Camelina sativa (L.) Crantz, also called gold-of-pleasure, false flax, or linseed dodder, is an oilseed crop belonging to the tribe Camelineae of the mustard family (Brassicaceae). Camelina is a hardy plant that adapts very well to different types of soil and grows best in cool semi-arid climates. The great potential of this crop is also being exploited to obtain a sustainable feedstock for its different applications, and to improve dryland agriculture. Camelina can be used to improve the quality of foods, such as dairy products and meat, and the consumption of its oil has potential benefits for human health.
- Camelina sativa
- oilseed crops
- PUFA
- cover crop
1. Introduction
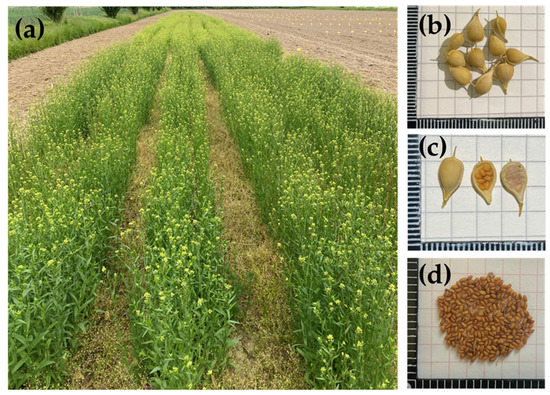
2. Cultivation
3. Uses and Potential
| Uses | Details | References | |
|---|---|---|---|
| Human nutrition | Food | [20][22][23][24][25][26] | |
| Diet supplements | |||
| Animal feed | Bird | Chicken broilers | [9][27][28][29][30][31][32] |
| Laying hens | |||
| Mammals | Cows | [33][34][35][36][37][38][39][40][40] | |
| Swine | |||
| Sheep | |||
| Rabbit | |||
| Swine | |||
| Fish | Salmon | [41][42][43][44][45][46][47][48][49] | |
| Trout | |||
| Other fish | |||
| Chemicals | Polymers | [50][51][52][53][54][55][56][57] | |
| Adhesives | |||
| Resins | |||
| Cosmetics ingredients | |||
| Fuels | Biodiesel | [1][3][5][21][57][58][59][60][61][62][63][64][65] | |
| Jet fuel | |||
This entry is adapted from the peer-reviewed paper 10.3390/plants12030570
References
- Zubr, J. Oil-Seed Crop: Camelina Sativa. Ind. Crops Prod. 1997, 6, 113–119.
- Vollmann, J.; Moritz, T.; Kargl, C.; Baumgartner, S.; Wagentristl, H. Agronomic Evaluation of Camelina Genotypes Selected for Seed Quality Characteristics. Ind. Crops Prod. 2007, 26, 270–277.
- Berti, M.; Gesch, R.; Eynck, C.; Anderson, J.; Cermak, S. Camelina Uses, Genetics, Genomics, Production, and Management. Ind. Crops Prod. 2016, 94, 690–710.
- Francis, A.; Warwick, S.I. The Biology of Canadian Weeds. 142. Camelina Alyssum (Mill.) Thell.; C. Microcarpa Andrz. Ex DC.; C. Sativa (L.) Crantz. Can. J. Plant Sci. 2009, 89, 791–810.
- Sainger, M.; Jaiwal, A.; Sainger, P.A.; Chaudhary, D.; Jaiwal, R.; Jaiwal, P.K. Advances in Genetic Improvement of Camelina sativa for Biofuel and Industrial Bio-Products. Renew. Sustain. Energy Rev. 2017, 68, 623–637.
- Ghamkhar, K.; Croser, J.; Aryamanesh, N.; Campbell, M.; Kon’kova, N.; Francis, C. Camelina (Camelina sativa (L.) Crantz) as an Alternative Oilseed: Molecular and Ecogeographic Analyses. Genome 2010, 53, 558–567.
- Falk, K.C. Camelina (Camelina Sativa). Biofuel Crops Prod. Physiol. Genet. 2013, 369–391.
- Larsson, M. Cultivation and Processing of Linum Usitatissimum and Camelina sativa in Southern Scandinavia during the Roman Iron Age. Veg. Hist. Archaeobotany 2013, 22, 509–520.
- Neupane, D.; Lohaus, R.H.; Solomon, J.K.Q.; Cushman, J.C. Realizing the Potential of Camelina sativa as a Bioenergy Crop for a Changing Global Climate. Plants 2022, 11, 772.
- Abbass, K.; Qasim, M.Z.; Song, H.; Murshed, M.; Mahmood, H.; Younis, I. A Review of the Global Climate Change Impacts, Adaptation, and Sustainable Mitigation Measures. Environ. Sci. Pollut. Res. 2022, 29, 42539–42559.
- Rostami Ahmadvandi, H.; Faghihi, A. Adapted Oilseed Crops with the Ability to Grow Economically in Dryland Conditions in Iran. Agrotech. Ind. Crops 2021, 1, 122–128.
- Gao, Y.; Jiang, C.; Zhang, Y.; Liu, L.; Wang, Y.; Kim, D.-S.; Yu, J.; Diao, J.; Wu, N.; Chen, M.; et al. Agronomic Performance of Camelina Genotypes Selected for Seed Yield and Quality Characteristics in Eastern China. Ind. Crops Prod. 2022, 184, 115077.
- Zanetti, F.; Eynck, C.; Christou, M.; Krzyżaniak, M.; Righini, D.; Alexopoulou, E.; Stolarski, M.J.; Van Loo, E.N.; Puttick, D.; Monti, A. Agronomic Performance and Seed Quality Attributes of Camelina (Camelina sativa L. Crantz) in Multi-Environment Trials across Europe and Canada. Ind. Crops Prod. 2017, 107, 602–608.
- Gore, M.; Kurt, O. Evaluation of Camelina Genotypes Grown in Winter at Different Sowing Times in Northern Turkey Ecological Conditions in Terms of Yield and Oil Ratio. Agrotech. Ind. Crops 2022, 31, 1397–1404.
- Blackshaw, R.; Johnson, E.; Gan, Y.; May, W.; McAndrew, D.; Barthet, V.; McDonald, T.; Wispinski, D. Alternative Oilseed Crops for Biodiesel Feedstock on the Canadian Prairies. Can. J. Plant Sci. 2011, 91, 889–896.
- Berti, M.; Gesch, R.; Johnson, B.; Ji, Y.; Seames, W.; Aponte, A. Double- and Relay-Cropping of Energy Crops in the Northern Great Plains, USA. Ind. Crops Prod. 2015, 75, 26–34.
- Masella, P.; Martinelli, T.; Galasso, I. Agronomic Evaluation and Phenotypic Plasticity of Camelina sativa Growing in Lombardia, Italy. Crop Pasture Sci. 2014, 65, 453–460.
- Estakhr, A.; Ranjbar, G. The Preliminary Study of Camelina Compatibility as a New Oil Crop in the Temperate Region of Fars Province. Agrotech. Ind. Crops 2021, 1, 77–84.
- Zubr, J. Qualitative Variation of Camelina sativa Seed from Different Locations. Ind. Crops Prod. 2003, 17, 161–169.
- Sydor, M.; Kurasiak-Popowska, D.; Stuper-Szablewska, K.; Rogoziński, T. Camelina Sativa. Status Quo and Future Perspectives. Ind. Crops Prod. 2022, 187, 115531.
- Matteo, R.; D’Avino, L.; Ramirez-Cando, L.J.; Pagnotta, E.; Angelini, L.G.; Spugnoli, P.; Tavarini, S.; Ugolini, L.; Foschi, L.; Lazzeri, L. Camelina (Camelina sativa L. Crantz) under Low-Input Management Systems in Northern Italy: Yields, Chemical Characterization and Environmental Sustainability. Ital. J. Agron. 2020, 15, 132–143.
- Tejera, N.; Vauzour, D.; Betancor, M.B.; Sayanova, O.; Usher, S.; Cochard, M.; Rigby, N.; Ruiz-Lopez, N.; Menoyo, D.; Tocher, D.R.; et al. A Transgenic Camelina sativa Seed Oil Effectively Replaces Fish Oil as a Dietary Source of Eicosapentaenoic Acid in Mice. J. Nutr. 2016, 146, 227–235.
- West, A.L.; Miles, E.A.; Lillycrop, K.A.; Napier, J.A.; Calder, P.C.; Burdge, G.C. Genetically Modified Plants Are an Alternative to Oily Fish for Providing N-3 Polyunsaturated Fatty Acids in the Human Diet: A Summary of the Findings of a Biotechnology and Biological Sciences Research Council Funded Project. Nutr. Bull. 2021, 46, 60–68.
- Dharavath, R.N.; Singh, S.; Chaturvedi, S.; Luqman, S. Camelina sativa (L.) Crantz A Mercantile Crop with Speckled Pharmacological Activities. Ann. Phytomedicine Int. J. 2016, 5, 6–26.
- Mondor, M.; Hernández-Álvarez, A.J. Camelina sativa Composition, Attributes, and Applications: A Review. Eur. J. Lipid Sci. Technol. 2022, 124, 2100035.
- Musazadeh, V.; Dehghan, P.; Azadmard-Damirchi, S. Effectiveness of Co-Administration of Camelina Oil and Caloric Restriction on Cardiometabolic Risk Factors, Liver Function and Mental Health in Patients with Non-Alcoholic Fatty Liver Disease: A Blinded Randomized Controlled Trial Protocol. J. Nutr. Food Secur. 2022, 9, 379–387.
- Jaśkiewicz, T.; Sagan, A.; Puzio, I. Effect of the Camelina sativa Oil on the Performance, Essential Fatty Acid Level in Tissues and Fat-Soluble Vitamins Content in the Livers of Broiler Chickens. Livest. Sci. 2014, 165, 74–79.
- Orczewska-Dudek, S.; Pietras, M. The Effect of Dietary Camelina sativa Oil or Cake in the Diets of Broiler Chickens on Growth Performance, Fatty Acid Profile, and Sensory Quality of Meat. Animals 2019, 9, 734.
- De Marzo, D.; Laudadio, V.; Khan, R.U.; Tufarelli, V.; Maiorano, G. Feeding of Camelina sativa Seeds to Light-Type Gentile Di Puglia Lambs: Effect on Productive Performance and Muscle Fatty Acid Composition. Anim. Biotechnol. 2022, 1–7.
- Zlepkin, V.A.; Salomatin, V.V.; Ryadnov, A.A.; Zlepkina, N.A.; Mishurova, M.N.; Ryadnova, T.A.; Kurskaya, Y.A. Vegetable Oil Various Types Together with Enzyme Preparation Influence on Broiler Chickens’ Meat Productivity and Quality. IOP Conf. Ser. Earth Environ. Sci. 2022, 965, 012035.
- Ciurescu, G.; Idriceanu, L.; Gheorghe, A.; Ropotă, M.; Drăghici, R. Meat Quality in Broiler Chickens Fed on Cowpea (Vigna Unguiculata Walp) Seeds. Sci. Rep. 2022, 12, 9685.
- Lolli, S.; Grilli, G.; Ferrari, L.; Battelli, G.; Pozzo, S.; Galasso, I.; Russo, R.; Brasca, M.; Reggiani, R.; Ferrante, V. Effect of Different Percentage of Camelina sativa Cake in Laying Hens Diet: Performance, Welfare, and Eggshell Quality. Animals 2020, 10, 1396.
- Peiretti, P.G.; Mussa, P.P.; Prola, L.; Meineri, G. Use of Different Levels of False Flax (Camelina sativa L.) Seed in Diets for Fattening Rabbits. Livest. Sci. 2007, 107, 192–198.
- Colombini, S.; Broderick, G.A.; Galasso, I.; Martinelli, T.; Rapetti, L.; Russo, R.; Reggiani, R. Evaluation of Camelina sativa (L.) Crantz Meal as an Alternative Protein Source in Ruminant Rations. J. Sci. Food Agric. 2014, 94, 736–743.
- Colonna, M.A.; Giannico, F.; Tufarelli, V.; Laudadio, V.; Selvaggi, M.; De Mastro, G.; Tedone, L. Dietary Supplementation with Camelina sativa (L. Crantz) Forage in Autochthonous Ionica Goats: Effects on Milk and Caciotta Cheese Chemical, Fatty Acid Composition and Sensory Properties. Animals 2021, 11, 1589.
- Mierlita, D.; Daraban, S.; Lup, F.; Chereji, A. The Effect of Grazing Management and Camelina Seed Supplementation in the Diet on Milk Performance and Milk Fatty Acid Composition of Dairy Ewes. J. Food Agric. Environ. 2011, 9, 368–373.
- Tedone, L.; Giannico, F.; Tufarelli, V.; Laudadio, V.; Selvaggi, M.; De Mastro, G.; Colonna, M.A. Camelina sativa (L. Crantz) Fresh Forage Productive Performance and Quality at Different Vegetative Stages: Effects of Dietary Supplementation in Ionica Goats on Milk Quality. Agriculture 2022, 12, 91.
- Taranu, I.; Gras, M.; Pistol, G.C.; Motiu, M.; Marin, D.E.; Lefter, N.; Ropota, M.; Habeanu, M. ω-3 PUFA Rich Camelina Oil By-Products Improve the Systemic Metabolism and Spleen Cell Functions in Fattening Pigs. PLOS ONE 2014, 9, e110186.
- Juodka, R.; Nainienė, R.; Juškienė, V.; Juška, R.; Leikus, R.; Kadžienė, G.; Stankevičienė, D. Camelina (Camelina sativa (L.) Crantz) as Feedstuffs in Meat Type Poultry Diet: A Source of Protein and n-3 Fatty Acids. Animals 2022, 12, 295.
- Riaz, R.; Ahmed, I.; Sizmaz, O.; Ahsan, U. Use of Camelina sativa and By-Products in Diets for Dairy Cows: A Review. Animals 2022, 12, 1082.
- Hixson, S.M.; Parrish, C.C.; Anderson, D.M. Full Substitution of Fish Oil with Camelina (Camelina Sativa) Oil, with Partial Substitution of Fish Meal with Camelina Meal, in Diets for Farmed Atlantic Salmon (Salmo Salar) and Its Effect on Tissue Lipids and Sensory Quality. Food Chem. 2014, 157, 51–61.
- Ruiz-Lopez, N.; Haslam, R.P.; Napier, J.A.; Sayanova, O. Successful High-level Accumulation of Fish Oil Omega-3 Long-chain Polyunsaturated Fatty Acids in a Transgenic Oilseed Crop. Plant J. 2014, 77, 198–208.
- Xue, X.; Hixson, S.M.; Hori, T.S.; Booman, M.; Parrish, C.C.; Anderson, D.M.; Rise, M.L. Atlantic Salmon (Salmo Salar) Liver Transcriptome Response to Diets Containing Camelina sativa Products. Comp. Biochem. Physiol. Part D Genom. Proteom. 2015, 14, 1–15.
- Betancor, M.B.; Sprague, M.; Sayanova, O.; Usher, S.; Campbell, P.J.; Napier, J.A.; Caballero, M.J.; Tocher, D.R. Evaluation of a High-EPA Oil from Transgenic Camelina sativa in Feeds for Atlantic Salmon (Salmo Salar L.): Effects on Tissue Fatty Acid Composition, Histology and Gene Expression. Aquaculture 2015, 444, 1–12.
- Tocher, D.R. Omega-3 Long-Chain Polyunsaturated Fatty Acids and Aquaculture in Perspective. Aquaculture 2015, 449, 94–107.
- Betancor, M.B.; Sprague, M.; Montero, D.; Usher, S.; Sayanova, O.; Campbell, P.J.; Napier, J.A.; Caballero, M.J.; Izquierdo, M.; Tocher, D.R. Replacement of Marine Fish Oil with de Novo Omega-3 Oils from Transgenic Camelina sativa in Feeds for Gilthead Sea Bream (Sparus Aurata L.). Lipids 2016, 51, 1171–1191.
- Wei, M.; Anderson, D.M.; Zhang, Z.; Colombo, S.M. High-Oil Residue Camelina Meal, a Viable Source of Protein at Low Levels in Diets for Juvenile Salmonids. Aquac. Nutr. 2020, 26, 558–567.
- Ruyter, B.; Bou, M.; Berge, G.M.; Mørkøre, T.; Sissener, N.H.; Sanden, M.; Lutfi, E.; Romarheim, O.-H.; Krasnov, A.; Østbye, T.-K.K. A Dose-Response Study with Omega-3 Rich Canola Oil as a Novel Source of Docosahexaenoic Acid (DHA) in Feed for Atlantic Salmon (Salmo Salar) in Seawater; Effects on Performance, Tissue Fatty Acid Composition, and Fillet Quality. Aquaculture 2022, 561, 738733.
- Toyes-Vargas, E.A.; Magallón-Barajas, F.J.; Parrish, C.C. Lipid Variations in Tilapia (Var. GIFT Oreochromis Sp.) Tissues Due to Dietary Replacement of Fish Oil with Camelina Oil (Camelina Sativa). Aquac. Res. 2022, 53, 2819–2832.
- Balanuca, B.; Stan, R.; Hanganu, A.; Lungu, A.; Iovu, H. Design of New Camelina Oil-Based Hydrophilic Monomers for Novel Polymeric Materials. J. Am. Oil Chem. Soc. 2015, 92, 881–891.
- Li, N.; Qi, G.; Sun, X.S.; Xu, F.; Wang, D. Adhesion Properties of Camelina Protein Fractions Isolated with Different Methods. Ind. Crops Prod. 2015, 69, 263–272.
- Li, Y.; Sun, X.S. Camelina Oil Derivatives and Adhesion Properties. Ind. Crops Prod. 2015, 73, 73–80.
- Kim, N.; Li, Y.; Sun, X.S. Epoxidation of Camelina sativa Oil and Peel Adhesion Properties. Ind. Crops Prod. 2015, 64, 1–8.
- Nosal, H.; Nowicki, J.; Warzała, M.; Nowakowska-Bogdan, E.; Zarębska, M. Synthesis and Characterization of Alkyd Resins Based on Camelina sativa Oil and Polyglycerol. Prog. Org. Coat. 2015, 86, 59–70.
- Piravi-vanak, Z.; Azadmard-Damirchi, S.; Kahrizi, D.; Mooraki, N.; Ercisli, S.; Savage, G.P.; Rostami Ahmadvandi, H.; Martinez, F. Physicochemical Properties of Oil Extracted from Camelina (Camelina Sativa) Seeds as a New Source of Vegetable Oil in Different Regions of Iran. J. Mol. Liq. 2022, 345, 117043.
- Mališová, M.; Horňáček, M.; Hudec, P.; Mikulec, J.; Slezáčková, M.; Hájeková, E. Preparation and Characterization of K-Loaded Mg/Al Mixed Oxides Obtained from Hydrotalcites for Transesterification of Camelina sativa Oil. Chem. Pap. 2022, 76, 7585–7596.
- Arshad, M.K.; Mohanty, A.; Acker, R.V.; Riddle, R.; Todd, J.; Khalil, H.; Misra, M. Valorization of Camelina Oil to Biobased Materials and Biofuels for New Industrial Uses: A Review. RSC Adv. 2022, 12, 27230–27245.
- Shonnard, D.R.; Williams, L.; Kalnes, T.N. Camelina-Derived Jet Fuel and Diesel: Sustainable Advanced Biofuels. Environ. Prog. Sustain. Energy 2010, 29, 382–392.
- Campbell, M.C.; Rossi, A.F.; Erskine, W. Camelina (Camelina sativa (L.) Crantz): Agronomic Potential in Mediterranean Environments and Diversity for Biofuel and Food Uses. Crop Pasture Sci. 2013, 64, 388.
- Gesch, R.W.; Archer, D.W. Double-Cropping with Winter Camelina in the Northern Corn Belt to Produce Fuel and Food. Ind. Crops Prod. 2013, 44, 718–725.
- Li, X.; Mupondwa, E. Life Cycle Assessment of Camelina Oil Derived Biodiesel and Jet Fuel in the Canadian Prairies. Sci. Total Environ. 2014, 481, 17–26.
- Obour, A.K.; Sintim, H.Y.; Obeng, E.; Jeliazkov, D.V. Oilseed Camelina (Camelina sativa L. Crantz): Production Systems, Prospects and Challenges in the USA Great Plains. Adv. Plants Agric. Res. 2015, 2, 68–76.
- Yang, J.; Caldwell, C.; Corscadden, K.; He, Q.S.; Li, J. An Evaluation of Biodiesel Production from Camelina sativa Grown in Nova Scotia. Ind. Crops Prod. 2016, 81, 162–168.
- Bacenetti, J.; Restuccia, A.; Schillaci, G.; Failla, S. Biodiesel Production from Unconventional Oilseed Crops (Linum Usitatissimum L. and Camelina sativa L.) in Mediterranean Conditions: Environmental Sustainability Assessment. Renew. Energy 2017, 112, 444–456.
- Ahmad, M.; Waraich, E.A.; Hafeez, M.B.; Zulfiqar, U.; Ahmad, Z.; Iqbal, M.A.; Raza, A.; Slam, M.S.; Rehman, A.; Younis, U.; et al. Changing Climate Scenario: Perspectives of Camelina sativa as Low-Input Biofuel and Oilseed Crop. In Global Agricultural Production: Resilience to Climate Change; Ahmed, M., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 197–236. ISBN 978-3-031-14973-3.
- Tanwar, B.; Goyal, A. Oilseeds: Health Attributes and Food Applications; Springer: Singapore, 2021; ISBN 9789811541933.
- Karvonen, H.M.; Aro, A.; Tapola, N.S.; Salminen, I.; Uusitupa, M.I.J.; Sarkkinen, E.S. Effect of -Linolenic AcidRich Camelina sativa Oil on Serum Fatty Acid Composition and Serum Lipids in Hypercholesterolemic Subjects. Metab.-Clin. Exp. 2002, 51, 1253–1260.
- Manninen, S.; Lankinen, M.; Erkkilä, A.; Nguyen, S.D.; Ruuth, M.; de Mello, V.; Öörni, K.; Schwab, U. The Effect of Intakes of Fish and Camelina sativa Oil on Atherogenic and Anti-Atherogenic Functions of LDL and HDL Particles: A Randomized Controlled Trial. Atherosclerosis 2019, 281, 56–61.
- Ibrahim, F.; El Habbasha, E.S. Chemical Composition, Medicinal Impacts and Cultivation of Camelina (Camelina Sativa): Review. Int.J. PharmTech Res. 2015, 8, 114–122.
- Russo, R.; Reggiani, R. Antinutritive Compounds in Twelve <I>Camelina sativa </I>Genotypes. Am. J. Plant Sci. 2012, 03, 1408–1412.
- Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzińska, M. Bioactive Compounds, Nutritional Quality and Oxidative Stability of Cold-Pressed Camelina (Camelina sativa L.) Oils. Appl. Sci. 2018, 8, 2606.
- Christodoulou, C.; Mavrommatis, A.; Mitsiopoulou, C.; Symeon, G.; Dotas, V.; Sotirakoglou, K.; Kotsampasi, B.; Tsiplakou, E. Assessing the Optimum Level of Supplementation with Camelina Seeds in Ewes’ Diets to Improve Milk Quality. Foods Basel Switz. 2021, 10, 2076.
- Nain, S.; Oryschak, M.A.; Betti, M.; Beltranena, E. Camelina sativa Cake for Broilers: Effects of Increasing Dietary Inclusion from 0 to 24% on Tissue Fatty Acid Proportions at 14, 28, and 42 d of Age. Poult. Sci. 2015, 94, 1247–1258.
- Russo, R.; Galasso, I.; Reggiani, R. Variability in Glucosinolate Content among Camelina Species. Am. J. Plant Sci. 2014, 05, 294–298.
- King, K.; Li, H.; Kang, J.; Lu, C. Mapping Quantitative Trait Loci for Seed Traits in Camelina Sativa. Theor. Appl. Genet. 2019, 132, 2567–2577.
- Lokesh, K.; Sethi, V.; Nikolaidis, T.; Goodger, E.; Nalianda, D. Life Cycle Greenhouse Gas Analysis of Biojet Fuels with a Technical Investigation into Their Impact on Jet Engine Performance. Biomass Bioenergy 2015, 77, 26–44.
- Masella, P.; Galasso, I. A Comparative Cradle-to-Gate Life Cycle Study of Bio-Energy Feedstock from Camelina Sativa, an Italian Case Study. Sustainability 2020, 12, 9590.
- Fröhlich, A.; Rice, B. Evaluation of Camelina sativa Oil as a Feedstock for Biodiesel Production. Ind. Crops Prod. 2005, 21, 25–31.
- Ciubota-Rosie, C.; Ruiz, J.R.; Ramos, M.J.; Pérez, Á. Biodiesel from Camelina Sativa: A Comprehensive Characterisation. Fuel 2013, 105, 572–577.
- Angelini, L.G.; Abou Chehade, L.; Foschi, L.; Tavarini, S. Performance and Potentiality of Camelina (Camelina sativa L. Crantz) Genotypes in Response to Sowing Date under Mediterranean Environment. Agronomy 2020, 10, 1929.
- Zubr, J. Dietary Fatty Acids and Amino Acids of Camelina sativa Seed. J. Food Qual. 2003, 26, 451–462.
