers has been widely discussed. A geopolymer is defined as a polymer created by the partial dissolution of an aluminosilicate source in a user-friendly alkaline or acidic medium to construct three-dimensional polymeric networks. On the other hand, the mechanism for the creation of AABs is similar to that of ordinary Portland cement (OPC), where, instead of calcium silicate hydrate gel, potassium or sodium aluminosilicate hydrate is available. Davidovits makes a distinction even between a geopolymer cement and a binder, stating that cement refers to a binding system that hardens at room temperature (e.g., OPC), while a binder requires heat setting.
- alkali-activated binder
- precursor
- alkali activator
1. Introduction
The application of alkali-activated binders (AABs) was introduced due to the lack of ordinary Portland cement (OPC) availability in the post-World-War-II period [1]. Since the late 1990s, the usage of AABs has been investigated as a sustainable solution in the construction industry [2]. In the initial investigations of AABs’ environmental impacts, Davidovits stated that 1 tonne of geopolymer cement generates 0.18 tonnes of CO2, corresponding to an about five to six times lower value than OPC [3]. In comparison, Duxson et al. emphasised that the use of geopolymer binders can result in an 80% or even greater decrease in CO2 emissions compared to OPC [4].
-
One-part mixtures combine dry alkali powder, solid aluminosilicate raw material, and water. They are suitable for in situ applications due to their advantageous handling characteristics compared to two-part mixtures containing a viscous alkali solution. Since the usage and packaging of this type are similar to those of cement, its utilisation seems promising.
-
Two-part mixtures are formed by combining an aqueous alkali solution, solid aluminosilicate raw material, and water. They have been recognised as suitable for precast applications. However, they pose a disadvantage, as they require the handling of large amounts of corrosive, hazardous, and viscous alkali solutions, which has led to the development of one-part mixtures [5].
However, precursors can be naturally occurring or industrial and agricultural by-products and/or waste. They are mainly composed of SiO2, Al2O3, and CaO [6]. By-products from the energy and mining industries have received much attention with respect to their use as a potential feedstock for AABs [7]. Moreover, solid waste generated throughout mining activities has been recognised as causing severe environmental pollution, and its utilization as raw material would be highly beneficial [8]. The producers of these by-products can benefit from reductions in their required storage and rehabilitation costs if the by-product is disposed of as waste. Additionally, from their sale as raw materials, some economic profit is also possible [7]. Various types of agricultural waste have also been investigated as raw materials for producing AABs [6]. On the other hand, bulk chemicals (e.g., sodium hydroxide, sodium silicate, etc.) are commonly utilised as AAs, while there have been few investigations on the use of industrial and agricultural by-products and/or wastes as alternative alkali sources [1]. In general, alkali hydroxide (ROH), non-silicate salts of weak acids (R2CO3, R2S, and RF), and silicic salts of R2O·(n)SiO2 are widely used, where R corresponds to an alkali metal, i.e., Na, K, or Li [2].
2. Obstacles Related to Technical Performance
2.1. Precursors
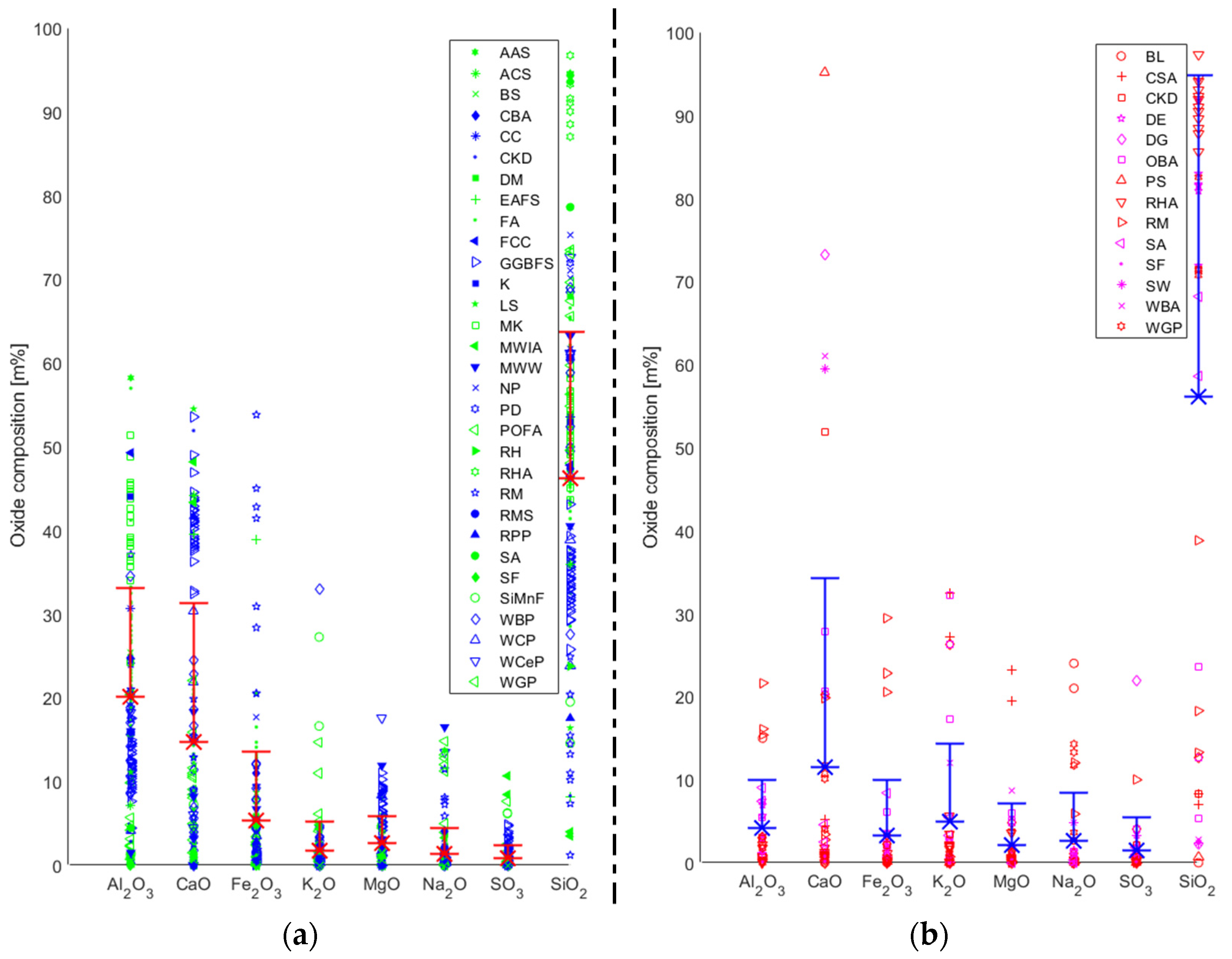
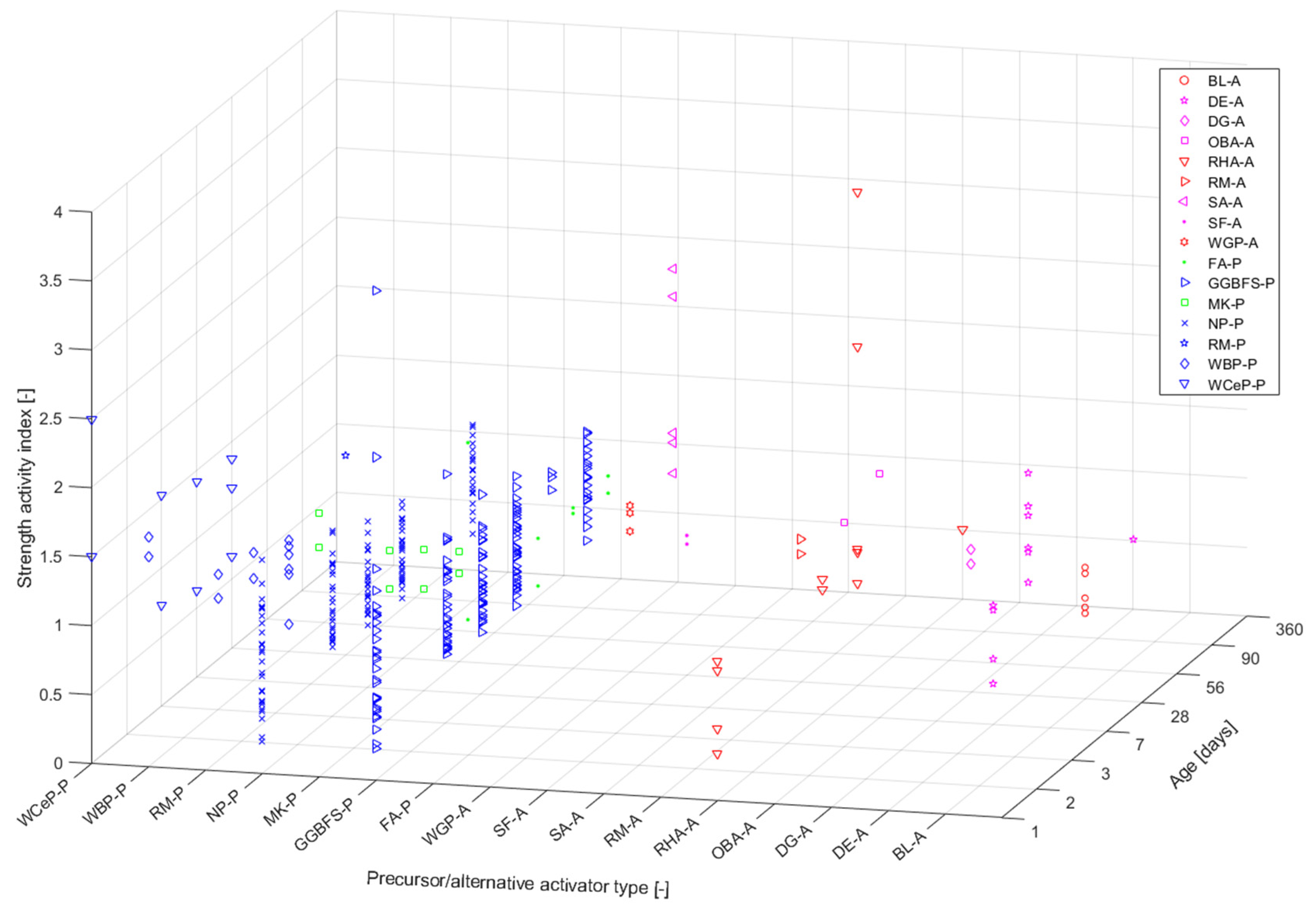
The reactivity of the precursors used to date cannot be simply based on their broad typologies. Silica and alumina from the source materials are among the most influential factors with respect to the material properties of AAB. Another oxide that significantly contributes to the reaction kinetics of AAB materials is that formed with calcium. It has been demonstrated that the workability of AAMs is reduced when a low-calcium precursor (e.g., fly ash) is partially/fully replaced by a high-calcium precursor (e.g., GGBFS). In general, the increase in the amount of calcium increases the rate of hydration (which is the opposite chemical process to polymerisation) while also consuming water. Due to these two effects, the viscosity can be increased, while the setting time can be decreased of the resulting AAM.
The reactivity of the precursors used to date cannot be simply based on their broad typologies. Silica and alumina from the source materials are among the most influential factors with respect to the material properties of AAB. Another oxide that significantly contributes to the reaction kinetics of AAB materials is that formed with calcium. It has been demonstrated that the workability of AAMs is reduced when a low-calcium precursor (e.g., fly ash) is partially/fully replaced by a high-calcium precursor (e.g., GGBFS). In general, the increase in the amount of calcium increases the rate of hydration (which is the opposite chemical process to polymerisation) while also consuming water. Due to these two effects, the viscosity can be increased, while the setting time can be decreased of the resulting AAM.
Moreover, heat curing is usually applied to low-calcium systems because of the slow polymerisation process, which is also one of the major disadvantages of utilising this kind of AAC cast in situ. However, when GGBFS is considered, air-dry curing can be applied [2].
2.2. Alkali Activators
3. Obstacles Related to Environmental Performance
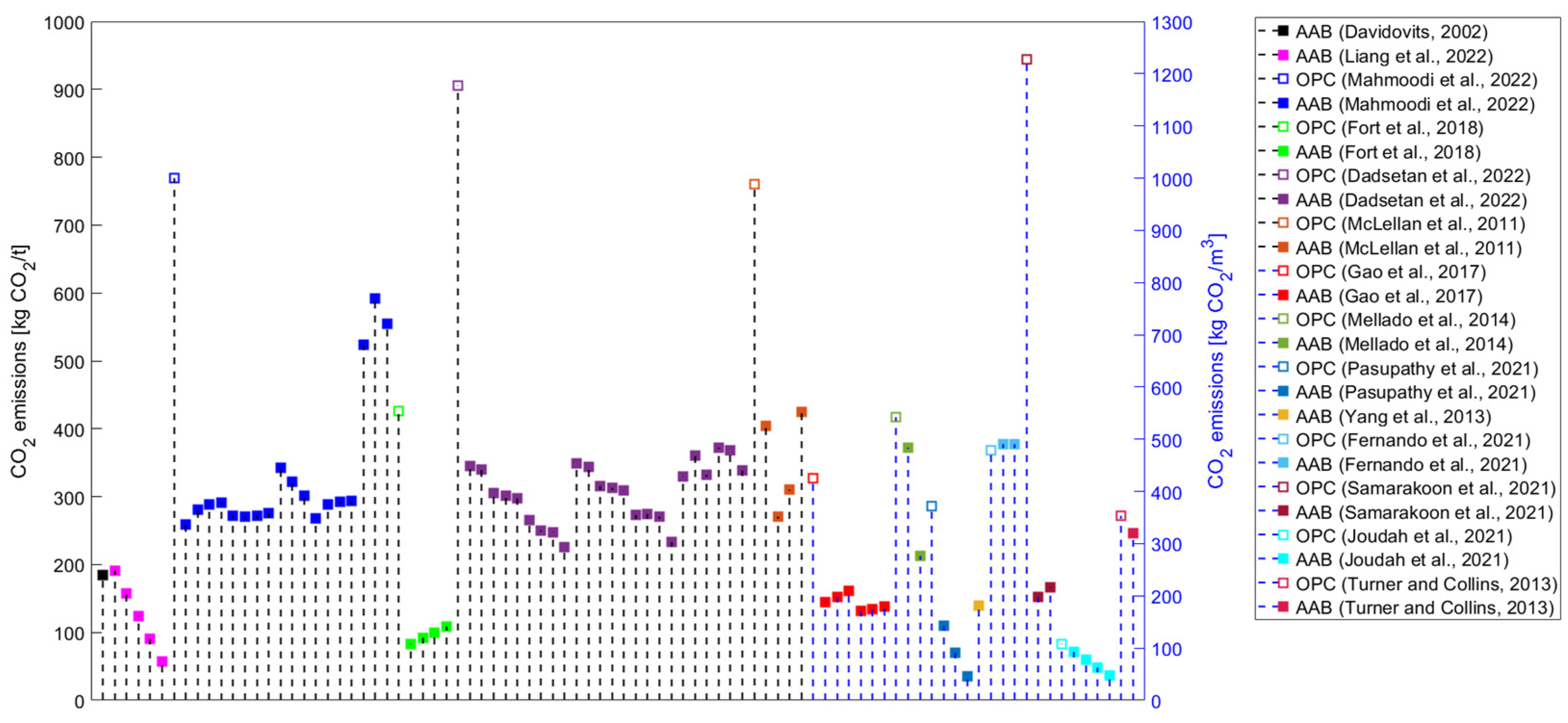
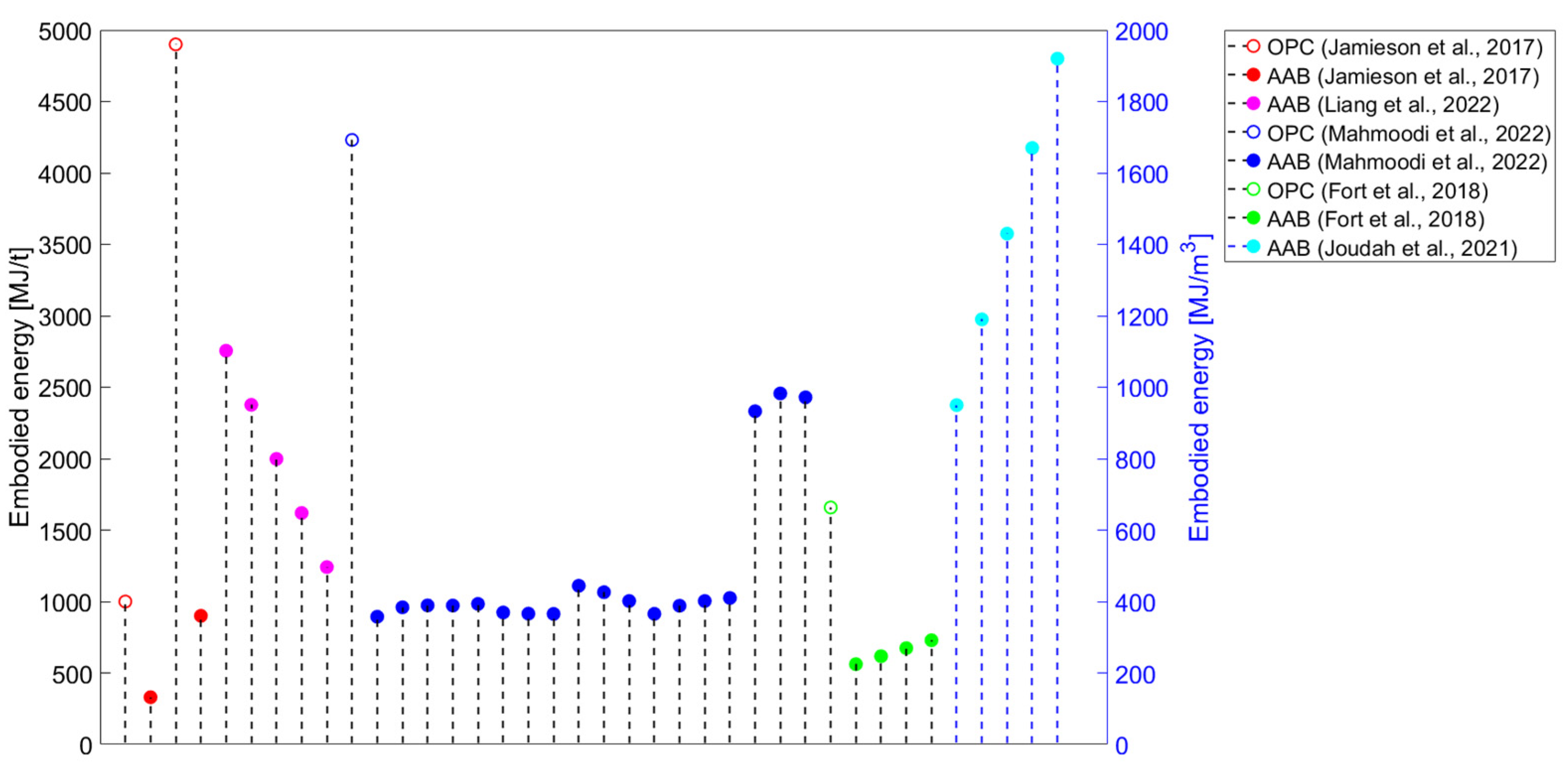
3.1. Precursors
3.2. Alkali Activators
3.3. Regional Context
4. Obstacles Related to Economic Performance
4.1. Precursors
4.2. Alkali Activators
4.3. Regional Context
This entry is adapted from the peer-reviewed paper 10.3390/ma16083121
References
- Adesanya, E.; Perumal, P.; Luukkonen, T.; Yliniemi, J.; Ohenoja, K.; Kinnunen, P.; Illikainen, M. Opportunities to Improve Sustainability of Alkali-Activated Materials: A Review of Side-Stream Based Activators. J. Clean. Prod. 2021, 286, 125558.
- Yang, K.-H.; Song, J.-K.; Song, K.-I. Assessment of CO2 Reduction of Alkali-Activated Concrete. J. Clean. Prod. 2013, 39, 265–272.
- Davidovits, J. Environmentally Driven Geopolymer Cement Applications. In Proceedings of the Geopolymer 2002 International Conference, Melbourne, Australia, 28 October 2002.
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The Role of Inorganic Polymer Technology in the Development of ‘Green Concrete’. Cem. Concr. Res. 2007, 37, 1590–1597.
- Abdulkareem, M.; Havukainen, J.; Nuortila-Jokinen, J.; Horttanainen, M. Environmental and Economic Perspective of Waste-Derived Activators on Alkali-Activated Mortars. J. Clean. Prod. 2021, 280, 124651.
- Ibrahim, M.; Maslehuddin, M. An Overview of Factors Influencing the Properties of Alkali-Activated Binders. J. Clean. Prod. 2021, 286, 124972.
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and Carbon Emissions for Geopolymer Pastes in Comparison to Ordinary Portland Cement. J. Clean. Prod. 2011, 19, 1080–1090.
- Jiao, H.; Yang, W.; Ruan, Z.; Yu, J.; Liu, J.; Yang, Y. The Micro-Scale Mechanism of Tailings Thickening Processing from Metal Mines. Int. J. Miner. Metall. Mater. 2022.
- Lahoti, M.; Narang, P.; Tan, K.H.; Yang, E.-H. Mix Design Factors and Strength Prediction of Metakaolin-Based Geopolymer. Ceram. Int. 2017, 43, 11433–11441.
- Sood, D.; Hossain, K.M.A. Strength, Shrinkage and Early Age Characteristics of One-Part Alkali-Activated Binders with High-Calcium Industrial Wastes, Solid Reagents and Fibers. J. Compos. Sci. 2021, 5, 315.
- Xie, T.; Visintin, P.; Zhao, X.; Gravina, R. Mix Design and Mechanical Properties of Geopolymer and Alkali Activated Concrete: Review of the State-of-the-Art and the Development of a New Unified Approach. Constr. Build. Mater. 2020, 256, 119380.
- Weil, M.; Dombrowski, K.; Buchwald, A. 10—Life-Cycle Analysis of Geopolymers. In Geopolymers; Woodhead Publishing: Sawston, UK, 2009; pp. 194–210. ISBN 978-1-84569-449-4.
- Keppert, M.; Vejmelková, E.; Bezdička, P.; Doleželová, M.; Čáchová, M.; Scheinherrová, L.; Pokorný, J.; Vyšvařil, M.; Rovnaníková, P.; Černý, R. Red-Clay Ceramic Powders as Geopolymer Precursors: Consideration of Amorphous Portion and CaO Content. Appl. Clay Sci. 2018, 161, 82–89.
- Ramagiri, K.K.; Kar, A. Environmental Impact Assessment of Alkali-Activated Mortar with Waste Precursors and Activators. J. Build. Eng. 2021, 44, 103391.
- Ameri, F.; Zareei, S.A.; Behforouz, B. Zero-Cement vs. Cementitious Mortars: An Experimental Comparative Study on Engineering and Environmental Properties. J. Build. Eng. 2020, 32, 101620.
- Sarıdemir, M.; Çelikten, S. Effect of High Temperature, Acid and Sulfate on Properties of Alkali-Activated Lightweight Aggregate Concretes. Constr. Build. Mater. 2022, 317, 125886.
- Najimi, M.; Ghafoori, N. Engineering Properties of Natural Pozzolan/Slag Based Alkali-Activated Concrete. Constr. Build. Mater. 2019, 208, 46–62.
- Fořt, J.; Vejmelková, E.; Koňáková, D.; Alblová, N.; Čáchová, M.; Keppert, M.; Rovnaníková, P.; Černý, R. Application of Waste Brick Powder in Alkali Activated Aluminosilicates: Functional and Environmental Aspects. J. Clean. Prod. 2018, 194, 714–725.
- Liang, G.; Luo, L.; Yao, W. Reusing Waste Red Brick Powder as Partial Mineral Precursor in Eco-Friendly Binders: Reaction Kinetics, Microstructure and Life-Cycle Assessment. Resour. Conserv. Recycl. 2022, 185, 106523.
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Şahmaran, M. Optimized Application of Ternary Brick, Ceramic and Concrete Wastes in Sustainable High Strength Geopolymers. J. Clean. Prod. 2022, 338, 130650.
- Gao, X.; Yu, Q.L.; Lazaro, A.; Brouwers, H.J.H. Investigation on a Green Olivine Nano-Silica Source Based Activator in Alkali Activated Slag-Fly Ash Blends: Reaction Kinetics, Gel Structure and Carbon Footprint. Cem. Concr. Res. 2017, 100, 129–139.
- Fernando, S.; Gunasekara, C.; Law, D.W.; Nasvi, M.C.M.; Setunge, S.; Dissanayake, R. Life Cycle Assessment and Cost Analysis of Fly Ash–Rice Husk Ash Blended Alkali-Activated Concrete. J. Environ. Manag. 2021, 295, 113140.
- Mellado, A.; Catalán, C.; Bouzón, N.; Borrachero, M.V.; Monzó, J.M.; Payá, J. Carbon Footprint of Geopolymeric Mortar: Study of the Contribution of the Alkaline Activating Solution and Assessment of an Alternative Route. RSC Adv. 2014, 4, 23846–23852.
- Dadsetan, S.; Siad, H.; Lachemi, M.; Mahmoodi, O.; Sahmaran, M. Optimization and Characterization of Geopolymer Binders from Ceramic Waste, Glass Waste and Sodium Glass Liquid. J. Clean. Prod. 2022, 342, 130931.
- Dadsetan, S.; Siad, H.; Lachemi, M.; Mahmoodi, O.; Sahmaran, M. Development of Ambient Cured Geopolymer Binders Based on Brick Waste and Processed Glass Waste. Environ. Sci. Pollut. Res. 2022, 29, 80755–80774.
- Pasupathy, K.; Ramakrishnan, S.; Sanjayan, J. Formulating Eco-Friendly Geopolymer Foam Concrete by Alkali-Activation of Ground Brick Waste. J. Clean. Prod. 2021, 325, 129180.
- Turner, L.K.; Collins, F.G. Carbon Dioxide Equivalent (CO2-e) Emissions: A Comparison between Geopolymer and OPC Cement Concrete. Constr. Build. Mater. 2013, 43, 125–130.
- Joudah, Z.H.; Huseien, G.F.; Samadi, M.; Shukor Lim, N.H.A. Sustainability Evaluation of Alkali-Activated Mortars Incorporating Industrial Wastes. Mater. Today Proc. 2021, 46, 1971–1977.
- Samarakoon, M.H.; Ranjith, P.G.; Hui Duan, W.; Haque, A.; Chen, B.K. Extensive Use of Waste Glass in One-Part Alkali-Activated Materials: Towards Sustainable Construction Practices. Waste Manag. 2021, 130, 1–11.
- Jamieson, E.; van Riessen, A.; McLellan, B.; Penna, B.; Kealley, C.; Nikraz, H. Introducing Bayer Liquor–Derived Geopolymers. In Handbook of Low Carbon Concrete; Elsevier: Amsterdam, The Netherlands, 2017; pp. 159–193. ISBN 978-0-12-804524-4.
- ISO 14040; Environmental Management—Life Cycle Assessment—Principles and Framework. International Organization for Standardization (ISO): Geneve, Switzerland, 2006.
- Ouellet-Plamondon, C.; Habert, G. Life Cycle Assessment (LCA) of Alkali-Activated Cements and Concretes. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015; pp. 663–686. ISBN 978-1-78242-276-1.
- Migunthanna, J.; Rajeev, P.; Sanjayan, J. Investigation of Waste Clay Brick as Partial Replacement of Geopolymer Binders for Rigid Pavement Application. Constr. Build. Mater. 2021, 305, 124787.
- Provis, J.L. Alkali-Activated Materials. Cem. Concr. Res. 2018, 114, 40–48.
- Sandanayake, M.; Gunasekara, C.; Law, D.; Zhang, G.; Setunge, S. Greenhouse Gas Emissions of Different Fly Ash Based Geopolymer Concretes in Building Construction. J. Clean. Prod. 2018, 204, 399–408.
- Nguyen, L.; Moseson, A.J.; Farnam, Y.; Spatari, S. Effects of Composition and Transportation Logistics on Environmental, Energy and Cost Metrics for the Production of Alternative Cementitious Binders. J. Clean. Prod. 2018, 185, 628–645.
- Tong, K.T.; Vinai, R.; Soutsos, M.N. Use of Vietnamese Rice Husk Ash for the Production of Sodium Silicate as the Activator for Alkali-Activated Binders. J. Clean. Prod. 2018, 201, 272–286.
- Passuello, A.; Rodríguez, E.D.; Hirt, E.; Longhi, M.; Bernal, S.A.; Provis, J.L.; Kirchheim, A.P. Evaluation of the Potential Improvement in the Environmental Footprint of Geopolymers Using Waste-Derived Activators. J. Clean. Prod. 2017, 166, 680–689.
- Davidovits, J. Geopolymer Cement a Review. Geopolymer Inst. Tech. Pap. 2013, 21, 1–11.
