Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Sea buckthorn (Hippophae rhamnoides L. or Elaeagnus rhamnoides L.) is a plant that has long been used as a Chinese herbal medicine. This species is known to contain numerous bioactive components, including polyphenols, fatty acids, vitamins, and phytosterols. In experiments both in vitro and in vivo (ranging from cell lines to animal models and human patients), sea buckthorn has shown positive effects on symptoms of metabolic syndrome; evidence suggests that sea buckthorn treatment can decrease blood lipid content, blood pressure, and blood sugar levels, and regulate key metabolites.
- sea buckthorn
- metabolic syndrome
- bioactive compounds
1. Introduction
Sea buckthorn is a thorny, deciduous, dioecious shrub of the family Elaeagnaceae. It is consumed throughout the world for both nutritional and medicinal purposes. Sea buckthorn trees are usually 2–6 m tall, with rough bark and stout young branches that grow from the main trunk in the form of small, sharp spines. It has lance-shaped or linear leaves of 3–8 cm in length and <7 mm in width that are dark green on the adaxial surface and silvery gray on the abaxial surface. It produces yellow or orange spherical berries of 3–8 mm in diameter, which cluster together and are densely surrounded by sharp spines. The ovoid seeds are brown or gray in color, 3–4 mm in length, and covered by a shiny shell (Figure 1). Sea buckthorn fruits are used in popular foods such as bread, yogurt, and jam, and in beverages, such as tea. Sea buckthorn products are a source of bioactive substances, including polyphenols, fatty acids, vitamins, and phytosterols [1].

Figure 1. Sea buckthorn berries, leaves, seeds, and oil.
Sea buckthorn contains nearly 200 known nutrients and bioactive compounds, giving it beneficial nutritional properties [2]. The medicinal value of sea buckthorn was recognized by the Chinese medical system 3000 years ago, dating back to the Tang Dynasty [3]. According to records, in traditional Chinese medicine, sea buckthorn is used to treat diseases including circulatory system diseases, skin lesions, metabolic disorders and digestive system diseases [4]. The value of sea buckthorn in the treatment of gastrointestinal diseases, cardiovascular diseases, and burns was recorded in the Tibetan medicinal classic rGyud Bzi (The Four Books of Pharmacopoeia), a classic of Tibetan medicine [5], and was officially listed in the Chinese Pharmacopoeia in 1977 [6][7][8][9]. It reportedly has medical value due to properties that provide anti-oxidative, immune-regulatory, cardioprotective, anti-atherosclerotic, anti-bacterial, anti-viral, anti-inflammatory, anti-diabetic, anti-cancer, hepatoprotective, and skin-protective effects. Sea buckthorn may be a valuable tool in preventing or treating metabolic syndrome.
2. Efficacy and Mechanism of Sea Buckthorn Active Compounds in Treating Metabolic Syndrome
Metabolic syndrome is characterized by various metabolic abnormalities, including abdominal obesity, high blood pressure, hyperglycemia, and dyslipidemia. The key aspects of metabolic syndrome treatment include reducing abnormal lipogenesis; improving dyslipidemia and insulin resistance; and controlling blood sugar, blood pressure, and other metabolic pathways. The bioactive compounds in sea buckthorn positively regulate metabolism and ameliorate complications caused by metabolic disorders (Figure 2).
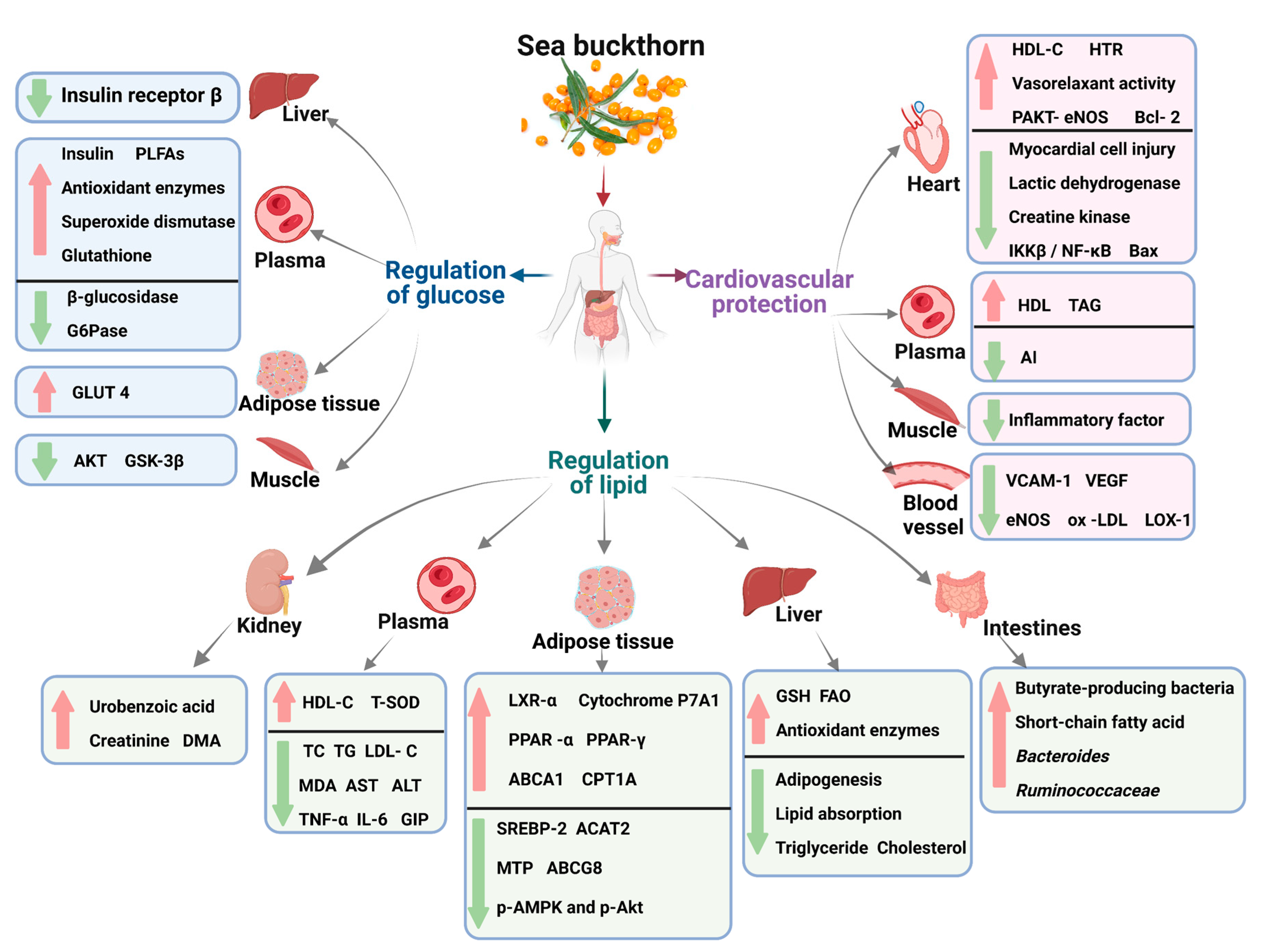
Figure 2. Schematic diagram of the effect of sea buckthorn on metabolic syndrome.
2.1. Clinical Trials of Sea Buckthorn
Sea buckthorn has been used in clinical trials to treat metabolic syndrome in recent years. A study in obese children assessed the effects of sea buckthorn pulp oil treatment (800 mg/d for 60 d) on inflammation, systemic redox status, and endothelial function. The treatment was shown to prevent atherosclerosis (AS) by strongly reducing triglycerides (TG), cholesterol, and blood pressure, and weakly reducing oxidative stress, inflammation, and insulin resistance [10]. Another human study investigated the effects of dietary supplementation with phenol-rich sea buckthorn powder. An analysis of plasma with nuclear magnetic resonance (NMR) fingerprinting after meals showed that the treatment delays postprandial lipid changes and inhibits increases of 3-hydroxy butanoic acid and N-acetyl glycoproteins compared to untreated controls [11].
The effects of sea buckthorn puree on the plasma metabolome and intestinal microflora have been assessed in patients with hypercholesterolemia. After 45 d of treatment, increases in blood glucose, lactate, and lipid levels negated the beneficial effects on sugar and lipid metabolism. This may have been due to the additional sugar intake caused by sea buckthorn puree consumption, which could have negatively affected the metabolism of people with hypercholesterolemia. However, after 90 d, the blood glucose and lactate levels decreased to below the baseline and the blood lipid levels returned to the baseline, potentially due to the actions of the bioactive ingredients (such as the phenolic compounds) in the sea buckthorn puree [12].
In a randomized, controlled, single-blind, three-way crossover study, sea buckthorn treatment was shown to improve blood glucose levels by 44.7% compared to a control group (p < 0.01), and to decrease the plasma insulin concentration at 30 and 45 min post-treatment by 39.6% (p < 0.01) and 16.5% (p < 0.05), respectively. [13]. A separate randomized, double-blind, two-way crossover study showed a slight decrease in fasting blood glucose (FBG) levels in patients with impaired glucose regulation (IGR) after consumption of sea buckthorn puree for 5 weeks [14]. As trans-palmitoleic acid (16:1n-7t) is associated with a lower incidence of type 2 diabetes, another study analyzed the effects of unmodified sea buckthorn oil and of a 16:1 sea buckthorn oil–n-7t mixture on serum phospholipid fatty acid (PLFA) levels. Both treatments moderately increased the PLFA concentrations in metabolically healthy adults, suggesting that sea buckthorn oil could prevent diabetes [15].
Another study showed that dietary supplementation with 0.75 mL of sea buckthorn seed oil per day effectively reduces dyslipidemia, cardiovascular risk factors, and hypertension in humans, which may be due to the presence of ω-3, -6, and -9 fatty acids in the oil. Improved antioxidant parameters may also be attributed to the high levels of β-carotene and vitamin E [16]. Sea buckthorn puree has also been assessed for its capacity to reduce blood lipid levels and other cardiovascular disease risk factors. It does not affect the serum levels of total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), or TG, but high-density lipoprotein cholesterol (HDL-C) levels show an overall decreasing trend after increasing at the start of treatment. Diastolic blood pressure (DBP) also decreases after sea buckthorn puree consumption, and hsCRP concentrations show a decreasing trend. These results show that long-term sea buckthorn puree consumption has anti-inflammatory effects and reduces blood pressure in patients with hypercholesterolemia [17]
2.2. Hyperlipidemia and Obesity
Lipids play important roles in providing energy and forming essential fatty acids. They are also integral components of human cells and tissues, such as cell membranes and myelin sheaths. Abnormalities in fat metabolism can result in metabolic disorders [18]. Lipid metabolism is also involved in the development of obesity.
Sea buckthorn supplementation has beneficial effects on lipid levels, which can be attributed to the presence of flavonoids (e.g., isorhamnetin, quercetin, and borneol), β-sitosterol, palmitoleic acid, and/or linolenic acid. In mice with hypercholesterolemia induced by a high-fat diet (HFD), treatment with sea buckthorn-derived flavone significantly reduces glucose, serum TC, and LDL-C levels in the blood and TC and TG concentrations in the liver [19]. In mice with hypercholesterolemia induced by phenol oxidative stress and a high-cholesterol diet (HCD), treatment with sea buckberry wine decreases oxidized glutathione (GSH) levels and liver lipid peroxidation. This treatment also increases superoxide dismutase (SOD) activity, levels of GSH and lipid peroxidation in the liver, and the ratio of HDL-C to LDL-C [20].
In another study, polyphenols isolated from sea buckthorn berries were orally administered to hyperlipidemic rats at a dose of 7–28 mg/kg. This significantly reduced the blood lipid levels, increased the antioxidant enzyme activity, and decreased the serum levels of tumor necrosis factor-α and interleukin (IL)-6. Furthermore, sea buckthorn berries have been shown to reduce vascular injury in hyperlipidemic rats by decreasing endothelial nitric oxide synthase (eNOS), lectin-like oxLDL receptor-1 (LOX-1), and intercellular adhesion molecule-1 (ICAM-1) expression in the aorta at both the mRNA and protein levels [21].
In HFD-induced obese mice, a 12-week treatment with 0.04% sea buckthorn flavone reversed obesity, liver steatosis, insulin sensitivity, and the inflammatory response. Specifically, the treatment increased energy expenditure and liver fatty acid oxidation (FAO) and inhibited adipose tissue formation, liver adipose formation, and liver fat absorption. Sea buckthorn flavonoids also inhibit plasma gastric inhibitory polypeptide (GIP) levels and liver glycogenase activity, which are regulated by secreted resistin and pro-inflammatory cytokines [22].
Sea buckthorn flavonoids can also regulate peroxide-activated receptor α (PPAR-α) and peroxide-activated receptor γ (PPAR-γ) expression in the liver and adipose tissue in a dose-dependent manner. In mice with HFD-induced obesity, this inhibits adipose tissue inflammation, decreasing obesity and reducing TG levels [23]. In mice fed a high-fat, high-fructose diet (HFFD), sea buckthorn flavonoids alleviate cognitive impairment by effectively normalizing insulin signaling and reducing neuroinflammation [24]. Sea buckthorn flavonoids also improve hyperlipidemia by promoting cholesterol conversion to bile acids and cholesterol effluent, inhibiting de novo cholesterol synthesis, and accelerating FAO [25].
In hypercholesterolemic golden hamsters, treatment with sea buckthorn seed oil (SBSO) downregulates acyl-CoA: cholesterol acyltransferase 2 (ACAT2), microsomal triacylglycerol transporter (MTP), and adenosine triphosphate binding box transporter 8 (ABCG8). SBSO supplementation also increases intestinal short-chain fatty acid production and neutral sterol excretion. A metagenomic analysis showed that dietary supplementation with sea buckthorn seed oil replacing 50% lard (SL) and replacing 100% lard (SH) positively regulates the relative abundance of Bacteroides S24-7, Ruminococcus, and Eubacteriaceae [26].
The hypolipidemic effects of sea buckthorn fruit oil (SBFO) extracts, which are rich in palmitoleic acid, have been studied in mice. Treatment with SBFO extract controls body weight and adipose tissue quality, reduces fat accumulation, and increases TC, TG, HDL-C, and non-HDL-C in a dose-dependent manner. SBFO extract also reduces hyperlipidemia-induced oxidative stress and liver damage by regulating antioxidant enzymes. Quantitative reverse transcription (qRT)-PCR and Western blot analyses indicate that SBFO extract influences expression of key genes in the adenosine monophosphate-activated protein kinase (AMPK) and Akt pathways, and promotes AMPK and Akt phosphorylation [27]. In adipocytes, sea buckthorn oil acts as an insulin sensitizer. Furthermore, it promotes 3T3-L1 preadipocyte proliferation and differentiation; it also increases insulin sensitivity, glucose transporter 4 (GLUT4) expression, and glucose uptake, potentially through AMPK and Akt activation [28].
2.3. Hyperglycemia and Diabetes
Diabetes is associated with abnormal carbohydrate, fat, and protein metabolism due to defects in insulin secretion, insulin action, or both. If not properly controlled, diabetes can lead to many complications, such as hyperlipidemia, hypertension, AS, hyperinsulinemia, retinopathy, kidney disease, and peripheral neuropathy [29].
Several studies have evaluated the potential of sea buckthorn supplementation as a treatment for diabetes. The results of a study on the antidiabetic activity of sea buckthorn pulp oil on human islet cells showed that sea buckthorn pulp oil enhanced the efficacy of glucose-induced insulin secretion by activating G protein-coupled receptors in pancreatic β-cells. Among the fatty acids of sea buckthorn pulp oil, palmitoleic acid had the highest activity [30]. Zhang et al. investigated the effects of sea buckthorn seed residue water extract on blood glucose and lipid levels and on antioxidant-related parameters in streptozotocin-induced diabetic rats. The rats were divided into four groups: normal control, diabetic control, diabetic treated with 5 mg/kg glyburide, and diabetic treated with 400 mg/kg sea buckthorn seed residue extract. The latter group showed significantly reduced blood glucose, TG, and nitric oxide levels. In addition, the serum SOD activity and GSH levels were significantly increased. This demonstrates the potential hypoglycemic, TG-lowering, and antioxidant effects of sea buckthorn supplements. Furthermore, it suggests that sea buckthorn may prevent some diabetic complications associated with hyperlipidemia and oxidative stress [31].
Sea buckthorn leaf extract has shown strong antioxidant and α-glucosidase-inhibitory activity. Six compounds have been isolated from the leaf extract: kaempferol-3-O-β-D-(600-O-coumaryl) glycoside, 1-feruloyl-β-D-glucopyranoside, isorhamnetin-3-O-glucoside, quercetin 3-O-β-D-glucopyranoside, quercetin 3-O-β-D-glucopyranosyl-7-O-α-L-rhamnopyranoside, and isorhamnetin-3-O-rutinoside. In a comparison of sea buckthorn leaf extracts generated with a range of polar and nonpolar solvents, butanol leaf extracts were shown to contain the largest number of phenolic compounds, have the highest free radical scavenging activity, and demonstrate the strongest α-glucosidase inhibition [32]. Methanol leaf extracts also positively affect antioxidant and antidiabetic activities in normal and alloxan diabetic Wistar rats in vitro; compared to a diabetic control group, the FBG levels were decreased in alloxan-induced diabetic rats who were intragastrically administered sea buckthorn leaf extract. Furthermore, levels of the endogenous antioxidant enzymes SOD and GSH peroxidase were significantly increased and malondialdehyde levels were significantly decreased in sea buckthorn-treated diabetic rats. These results indicate that a methanol extract of sea buckthorn leaf enhances antioxidant defenses against reactive oxygen species produced as a result of hyperglycemia [33].
L-resveratrol extract (SQE) and L-resveratrol standard (QS) extracted from sea buckthorn leaves show good inhibitory activity against α-amylase. Kinetic studies have shown that the enzyme inhibition is competitive. Glucose consumption by SQE and QS reduce the total TG and non-esterified fatty acid (NEFA) contents in cells with elevated insulin resistance (IR). In addition, SQE and QS downregulate glucose 6-phosphatase and upregulate PPARα. These results suggest that SQE and QS play important roles in regulating glycolipid metabolism [34].
Four unique branched-chain amino acid polypeptides have been identified in sea buckthorn seed proteins: Leu/Ile-Pro-Glu-Asp-Pro, Asp-Leu/Ile-Val-Gly-Glu, Leu/Ile-Pro, and Leu/Ile-Pro-Leu/Ile. In experiments investigating the hypoglycemic activity in db/db mice with type 2 diabetes, oral administration of any of these four branched amino acid peptides significantly reverses symptoms of diabetes and reduces FBG levels via GLUT4 upregulation. The branched-chain amino acid polypeptides also significantly increase muscle glycogen content by downregulating protein kinase B (AKT) and glycogen synthetase 3β (GSK-3β) and increasing glycogen synthetase (GS) activity. In addition, they significantly upregulate phosphatidylinositol 3-kinase (PI3K) at the protein level [35].
Sea buckthorn proteins also exert regulatory effects on intestinal microbes. In mice, treatment with sea buckthorn proteins significantly decreases bodyweight and blood glucose levels and recovers normal levels of Bifidobacterium, Lactobacillus, Bacillus, and Clostridium globularis. A metagenomic sequencing analysis has revealed differences in the intestinal microbial community as a result of sea buckthorn treatment. Amplified ribosomal DNA restriction analysis (ARDRA) confirmed that sea buckthorn protein supplementation in mice increases intestinal microbial diversity, as measured by the Shannon (H) and Simpson (E) indices [36].
L-quebrachitol may contribute to the hypoglycemic effects of sea buckthorn. In rats, treatment with sea buckthorn juice reduces food intake, weight gain, random blood glucose levels, and insulin receptor β expression in the liver. Sea buckthorn juice also significantly improves glucose tolerance and pancreatic tissue integrity. Enrichment of sea buckthorn juice with L-quebrachitol alcohol increases fasting plasma insulin levels and influences random blood glucose levels, glucose tolerance, and pancreatic tissue, similar to unenriched sea buckthorn juice [37].
In a study of the hypoglycemic effects of sea buckthorn seed protein (SSP) in streptozotocin (STZ)-induced diabetic ICR mice, SSP showed a significant hypoglycemic effect; compared to the diabetic control mice, the diabetic mice treated with SSP had reduced body weight, FBG, inflammatory factors and insulin (SIN), and lipid content [38]. The effects of sea buckthorn fruit oil extract on type 2 diabetes have also been investigated in vitro using HepG2 cells and diabetic rats. These studies show that sea buckthorn fruit oil extract effectively improves the glucose intake in insulin-resistant cells. Furthermore, it significantly reduces the blood glucose and insulin indices of T2 DM SD rats by regulating the phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway. Western blot and qRT-PCR analyses showed that sea buckthorn extracts promote PI3K and glycogen synthesis (GS) expression, but inhibit GSK-3β expression [39].
2.4. Hypertension and Cardiovascular Disease
Hypertension is related to specific metabolic processes, such as insulin resistance and compensatory hyperinsulinemia. Platelet aggregation, endothelial function, and intracellular free calcium levels ([Ca2+] i) may influence hypertension development. Improved insulin sensitivity and [Ca2+] i could lead to antihypertensive effects [40][41].
There is some evidence that sea buckthorn supplementation can lower blood pressure and prevent cardiovascular diseases. Flavonoids are a type of polyphenol that are naturally found in fruits and vegetables, including sea buckthorn. The most abundant flavonoids in sea buckthorn fruits and leaves are isorhamnetin and quercetin, respectively [42]. The antioxidant properties of flavanols indicate that they reduce the risk of cardiovascular disease. Treatment with sea buckthorn total flavonoids protects against myocardial ischemia-reperfusion, tumors, oxidative damage, and aging [43]. Sea buckthorn flavonoids also protect endothelial cells from oxidative LDL-induced damage [44]. In rats susceptible to hypertension, treatment with 0.7 g/kg dried sea buckthorn fruit powder for 60 d improves metabolic processes and alleviates hypertensive stress [45]. Feeding rats a diet high in sucrose significantly increases their systolic blood pressure, plasma insulin and TG levels, and angiotensin II levels in the heart and kidneys. However, supplementation with sea buckthorn seed extract has an antihypertensive effect, which is achieved through blocking of the angiotensin II pathway and improvement of insulin sensitivity [41].
The cardioprotective properties of sea buckthorn oil have been attributed to its high unsaturated fatty acid content [46][47][48]. Sea buckthorn oil protects against myocardial ischemia-reperfusion injury in rats by activating the protein kinase B (Akt)-endothelial nitric oxide synthase (ENOS) signaling pathway. Pretreatment with 20 mg/kg pulp oil stabilizes cardiac function and myocardial GSH levels; it also significantly inhibits lipid peroxidation. Sea buckthorn oil also improves hemodynamics and systolic function, reduces tumor necrosis factor levels, and inhibits lactate dehydrogenase activity, which is a marker of myocardial cell damage [49]. In studies of human subjects with normal lipid levels, sea buckthorn pulp and seed oil exhibit anti-aggregation activity, suggesting that these substances may have beneficial effects on the cardiovascular system as antiplatelet/anti-aggregation factors [50].
Sea buckthorn seed oil has been shown to have significant anti-atherosclerotic properties; one study showed that injection of 1 mL of this oil decreased blood concentrations of TG, TC, LDL-C, and HDL-C. The cardioprotective effects of the oil are likely due to the unsaturated fatty acid, tocopherol, phytosterol, and β-carotene contents; when taken in combination, these compounds may have a synergistic effect on cardiovascular health [50]. In individuals with cardiovascular risk, consumption of sea buckthorn fruit extract significantly reduces TC and LDL-C levels and increases HDL-C levels. However, this effect has not been observed in healthy subjects. The cardioprotective effects of the fruit are likely due to compounds including β-sitosterols and flavonoids [51].
This entry is adapted from the peer-reviewed paper 10.3390/foods12101985
References
- Dong, K.; Binosha Fernando, W.M.A.D.; Durham, R.; Stockmann, R.; Jayasena, V. Nutritional value, health-promoting benefits and food application of sea buckthorn. Food Rev. Int. 2021, 2122–2137.
- Wang, Z.; Zhao, F.; Wei, P.; Chai, X.; Hou, G.; Meng, Q. Phytochemistry, health benefits, and food applications of sea buckthorn (Hippophae rhamnoides L.): A comprehensive review. Front. Nutr. 2022, 9, 1036295.
- Olas, B. The beneficial health aspects of sea buckthorn (Elaeagnus rhamnoides (L.) A. Nelson) oil. J. Ethnopharmacol. 2018, 213, 183–190.
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278.
- Wang, K.; Xu, Z.; Liao, X. Bioactive compounds, health benefits and functional food products of sea buckthorn: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6761–6782.
- Ren, R.; Li, N.; Su, C.; Wang, Y.; Zhao, X.; Yang, L.; Li, Y.; Zhang, B.; Chen, J.; Ma, X. The bioactive components as well as the nutritional and health effects of sea buckthorn. RSC Adv. 2020, 10, 44654–44671.
- Wen, P.; Zhao, P.; Qin, G.; Tang, S.; Li, B.; Zhang, J.; Peng, L. Genotoxicity and teratogenicity of seabuckthorn (Hippophae rhamnoides L.) berry oil. Drug. Chem. Toxicol. 2020, 43, 391–397.
- Zuchowski, J. Phytochemistry and pharmacology of sea buckthorn (Elaeagnus rhamnoides; syn. Hippophae rhamnoides): Progress from 2010 to 2021. Phytochem. Rev. 2023, 22, 3–33.
- Ciesarova, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolkova, B.; Koplik, R.; Belajova, E.; Kukurova, K.; Dasko, L.; Panovska, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 9170.
- Virgolici, B.; Lixandru, D.; Casariu, E.D.; Stancu, M.; Greabu, M.; Totan, A.; Miricescu, D.; Mohora, M. Sea buckthorn pulp oil treatment prevents atherosclerosis in obese children. ISRN Oxid. Med. 2013, 2013, 164941.
- Lindstedt, A.; Jarvinen, R.; Sinkkonen, J.; Lehtonen, H.-M.; Graca, G.; Viitanen, M.; Gil, A.M.; Kallio, H. Postprandial response on fatty meal is affected by sea buckthorn (Hippophae rhamnoides) supplementation: NMR metabolomics study. Food Res. Int. 2014, 58, 23–34.
- Chen, K.; Zhou, F.; Zhang, J.; Li, P.; Zhang, Y.; Yang, B. Dietary supplementation with sea buckthorn berry puree alters plasma metabolomic profile and gut microbiota composition in hypercholesterolemia population. Foods 2022, 11, 2481.
- Mortensen, M.W.; Spagner, C.; Cuparencu, C.; Astrup, A.; Raben, A.; Dragsted, L.O. Sea buckthorn decreases and delays insulin response and improves glycaemic profile following a sucrose-containing berry meal: A randomised, controlled, crossover study of Danish sea buckthorn and strawberries in overweight and obese male subjects. Eur. J. Nutr. 2018, 57, 2827–2837.
- Ren, Z.; Gong, H.; Zhao, A.; Zhang, J.; Yang, C.; Wang, P.; Zhang, Y. Effect of sea buckthorn on plasma glucose in individuals with impaired glucose regulation: A two-stage randomized crossover intervention study. Foods 2021, 10, 40804.
- Huang, N.K.; Matthan, N.R.; Galluccio, J.M.; Shi, P.; Lichtenstein, A.H.; Mozaffarian, D. Supplementation with seabuckthorn oil augmented in 16:1n-7t increases serum trans-palmitoleic acid in metabolically healthy adults: A randomized crossover dose-escalation study. J. Nutr. 2020, 150, 1388–1396.
- Vivek, V.; Kalpana, B.; Ashish, K.; Kumar Hota, S.; Prakash Chaurasia, O.; Bhuvnesh, K. Effect of seabuckthorn seed oil in reducing cardiovascular risk factors: A longitudinal controlled trial on hypertensive subjects. Clin. Nutr. 2017, 36, 1231–1238.
- Zhou, F.; Zhang, J.; Zhao, A.; Zhang, Y.; Wang, P. Effects of sea buckthorn puree on risk factors of cardiovascular disease in hypercholesterolemia population: A double-blind, randomized, placebo-controlled trial. Anim. Biotechnol. 2022, 33, 955–963.
- Cheng, C.; Li, Z.; Zhao, X.; Liao, C.; Quan, J.; Bode, A.M.; Cao, Y.; Luo, X. Natural alkaloid and polyphenol compounds targeting lipid metabolism: Treatment implications in metabolic diseases. Eur. J. Pharmacol. 2020, 870, 2922.
- Jiesi, W.; Wen, Z.; Dan, Z.; Xinglei, Z.; Xiufeng, P.; Weijing, Q. Hypolipidaemic and hypoglycaemic effects of total flavonoids from seed residues of Hippophae rhamnoides L. in mice fed a high-fat diet. J. Sci. Food Agric. 2011, 91, 1446–1451.
- Bharti, N.; Rajdeep, K.; Gargi, D. Protective effects of a novel sea buckthorn wine on oxidative stress and hypercholesterolemia. Food Funct. 2013, 4, 240–248.
- Yang, F.; Suo, Y.; Chen, D.; Tong, L. Protection against vascular endothelial dysfunction by polyphenols in sea buckthorn berries in rats with hyperlipidemia. Biosci. Trends 2016, 10, 188–196.
- Kwon, E.Y.; Lee, J.; Kim, Y.J.; Do, A.; Choi, J.Y.; Cho, S.J.; Jung, U.J.; Lee, M.K.; Park, Y.B.; Choi, M.S. Seabuckthorn leaves extract and flavonoid glycosides extract from seabuckthorn leaves ameliorates adiposity, hepatic steatosis, insulin resistance, and inflammation in diet-induced obesity. Nutrients 2017, 9, 569.
- Xin, Y.; Qian, W.; Zeng-Run, P.; Meng-Ran, P.; Wen, Z. Flavonoid-enriched extract from Hippophae rhamnoides seed reduces high fat diet induced obesity, hypertriglyceridemia, and hepatic triglyceride accumulation in C57BL/6 mice. Pharm. Biol. 2017, 55, 1207–1214.
- Mulati, A.; Ma, S.; Zhang, H.; Ren, B.; Zhao, B.; Wang, L.; Liu, X.; Zhao, T.; Kamanova, S.; Sair, A.T.; et al. Sea buckthorn flavonoids alleviate high-fat and high-fructose diet-induced cognitive impairment by inhibiting insulin resistance and neuroinflammation. J. Agric. Food Chem. 2020, 68, 5835–5846.
- Xiao, P.-T.; Liu, S.-Y.; Kuang, Y.-J.; Jiang, Z.-M.; Lin, Y.; Xie, Z.-S.; Liu, E.H. Network pharmacology analysis and experimental validation to explore the mechanism of sea buckthorn flavonoids on hyperlipidemia. J. Ethnopharmacol. 2021, 264, 3380.
- Wangjun, H.; Zouyan, H.; Hanyue, Z.; Jianhui, L.; Erika, K.; Yimin, Z.; Ka Ying, M.; Wen-Sen, H.; Zhen-Yu, C. Sea buckthorn seed oil reduces blood cholesterol and modulates gut microbiota. Food Funct. 2019, 10, 5669–5681.
- Shan, G.; Gaoshuang, H.; Dong, L.; Mingzhe, S.; Dehua, M. Anti-hyperlipidemia effect of sea buckthorn fruit oil extract through the AMPK and Akt signaling pathway in hamsters. J. Funct. Foods 2020, 66, 103837.
- Ting, Z.; Xuze, Q.; Yuxin, C.; Jianxin, Z.; Junxing, Z. Sea buckthorn (Hippophae rhamnoides L.) oil enhances proliferation, adipocytes differentiation and insulin sensitivity in 3T3-L1 cells. Food Sci. Biotechnol. 2020, 29, 1511–1518.
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.S.; Smejkal, K.; Malanik, M.; et al. Berberine in cardiovascular and metabolic diseases: From mechanisms to therapeutics. Theranostics 2019, 9, 1923–1951.
- Korkus, E.; Dąbrowski, G.; Szustak, M.; Czaplicki, S.; Madaj, R.; Chworoś, A.; Koziołkiewicz, M.; Konopka, I.; Gendaszewska-Darmach, E. Evaluation of the anti-diabetic activity of sea buckthorn pulp oils prepared with different extraction methods in human islet EndoC-betaH1 cells. NFS J. 2022, 27, 54–66.
- Zhang, W.; Zhao, J.; Wang, J.; Pang, X.; Zhuang, X.; Zhu, X.; Qu, W. Hypoglycemic effect of aqueous extract of sea buckthorn (Hippophae rhamnoides L.) seed residues in streptozotocin-induced diabetic rats. Phytother. Res. 2010, 24, 228–232.
- Ju-Sung, K.; Yong-Soo, K.; Yeo-Jin, S.; Myong-Jo, K. Isolation and identification of sea buckthorn (Hippophae rhamnoides) phenolics with antioxidant activity and alpha-glucosidase inhibitory effect. J. Agric. Food Chem. 2011, 59, 138–144.
- Middha, S.K.; Usha, T.; Basistha, B.C.; Goyal, A.K. Amelioration of antioxidant potential, toxicity, and antihyperglycemic activity of Hippophae salicifolia D. Don leaf extracts in alloxan-induced diabetic rats. 3 Biotech 2019, 9, 308.
- Mu, Y.; Tao, C.; Yuan, W.; Lv, Z. Sea buckthorn leaf l-quebrachitol extract for improved glucose and lipid metabolism in insulin resistant HepG2 Cells. Bioresources 2022, 17, 527–542.
- Xiping, Z.; Wei, W.; Chun, C. Hypoglycemic effect of hydrophobic BCAA peptides is associated with altered PI3K/Akt protein expression. J. Agric. Food Chem. 2021, 69, 4446–4452.
- Huaibo, Y.; Fangfang, S.; Lina, M.; Wenjuan, W. Effect of sea buckthorn protein on the intestinal microbial community in streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2018, 107, 1168–1174.
- Xue, Y.; Miao, Q.; Zhao, A.; Zheng, Y.; Zhang, Y.; Wang, P.; Kallio, H.; Yang, B. Effects of sea buckthorn (Hippophae rhamnoides) juice and L-quebrachitol on type 2 diabetes mellitus in db/db mice. J. Funct. Foods 2015, 16, 223–233.
- Huaibo, Y.; Xiping, Z.; Wenjuan, W.; Lina, M.; Deyi, C.; Cuan, Z. Hypoglycemic and anti-inflammatory effects of sea buckthorn seed protein in diabetic ICR mice. Food Funct. 2016, 7, 1610–1615.
- Shan, G.; Qing, G.; Chengguang, Q.; Rui, S.; Zesheng, Z. Sea buckthorn fruit oil extract alleviates insulin resistance through the PI3K/Akt signaling pathway in type 2 diabetes mellitus cells and rats. J. Agric. Food Chem. 2017, 65, 1328–1336.
- Shuxian, C.; Jianmin, T.; Shanshan, W.; Ling, L. Kaempferol protects lipopolysaccharide-induced inflammatory injury in human aortic endothelial cells (HAECs) by regulation of miR-203. Biomed. Pharmacother. 2019, 115, 108888.
- Pang, X.; Zhao, J.; Zhang, W.; Zhuang, X.; Wang, J.; Xu, R.; Xu, Z.; Qu, W. Antihypertensive effect of total flavones extracted from seed residues of Hippophae rhamnoides L. in sucrose-fed rats. J. Ethnopharmacol. 2008, 117, 325–331.
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011.
- Eccleston, C.; Baoru, Y.; Tahvonen, R.; Kallio, H.; Rimbach, G.; Minihane, A.M. Effects of an antioxidant-rich juice (sea buckthorn) on risk factors for coronary heart disease in humans. J. Nutr. Biochem. 2002, 13, 346–354.
- Bao, M.; Lou, Y. Flavonoids from seabuckthorn protect endothelial cells (EA.hy926) from oxidized low-density lipoprotein induced injuries via regulation of LOX-1 and eNOS expression. J. Cardiovasc. Pharmacol. 2006, 48, 834–841.
- Koyama, T.; Taka, A.; Togashi, H. Effects of a herbal medicine, Hippophae rhamnoides, on cardiovascular functions and coronary microvessels in the spontaneously hypertensive stroke-prone rat. Clin. Hemorheol. Microcirc. 2009, 41, 17–26.
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204.
- Hsu, Y.W.; Tsai, C.F.; Chen, W.K.; Lu, F.J. Protective effects of seabuckthorn (Hippophae rhamnoides L.) seed oil against carbon tetrachloride-induced hepatotoxicity in mice. Food Chem. Toxicol. 2009, 47, 2281–2288.
- Olas, B. The multifunctionality of berries toward blood platelets and the role of berry phenolics in cardiovascular disorders. Platelets 2017, 28, 540–549.
- Suchal, K.; Bhatia, J.; Malik, S.; Malhotra, R.K.; Gamad, N.; Goyal, S.; Nag, T.C.; Arya, D.S.; Ojha, S. Seabuckthorn pulp oil protects against myocardial ischemia-reperfusion injury in rats through activation of Akt/eNOS. Front. Pharmacol. 2016, 7, 155.
- Johansson, A.K.; Korte, H.; Yang, B.R.; Stanley, J.C.; Kallio, H.P. Sea buckthorn berry oil inhibits platelet aggregation. J. Nutr. Biochem. 2000, 11, 491–495.
- Guo, X.-F.; Yang, B.; Cai, W.; Li, D. Effect of sea buckthorn (Hippophae rhamnoides L.) on blood lipid profiles: A systematic review and meta-analysis from 11 independent randomized controlled trials. Trends Food Sci. Technol. 2017, 61, 1–10.
This entry is offline, you can click here to edit this entry!
