Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Even in the absence of strong indications deriving from clinical studies, the removal of mediators is increasingly used in septic shock and in other clinical conditions characterized by a hyperinflammatory response. Despite the different underlying mechanisms of action, they are collectively indicated as blood purification techniques. Their main categories include blood- and plasma processing procedures, which can run in a stand-alone mode or, more commonly, in association with a renal replacement treatment.
- septic shock
- sepsis mediators
- hemofiltration
- blood purification techniques
1. Introduction
Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection [1] that is caused by the release of a huge and only partially known number of mediators produced during the interaction between the infecting germ and the patient’s immune system. The possible role of endogenous toxic substances in the pathogenesis of diseases is not a new concept, because since ancient times it was believed that many, if not all, disturbances affecting the humanity were caused by these agents. Consequently, their removal was considered an appropriate therapeutic target; with this aim, bloodletting gained wide popularity, becoming the first procedure of blood purification (BP). However, when in the second half of the 19th century it became clear that an exceedingly high number of microorganisms were responsible for many diseases previously treated with this approach, its use rapidly declined. At present, bloodletting is limited to rather uncommon conditions, including hemochromatosis and polycythemia. The modern era of BP arose in the 1940s, when Kollf et al. started to treat patients with acute or chronic kidney injury (AKI and CKI, respectively) using a cellophane membrane perfused by the patients’ blood to remove uremic toxins [2]. It is remarkable that this approach should be unacceptable in the era of evidence-based medicine (EBM) and Ethical Committees, as the first 16 patients died during the procedures or immediately thereafter and only the 17th patient survived and was discharged home [2]. Some decades later, it appeared that the systemic disturbances associated with severe infections, including fever, arterial hypotension, multiple organ failure, etc., could be ascribed more to the interaction between the host’s immune system and the infecting agent than to the latter only. Moreover, this reaction appeared to be at least partially determined by circulating factors, as the fluid removed from the bloodstream of septic and trauma patients using a cuprophan membrane was able to induce an intense proteolysis in isolated rat limbs, indicating the presence of a filtrable and transmissible factor able to cause the same muscle alterations observed in critical conditions [3]. From then on, an ever-increasing number of substances with both pro- and anti-inflammatory properties produced in these clinical circumstances have been identified [4], and it was hypothesized that their neutralization could positively influence the clinical course of sepsis and septic shock and/or other clinical conditions characterized by an uncontrolled inflammatory reaction. Conversely, in patients with a prolonged length-of-stay (LoS) in the Intensive Care Unit (ICU), these mediators are replaced or, better to say, are counterbalanced by the action of substances with anti-inflammatory capabilities, making them susceptible to infections sustained by low-virulence strains such as Acinetobacter baumanii, and to the reactivation of viruses, including Cytomegalovirus and Herpes viruses.
Aiming to neutralize the pro-inflammatory mediators, two different strategies have been developed. The first consists in the administration of inhibitors of a specific mediator or in the blockade of their cellular receptor; however, the results of many randomized controlled trials (RCTs) in ICU patients were largely below the expectations derived from experimental investigations and small Phase I human studies. However, some subgroup analyses indicated an increased survival of patients with elevated blood levels of the specific mediator targeted by the study substance. The use of inhibitors is advocated in the treatment of clinical conditions characterized by their persistent low-level production, including different rheumatologic and chronic inflammatory intestinal diseases.
The second strategy is based on the extracorporeal elimination of germ-derived substances, such as endotoxin or bloodborne mediators produced by the host via different mechanisms, including (a) their convective removal through an artificial membrane used also in Continuous Renal Replacement Treatments (CRRT) whose cutoff value is compatible with their molecular weight (MW); or (b) their adsorption on the membrane surface (Figure 1).
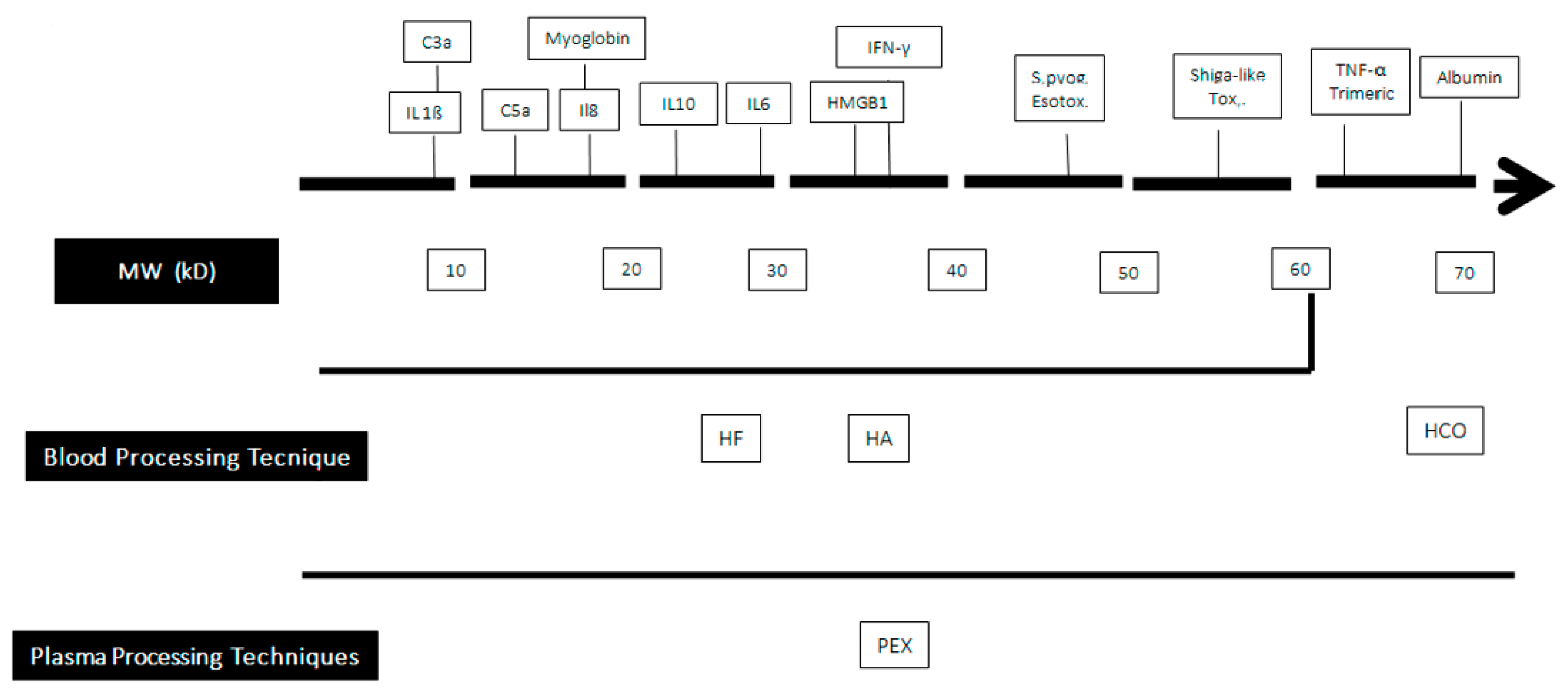
Figure 1. Clearance capabilities of blood purification techniques according to the molecular weight of some bloodborne substances. MW: Molecular Weight; HF: Hemofiltration; HA: hemadsorption; PEX: plasma exchange; HCO: high cut-off membrance.C3a: Activated Complement factor 3; IL: interleukin; HMG 1: High Mobility Group Box 1; IFN-γ: γ Interferon.
2. Rationale of Blood Purification
Different mechanisms have been hypothesized to explain the possible beneficial effects of the elimination of mediators from the bloodstream [5], including:
- (a)
-
The lowering of both pro- and anti-inflammatory mediators below a threshold level, thus limiting the associated organ damage [6];
- (b)
-
The passage of mediators from the tissues to the blood and their subsequent extracorporeal clearance along a concentration gradient [7];
- (c)
-
The restoration of a cytokine gradient between the tissues and the blood, promoting leukocyte chemotaxis [8];
- (d)
-
The interaction between the membrane and the immune cells, as demonstrated by the modulation of surface molecules during different BP procedures [9].
It is likely that multiple mechanisms (i.e., a + b), maybe in different time windows, cooperate to achieve the therapeutic effect of BP.
3. Classification and Principles of Function of the BP Techniques
As stated above, different techniques are used to clear the mediators produced during septic shock or other clinical conditions characterized by elevated levels of inflammatory mediators, such as hemophagocytic syndrome (HS). Their removal is related to the characteristic (a) of the mediators, including their MW and the chemico-physical properties; and (b) of the device used, such as the cutoff value of the membrane, its surface of contact with the substrate to be processed, and the affinity for the substance to be cleared.
Thus, BP can be considered an umbrella term covering different techniques that can be primarily subdivided into blood- and plasma-processing procedures (Table 1). The factors influencing the efficacy of the BP differ according to their principle of function. Consequently, as far as the hemofiltration (HF)-based techniques are concerned, in which the mediators are eliminated by convection, the main determinant of removal is the production of ultrafiltrate (Qf), that, in turn, depends on the blood flowing inside the filter (Qb), the size of the pores, the subsequent sieving coefficient, the surface of the membranes used, and their chemico-physical properties. In contrast, only the Qb accounts for the efficacy of the absorption-based procedures [10]. Despite these differences, both families share a more- or less-pronounced time-dependent decay of the clearance capabilities, and their use can last from 2 to 24 h before the exhaustion of the BP effect.
Table 1. Techniques used in BP. Legend: CPFA®: coupled plasma filtration and adsorption.
| Substrate | Technique/Brand | Mechanism |
|---|---|---|
| Blood | Ultrafiltration | High-volume ultrafiltration |
| High-cutoff membrane | ||
| Hemoadsorption | Toraymixin® | |
| oXyris® | ||
| Cytosorb® | ||
| Seraph® | ||
| Plasma | Plasma exchange | |
| Ultrafiltration + plasma adsorption | CPFA® |
All BP procedures require a dedicated vascular access using a large-bore catheter and anticoagulation of the extracorporeal circuit using heparin or citrate.
3.1. Blood Processing Techniques
3.1.1. Hemofiltration (HF)
HF’s principle of functioning consists in the convective removal of H2O and solutes, including mediators, from the bloodstream by means of a synthetic membrane with a cutoff value of ~50–60 kDa, which are used also in CRRTs. The ultrafiltrate (UF) produced has the same electrolyte composition as the plasma. In fact, HF is an umbrella term covering multiple strategies that take advantage of the different amounts of UF considering the therapeutic target (see below). More recently, high-cutoff (HCO) membranes have been developed, but their use is associated with high albumin losses [11]; to overcome this problem, HCO membranes can be used in the diffusive rather than the in convective mode, or by slightly reducing their pore size and surfaces [12]. Independent of the characteristics of the membrane used, the volume of UF produced is related either to the aforementioned variables and to the blood flowing over it per unit time (Qb).
3.1.2. Hemoadsorption (HA)
HA consists in the adhesion of the circulating mediators on the surface of a membrane able to capture them. Four HA techniques have been developed so far [5][10][13]. The first takes advantage of an adsorbing column containing multiple polymixin-immobilized fibers (Toraymixin®, Toray Industries, Tokyo, Japan) arrayed into a cartridge to remove the endotoxin molecules from the Qb. Due to this characteristic, its use has been advocated in the treatment of septic shock caused by Gram-negative bacteria only.
The second technique consists in a cartridge containing a synthetic resin constituted by polystyrene and divinylbenzene microbeads (Cytosorb®, Cytosorbents Corporation, Monmouth Junction, NJ, USA; Aferetica s.r.l., Bologna, Italy). The wide adsorptive surface (~40.000 m2) is able to adsorb hydrophobic pro- and anti-inflammatory mediators with MWs ranging from 5 to 60 kD. Cytosorb® represents an evolution of coupled plasma filtration and adsorption (CPFA; see below), as it uses the same binding resin that is arranged in microtubules instead that in microbeads.
The efficacy of Cytosorb® is concentration-dependent, as substances present in large concentrations are removed more efficiently than those with lower blood levels. Cytosorb® can run in a stand-alone mode or can be associated with a Continuous Renal Replacement Treatment (CRRT) or with an extracorporeal membrane oxygenation (ECMO) apparatus.
The third technique is based on a filter containing a modified AN69 membrane associated with a positively charged polyethyleneimine polymer able to absorb both endotoxin and several different septic mediators (oXiris®, Baxter, Meyzieu, France) from the bloodstream, while simultaneously providing CRRT.
The final technique consists in an HA device (Seraph 100®, ExThera Medical Corp, Martinez, CA, USA) packed with polymer beads covered with covalent end-point heparin ultra-high-MW polyethylene. This design mimics the heparan sulfate attached on the cell surface, allowing the in vitro binding of toxins, bacteria, and Antithrombin III, thus clearing them from the bloodstream [14]. Due to these properties, the US Food and Drug Administration (FDA) recently approved its use for the treatment of COVID-19 patients.
3.2. Plasma Processing Techniques
Three techniques are currently used. They include:
-
Plasmapheresis (PF), which is based on the selective removal of one or more plasma components (lipoproteins, paraproteins, etc.), and is not currently used in the treatment of septic shock;
-
Plasma exchange (PEX), consisting in the removal of one or more volumes of plasma, which is replaced with donors’ plasma or albumin. The rationale of PEX consists in the removal of “toxic substances” and the supply of a large amount of plasma components whose absence is considered responsible for the disorder (i.e., ADAMTS 13 for patients with thrombotic thrombocytopenic purpura [15]). Ideally, the best candidate substance for removal by PEX should have a high MW, small volume of distribution, long half-life, and low turnover rate [15];
-
Coupled plasma filtration and adsorption (CPFA), which basically consists in a three-step process: (1) the partial extraction of plasma from the blood via a plasma filter; (2) its processing within a cartridge, where a number of mediators are absorbed by a synthetic resin arranged in microtubules; and (3) reinfusion of the purified plasma upstream of a second filter used for continuous veno-venous hemodiafiltration in cases of concomitant AKI. The adsorptive capabilities of the resin are exhausted after 10 h, but the CRRT can continue beyond this limit by excluding the plasma processing unit.
This entry is adapted from the peer-reviewed paper 10.3390/jcm12051723
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810.
- Wieringa, F.P.; Søndergaard, H.; Ash, S. Father of Artificial Organs—The story of medical pioneer Willem J. Kolff (1911–2009). Artif. Organs 2021, 45, 1136–1140.
- Clowes, G.H., Jr.; George, B.C.; Villee, C.A., Jr.; Saravis, C.A. Muscle proteolysis induced by a circulating peptide in patients with sepsis or trauma. N. Engl. J. Med. 1983, 308, 545–552.
- Wiersinga, W.J.; Leopold, S.J.; Cranendonk, D.R.; van der Poll, T. Host innate immune responses to sepsis. Virulence 2014, 5, 36–44.
- Girardot, T.; Schneider, A.; Rimmelé, T. Blood Purification Techniques for Sepsis and Septic AKI. Semin. Nephrol. 2019, 39, 505–514.
- Ronco, C.; Tetta, C.; Mariano, F.; Wratten, M.L.; Bonello, M.; Bordoni, V.; Cardona, X.; Inguaggiato, P.; Pilotto, L.; d’Intini, V.; et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: The peak concentration hypothesis. Artif. Organs 2003, 27, 792–801.
- Lee, P.A.; Matson, J.R.; Pryor, R.W.; Hinshaw, L.B. Continuous arteriovenous hemofiltration therapy for Staphylococcus aureus-induced septicemia in immature swine. Crit. Care Med. 1993, 21, 914–924.
- Rimmelé, T.; Kellum, J.A. Clinical review: Blood purification for sepsis. Crit. Care 2011, 15, 205.
- Ma, S.; Xu, Q.; Deng, B.; Zheng, Y.; Tian, H.; Wang, L.; Ding, F. Granulocyte and monocyte adsorptive apheresis ameliorates sepsis in rats. Intensive Care Med. Exp. 2017, 5, 18.
- Monard, C.; Rimmelé, T.; Ronco, C. Extracorporeal Blood Purification Therapies for Sepsis. Blood Purif. 2019, 47 (Suppl. 3), 1–14.
- Morgera, S.; Rocktaschel, J.; Haase, M.; Lehmann, C.; von Heymann, C.; Ziemer, S.; Priem, F.; Hocher, B.; Gohl, H.; Kox, W.J.; et al. Intermittent high permeability hemofiltration in septic patients with acute renal failure. Intensive Care Med. 2003, 29, 1989–1995.
- Villa, G.; Chelazzi, C.; Morettini, E.; Zamidei, L.; Valente, S.; Caldini, A.L.; Zagli, G.; De Gaudio, A.R.; Romagnoli, S. Organ dysfunction during continuous veno-venous high cut-off hemodialysis in patients with septic acute kidney injury: A prospective observational study. PLoS ONE 2017, 12, e0172039.
- Ankawi, G.; Fan, W.; Pomarè Montin, D.; Lorenzin, A.; Neri, M.; Caprara, C.; de Cal, M.; Ronco, C. A New Series of Sorbent Devices for Multiple Clinical Purposes: Current Evidence and Future Directions. Blood Purif. 2019, 47, 94–100.
- Eden, G.; Schmidt, J.J.; Büttner, S.; Kümpers, P.; Hafer, C.; Rovas, A.; Koch, B.F.; Schmidt, B.M.W.; Kielstein, J.T. Safety and efficacy of the Seraph® 100 Microbind® Affinity Blood Filter to remove bacteria from the blood stream: Results of the first in human study. Crit. Care 2022, 17, 181.
- Bauer, P.R.; Ostermann, M.; Russell, L.; Robba, C.; David, S.; Ferreyro, B.L.; Cid, J.; Castro, P.; Juffermans, N.P.; Montini, L.; et al. Investigators. Plasma exchange in the intensive care unit: A narrative review. Intensive Care Med. 2022, 48, 1382–1396.
This entry is offline, you can click here to edit this entry!
