Cyanobacterial toxins, also known as cyanotoxins, represent a significant hazard to human, animal, and environmental well-being. This research aims to present a comprehensive overview of the challenges associated with detecting and characterizing cyanotoxins through the utilization of various biotests. By exploring the utility of alternative aquatic model organisms and in vitro tests employing cultured cells, the importance of adopting a multi-level approach when investigating cyanotoxicity was highlioghted.
- cyanobacteria
- cyanotoxins
- toxicity assessment
- biotests
1. Introduction
2. Bioassays with Vertebrate Animal Models
In the assessment of cyanobacterial toxicity, researchers often rely on vertebrate bioassays, which involve exposing living organisms to different levels of cyanotoxins and observing their physiological responses. Vertebrate bioassays offer several advantages over other methods of cyanotoxicity testing. They provide a more realistic and comprehensive representation of the effects of cyanotoxins on living organisms, taking into account factors such as metabolism, pharmacokinetics, and route of exposure [12]. Moreover, vertebrate bioassays might help to identify the specific mechanisms by which cyanotoxins cause toxicity, which can aid the development of effective treatment and prevention strategies [13]. Another advantage of vertebrate bioassays is their ability to simulate the effects of chronic exposure to cyanotoxins, which is often more relevant to real-world scenarios than acute exposure [14]. Despite their many benefits, vertebrate bioassays also have several limitations that must be considered. One significant challenge is the ethical concern associated with the use of live animals in research. Additionally, there is inter-species variability in the response to a toxin [9], which introduces a degree of uncertainty into the acquired data. Depending on whether a terrestrial or an aquatic model organism is selected, exposure routes can include oral ingestion or gavage, immersion in water containing purified cyanotoxins or crude extracts, or intraperitoneal injection . The choice of exposure route can significantly impact the toxic potential of cyanotoxins and the severity of their effects. Studies have demonstrated notable differences in the effects caused by various exposure routes, emphasizing the importance of carefully selecting the appropriate model organism and exposure method for meaningful toxicity testing [15][16].
2.1. Mouse Bioassay
In this assay, adult mice are usually intraperitoneally (i.p.) injected or orally exposed to a sample and monitored for up to 24 h for toxicity-related symptoms. The observed symptoms and mortality are primarily used to determine the toxicity of bloom water samples in a qualitative manner, labeling a bloom as „toxic” or „non-toxic” [17][18][19], although the toxic symptoms and pathology observed in the exposed animals can indicate the class of the toxins [20][19]. Moreover, biological potency of the identified cyanotoxins can be established by calculating the LC50 values, and the assay can potentially be calibrated against a specific toxin such as microcystin-LR and, therefore, produce results in terms of microcystin-LR toxicity equivalents [20]. Mouse bioassay has been instrumental in determining the LC50 values of various types of cyanotoxins, including microcystins, cylindrospermopsin, nodularin, and saxitoxins. The reported 24 h LC50 values for microcystin-LR, for example, range from 5 to 10.9 mg/kg [21][22][23][24] and 25 to 158 µg/kg [21][22][23][24][25][26] for oral and intraperitoneal administration, respectively. The liver and hepatocyte damage induced by both routes is similar, with hemorrhage and apoptosis being prominent [9][25][27][28]. Additionally, exposure to microcystin causes liver congestion with blood, increased liver/body weight ratios, and reduced serum glucose and total protein levels in the affected mice [22][24][29]. The LC50 values established for mice after nodularin exposure (intraperitoneal) range from 30 to 50 μg/kg [30][31][32]. Cylindrospermopsin, on the other hand, has been shown to cause cell death in mice, with a 24 h LC50 of approximately 2 mg/kg after intraperitoneal injection [33][32][34] and 0.2 mg/kg after 5–6 days of exposure [35]. Ingestion of CYN in mice can lead to liver damage, but it may also affect other organs, including the kidney, lungs, thymus, spleen, adrenal glands, and heart. This is especially true in cases where CYN-containing cell extracts are injected intraperitoneally, which can be more toxic and demonstrate a wider range of toxicity than pure CYN [32][36][37][38]. The toxicity of anatoxins appears to be largely dependent on the exposure route. Anatoxin-a and homoanatoxin-a have an LC50 range of approximately 200–250 µg/kg in mice (intraperitoneal), 730 µg/kg for dihydroanatoxin-a, and 20 µg/kg for guanitoxin [39][40]. However, when orally administered, the toxicity of dihydroanatoxin-a has been proven to be higher (feeding—8 mg/kg; gavage—2.5 mg/kg) than that of anatoxin-a (feeding—25 mg/kg; gavage—10 mg/kg) [38]. Finally, saxitoxins have LC50 values of 5–10 μg/kg (intraperitoneal) in mice [40]. As can be seen, much of the essential information on cyanobacterial toxicity and modes of action for different toxin groups available today were obtained using the mouse bioassay. Even today, this test remains a valuable tool for the initial screening of highly concentrated cyanobacterial samples of unknown toxicity. However, it is not appropriate for cyanotoxin quantification in water samples due to its lack of sensitivity and precision at low concentrations [20]. Additionally, the number of mice required for meaningful testing procedures would be impractical and is widely considered unethical. Therefore, an alternative method should be used for the sensitive quantification of cyanotoxins in water samples, particularly when testing for microcystins at concentrations around 1 μg/L, the value mentioned in the provisional drinking water guideline of the World Health Organization. Overall, the mouse bioassay can be useful in combination with other analytical methods for a comprehensive assessment of cyanobacterial toxicity.
2.2 Aquatic Vertebrate Animal Models
Fish species such as zebrafish (Danio rerio), fathead minnow (Pimephales promelas), rainbow trout (Oncorhynchus mykiss), Japanese medaka (Oryzias latipes), Nile tilapia (Oreochromis niloticus), and the common carp (Cyprinus carpio) are used as animal models in cyanobacterial toxicity studies, providing possible insights into the potential effects of toxins on human health. Smaller teleost fish species such as zebrafish and medaka are relatively easy to maintain and breed, allowing large numbers of individuals to be generated quickly for statistically significant sample sizes at low costs [41][42]. They also have a relatively short lifespan and develop rapidly, which simplifies the study of the effects of toxins on different developmental stages. Furthermore, some fish species, such as the zebrafish, show a high degree of genetic, anatomical, and physiological similarity with mammals [42]. This similarity allows researchers to investigate toxicological effects on fish organ systems and extrapolate the results to predict potential effects in humans with increased accuracy. Numerous studies have been conducted to investigate the effects of experimental intoxications in mammals and fish which have revealed significant liver damage, manifested as hemorrhages, apoptosis, cellular hypertrophy, and glycogen depletion, along with the occurrence of apoptotic cells and dissociation of liver sheets in response to microcystin exposure [32][43][44][45][46][47][48]. As is the case with mammalian organisms, fish liver is an ideal organ for studying cyanobacterial hepatotoxins, as it is the primary target of these toxins and also the principal detoxification organ, reflecting the organism’s overall response to xenobiotics. Moreover, liver function is tied to the regulation of reproduction in oviparous fish through the synthesis of hepatic vitellogenin (vtg) [47]. Therefore, fish liver proteome and transcriptome analysis can potentially provide insights into the reproductive toxicity of different cyanotoxins. In vivo studies indicate that microcystin exposure alters the morphology of fish liver cells [15][49][50][51]. However, though the morphological alterations found in fish livers following microcystin exposure resemble those produced in rat or mouse liver cells, there is evidence to suggest that fish may be less sensitive towards MC toxicity [52][53], possibly due to their long evolutionary history of adaptation to aquatic environments where they have been exposed to these toxins. Interestingly, toxicity studies have also suggested that embryos may be more susceptible to cyanobacterial toxins than juvenile and adult fish [54][55], although direct ambient environmental exposure of fish embryos to toxicants may be limited due to the highly resistant chorion of the embryo, which reduces microcystin penetration. Improved microinjection technologies have been developed [55] which are less aggressive to the embryos, resulting in very low mortality and providing a reliable means of exposure to toxicants for research purposes.
Fish, however, serve as more than just predictors of toxic agents’ direct impact on humans. Taking into account their critical role in both ecosystem function and human food provision, it is clear why an extensive amount of research has been conducted on cyanotoxin accumulation in fish muscle tissues, as well as histopathological changes in fish organs [12][56]. Fish, especially species that actively feed on phytoplankton, are directly exposed to possible hepatotoxins by ingesting contaminated organisms and/or passively via their epithelium (gills and skin) when hepatotoxins are dissolved in water [12]. Model organisms that incorporate both human and environmental health aspects are particularly valuable in the study of cyanotoxins given the connection between the two.
3. Bioassays with Invertebrate Animal Models
In recent years, the interest in using invertebrate models for toxicity testing of cyanobacterial metabolites has been growing, as they offer several advantages over traditional mammalian models, including cost-effectiveness, high throughput, and fewer ethical concerns [48][46]. Moreover, invertebrate models can provide valuable insights into the sublethal effects of cyanobacterial toxins on the environment and ecosystem. In this chapter, the researchers review the current state of knowledge on invertebrate toxicity testing of cyanobacterial toxicity, focusing on the strengths and limitations of various invertebrate models and their suitability for different types of toxicity assessments and samples.
3.1. Artemia salina Bioassay
Brine shrimp lethality bioassay [57][58][59] is a fast and inexpensive test with no culture maintenance required, as A. salina cysts (eggs), which are commercially available, can be stored for several years at −20 °C once freeze-dried, readily hatched from this state within 24 h into nauplii (larvae), and used in experiments without the need for special equipment. Uniformity of the experimental groups can be ensured by using larvae in the same developmental stage, as well as by taking into consideration their geographical origin, as these factors can influence growth, reproduction, and survival rates. Testing procedures which employ a high-throughput approach have been described in the past [57] and have enabled rapid analysis of a large number of samples and dilutions in a single plate. This is convenient, as this assay has mostly been established as a rapid and inexpensive substitute for cytotoxicity assays, especially valuable in laboratories not equipped with cell culture facilities. Tests can be conducted by introducing neonates into microtiter plates containing artificial salt water and the test solution and incubating the plates at 30 °C under illumination for 48 h [59][60]. The main response criterion is mortality (lack of motility), which is recorded via microscopic examination after 24 and 48 h of exposure and from which LC50 values are calculated. Studies comparing the successfulness of the Artemia bioassay with that of the mouse bioassay in testing toxic cyanobacterial metabolites have shown great similarity and a good correlation between the obtained results, confirming the reliability of this test for the investigation of cyanobacterial toxicity [61][62][63]. However, research data on the correlation between the sensitivity of the brine shrimp assay and some tumor cell lines in determining cytotoxic potential have been conflicting and will be discussed in the following chapter. One of the recognized downsides of the test is the decreased solubility and bioavailability of some substances in a saline medium, which is necessary for the normal functioning of brine shrimp [62][63]. Additionally, some inconsistencies could potentially arise when conducting tests using toxins associated with freshwater cyanobacteria in a saline environment [64], though such issues have not yet been reported.
3.2. Daphnia sp. Bioassays
Species of the genus Daphnia (Müller) are ubiquitous in temperate freshwaters and represent important indicators of changes in various aquatic environments. Within these habitats, they are often the primary grazers of algae, bacteria, and protozoa and, as such, represent an integral ecological component [65]. Their application in toxicological studies started as early as 1920s and has become a widely implemented practice when it comes to the environmental monitoring of pollutants with the adoption of guidelines concerned with acute [66] and chronic [67] toxicity testing. Among various characteristics that contribute to them being valuable model organisms in toxicity studies, including fast reproduction, large clutch size, ease of culture maintenance, and high sensitivity to the presence of environmental contaminants, is the ability of clonal reproduction [68]. Because of this trait, it is possible to achieve and maintain genetic uniformity in cultures, providing a stable, constant genetic background to which experimental results can be compared. This aspect has been further potentiated by the development of Daphnia genome [69]. Immobilization is the most commonly used endpoint in toxicity assays for these species, but they have also shown a particular sensitivity to sub-lethal concentrations of harmful substances. This sensitivity enables early detection and monitoring of toxin-induced changes during intoxication [70]. Bioassays with Daphnia species have been of great use in cyanobacterial toxicity testing. Besides the broad number of endpoints used for daphnids, in recent years, numerous studies have explored the alterations in gene expression induced by cyanotoxins in Daphnia [71][72][73][74][75], though the molecular mechanisms underlying their response to toxic cyanobacteria remain largely unresolved. Investigating gene expression and identifying novel biomarkers in ecologically relevant organisms, such as Daphnia, can provide valuable insights into environmental toxicity and offer information for ecological risk assessment. Moreover, gene expression profiling and determining toxicity modes can help to address adverse phenotypic outcomes linked to specific gene functions. Therefore, gene expression analyses can improve the understanding of the mechanisms by which cyanotoxins elicit or modulate adverse effects in daphnids, and they can help identify sensitive biomarkers responsive to cyanotoxicity. Connecting these findings with changes observable at higher levels of biological organization could provide a deeper understanding of cyanobacterial toxicity in the context of the entire organism. This understanding may also enable the detection of toxic effects at early stages in the toxic effects cascade.
3.3. Thamnocephalus platyurus Bioassay
The commercially available biotest employing larvae of the freshwater anostracan crustacean Thamnocephalus platyurus (fairy shrimp) has successfully been used in the screening of cyanobacterial toxicity, especially that of CYN and MC [76]. This is a cost-effective and standardized bioassay applicable to chemical substances, surface waters, wastewaters, groundwaters, aqueous extracts, and cyanotoxins. Thamnotoxkit F® provides all necessary materials to conduct six 24 h mortality tests in a multiwell plate, using instar II-III larvae which are hatched from cysts. Guidelines provided in ISO 14380:2011 [77] outline a method for determining the lethal effects of toxicants on Thamnocephalus platyurus and a rapid test for sublethal effects after 1 h of exposure. A 60-min feeding inhibition test and 24-h mortality test are performed according to the Rapidtoxkit and Thamnotoxkit F® standard operational procedures, respectively. In an interlaboratory study conducted in 1997, Thamnotoxkit F® microbiotest was evaluated for its potential as a tool in monitoring procedures and early detection of toxic blooms [78]. Extracts of five toxin-producing cyanobacteria (Anabaena flos-aquae, Microcystis aeruginosa, Cylindrospermopsis raciborskii, Aphanizomenon flos-aquae, and Tychonema bourrellyi) were selected for the assessment and the concentration series used ranged from 0.3 to 5.0 mg/mL. The results showed that T. platiurus larvae were most sensitive to the extracts of Anabaena flos-aquae and M. aeruginosa, which caused 100% mortality in almost all the concentrations used, except for the highest dilution, in which the mortality rate was still around 73.5%. The extracts of C. raciborskii caused 100% mortality starting from a concentration of 1.0 mg/mL, while A. flos-aquae caused total mortality at a concentration of 3.0 mg/mL. The usefulness of Thamnotoxkit F® was demonstrated in detecting microcystin-LR in cyanobacterial bloom samples [79]. It was found to be more sensitive to the presence of this toxin than other assays, including the mouse bioassay. However, one of the main obstacles when implementing this assay into routine monitoring of bloom toxicity is the issue of hypersensitivity and lack of discriminative power when it comes to toxic and non-toxic cyanobacterial samples.
3.4. Chironomus Bioassays
Chironomus riparius, also known as the harlequin fly or non-biting midge, is commonly used in toxicity testing procedures designed to assess the effects of pollutants and toxic substances potentially harmful to benthic organisms. It is known that toxic secondary metabolites released during cyanobacterial blooms in aquatic ecosystems can be harmful to benthic invertebrates [46] because after the bloom, dead cyanobacterial cells settle at the bottom of the basin and contaminate the benthic community, causing harm to organisms, including chironomid larvae, which are abundant in these environments. This insect species is sensitive to changes in water quality and provides a rapid, cost-effective, and sensitive model for evaluating the toxicity of water samples and a useful tool for monitoring water quality and environmental health. The toxicity testing procedures have been designed to assess the effects of both acute [80] and prolonged [81][82][83] exposure of chironomids to waterborne agents. They are basically modifications of the previously described Daphnia sp. acute immobilization test. The test uses first instar larvae of C. riparius (recommended) or C. dilutus and C. yoshimatsui that are randomly selected from a batch culture. Despite being underrepresented in the literature, the use of Chironomus riparius as a model organism in cyanobacterial toxicity testing offers several advantages, including its high sensitivity to various toxic compounds, ease of maintenance in laboratory conditions, and the establishment of both acute and chronic testing procedures for this species. This species belongs to the most abundant and widely distributed insect group in freshwater [84] and, as a benthic species, it allows for the functional assessment of the threat posed by sediments polluted by toxic compounds. However, as there are many examples of successful rearing of dipteran larvae using monocultures of cyanobacteria, there is potentially a point to be made for decreased sensitivity of these organisms to cyanobacterial toxicity. Furthermore, differences in sensitivity may exist among different populations of the organism, and its responses may not be representative of other aquatic organisms.
4. In vitro Bioassays
The first in vitro study of cyanobacterial toxicity was published in 1981 [27], guided by the idea that liver deformations previously reported in poisoned animals would be reflected in the effects observed in the isolated hepatic cells. The authors exposed a primary culture of freshly isolated rat hepatocytes to a purified toxin isolated from a Microcystis aeruginosa bloom and observed the changes in the affected cells using scanning electron microscopy and phase-contrast microscopy. They found that incubation with the toxin caused cells to become deformed and that the severity of this effect was dependent on the applied dose and duration of exposure. Since this early publication, numerous in vitro studies of cyanobacterial toxicity have been published, and these studies have helped to shed light on the mechanisms of cyanobacterial toxicity and the factors that contribute to this toxicity. These in vitro models offer a versatile and flexible approach in cyanobacterial toxicity testing, and they align with the growing trend of minimizing the use of animals in toxicity testing, especially since the establishment of immortalized cell lines, which can renew themselves in artificial cultures indefinitely [85]. Primary cell cultures often maintain enzyme activity to a higher degree than immortalized cell lines, which allows for various investigations to be performed, including the determination of metabolic profiles and examination of inhibition and induction effects. Additionally, a single preparation of primary cells can be used to test a large number of samples, which can be more cost-effective than using whole animals [86]. Established cell lines, on the other hand, have the advantage of being more stable and easier to maintain over time compared with primary cells. This makes them more convenient to use in long-term studies or high-throughput screens [87]. Additionally, established cell lines are readily available and well-characterized, which may allow for more consistent and reliable results compared with primary cells [85]. Due to these advantages, vertebrate cell cultures are an increasingly popular replacement for animal testing in toxicity studies. However, it should be noted that both primary and established cell lines have limitations. Established cell lines, while stable and well-characterized, may not be fully representative of the target tissue or organ, particularly if the cell line was derived from a different tissue or species [88]. Furthermore, there is evidence of altered transport properties in certain cells due to the passaging process, which leads to decreased carrier permeability for specific compounds, thereby lowering their effectiveness [89]. Primary cell lines, on the other hand, are more difficult to maintain, prone to genetic drift, and have a limited lifespan [90]. Additionally, primary cells can be difficult to obtain, and the process of isolating them can potentially affect their response to toxins. Overall, the use of cell lines in cyanobacterial toxicity testing should be carefully considered, taking into account the specific goals of the study, the availability of appropriate cell lines, and the limitations of each system. In eukaryotic cells, MCs enter via transmembrane multispecific organic anion transporters, which are expressed in the liver, kidney, gastrointestinal tract, and brain [91][92], and once they enter the organism, they accumulate in the liver. Considering that MCs and CYNs, two of the most toxicologically relevant cyanotoxins, induce hepatic damage, many studies concerning cyanobacterial cytotoxicity were selected liver cell lines such as the human hepatocellular carcinoma HepG2. A higher toxicity of CYN than MC-LR in HepG2 cells was reported, with EC50 values (24 h) of ~4 and 90 μg/mL, respectively [93]. The obtained EC50 values in the case of CYN after 24 and 48 h were comparable with those obtained earlier [94][95], while in the case of MC-LR, cytotoxic effects were not found in HepG2 and other hepatic cell lines at concentrations up to 100 μg/mL (48–96 h) [96]. Such discrepancies in EC50 values could be due to experimental conditions (passage of cells, medium used, etc.) [92]. In terms of the cytotoxicity of cyanobacterial extracts to the HepG2 cell line, IC50 values in the range from 49 to 396 μg/mL were recorded [97]. Non-hepatic cell lines, including cancer cell lines, have also been utilized for evaluating cyanobacterial cytotoxicity (refer to Table 3). Among these, HeLa cells (human cervical epithelial adenocarcinoma cells) are most commonly used for conducting cytotoxicity and antitumor activity tests [98]. As cyanobacteria are known producers of neurotoxic compounds, some cell lines have been specifically used to estimate neurotoxicity. Human neuroblastoma cells SH-SY5Y have been used in several studies to test the cytotoxicity of BMAA (β-N-methylamino-L-alanine), a non-proteinogenic and toxic amino acid that may harm the nervous system and provoke neurodegenerative diseases such as Alzheimer’s and amyotrophic lateral sclerosis. Using this cell line, different effects were found, such as increased ROS and protein oxidation, upregulation of lysosomal enzymes and apoptosis, misincorporation of L-BMAA protein aggregation, etc., while in HepG2 and Caco-2 cells, BMAA did not affect the common proteinogenic amino acid metabolic pathways [99].
5. Limitations and Challenges in Cyanobacterial Toxicity Testing
Given the complexities of cyanobacterial toxicity, developing alternatives to mammalian assays with high predictability of in vivo effects is a difficult task. Bioassays play a crucial role in the investigation of substances produced by cyanobacteria, which may have unknown or insufficiently characterized effects. It is clear that the field of cyanobacterial toxicity testing faces several challenges, including ethical limitations in using various animal models, the varying sensitivity levels of different model organisms, the difficulty in extrapolating data to humans, the complexity of samples where identifying the specific compound responsible for toxicity is often challenging, and inability to accurately predict long-term effects of low-level exposure. These challenges require innovative approaches to ensure accurate and ethical testing while providing valuable insights into the potential risks of cyanobacterial toxins. The many bioassays that have been developed, from molecular to whole-organism levels, offer different complexities, and each approach has its own strengths and limitations [100][101][102]. However, a single test is often insufficient, and a combination of different testing methods may be necessary to fully assess the potential risks of exposure to various toxic cyanobacterial metabolites. Animal models continue to provide benefits because they share genetic and physiological similarities with humans, however, unforeseeable factors in animal organisms and the high cost of breeding and housing animals for research purposes are crucial aspects to consider [103][104]. On the other hand, human cell cultures can be cultivated as organotypic cultures to permit easier extrapolation of in vitro results to humans. However, obtaining and treating basic human cells in a safe and ethical manner remains a challenge. Furthermore, in vitro testing may not accurately depict the complexity of whole organisms and the interactions of diverse cell types and organs [105]. Despite their rapid responses, it is essential to validate bioassays’ performances, particularly if they are to be used in complex samples such as raw or treated drinking water or blooms. In vitro bioassays can provide insight into the biochemical processes underlying toxicity, while in vivo studies, despite ethical and technical concerns, are still necessary for risk assessment and guideline value derivation [106][107].
6. Tracking the Evolution of Bioassays for Cyanotoxin Testing
In this section, an overview of the historical and current research landscape in the field of cyanobacterial toxicity testing is provided. A comprehensive search of three major scientific databases, i.e., Scopus, PubMed, and Embase was conducted, focusing on publications that utilize some the most commonly applied animal models in cyanobacterial toxicity testing (Figure 1). The obtained references were categorized according to the year they were published, providing an insight into the historical development of bioassays for cyanotoxin testing and the frequency of their use from 1969 until today. Furthermore, a distribution of publications across different toxin classes was included, allowing for a visual representation of the current state of research in the field of cyanotoxin bioassay testing, and highlighting areas where further research would be needed. The main cyanotoxin classes included in the analysis were microcystins (MCs), cylindrospermopsins (CYN), all anatoxins, and guanitoxin grouped together and presented as ANTX and saxitoxins (STX). After combining the found publications from all three databases into a single document, duplicates and secondary source publications were removed, resulting in a total of 1067 original research articles used in the analysis. Among these, Artemia salina were used in 176 publications, with 38 studies focusing on microcystin (MC) testing, 10 on cylindrospermopsin (CYN), 6 on saxitoxin (STX), 3 on anatoxins (ANTX), and 119 on other toxic metabolites or uncharacterized samples such as crude extracts. Daphnia were the most commonly used model, with 446 publications utilizing various species of daphnids. Of these, 198 studies focused on MC testing, 41 on CYN, 14 on STX, 9 on ANTX, and 184 on extracts or other toxic metabolites. The mouse bioassay was used in 322 publications, with 181 focusing on MC testing, 33 on CYN, 16 on STX, 18 on ANTX, and 74 on other toxins or uncharacterized samples. Finally, zebrafish were used in 123 publications, with 71 studies focusing on MC testing, 11 on CYN, 12 on STX, 3 on ANTX, and 26 on other toxic metabolites or uncharacterized samples.
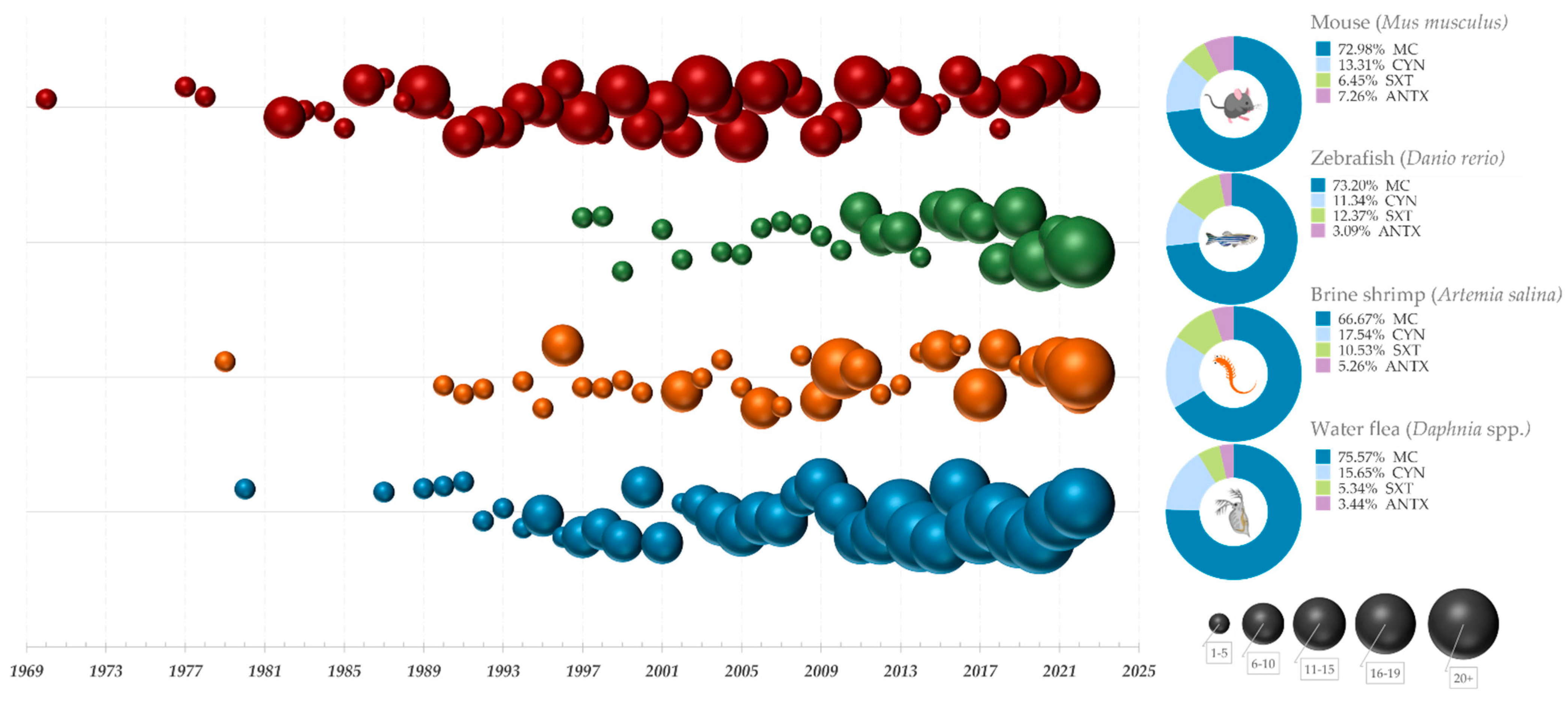
Figure 1. A historical overview of bioassays used in cyanobacterial toxicity testing. Graphs on the left side represent timelines for some of the most widely used bioassays, with the number of publications from each year, starting from 1969, represented by bubbles of different sizes and colors (Mouse–red; Zebrafish–green; Brine shrimp–orange; Water flea–blue). Legend in the lower right corner signifies 5 different bubble categories divided according to the number of publications they represent. The pie charts on the right depict the proportion of publications relating to major cyanotoxin groups.
The analysis of the number of publications over time revealed a gradual decline in the use of mice for cyanobacterial toxicity testing, particularly after the late 1990s and early 2000s. Simultaneously, there has been a significant increase in the utilization of alternative vertebrate and invertebrate models included in the analysis. This trend corresponds with some of the early articles and advisory statements that paved the way for the development and validation of alternative methods for toxicity testing of water samples, such as in vitro assays and bioassays using other non-mammalian model organisms [108]. The utilization of the Daphnia bioassay has significantly increased since the adoption of toxicity testing guidelines for these species in 2004 [66] and 2012 [67], with over 20 studies being published annually since 2017 and continuing to this day. The most-studied cyanotoxins in all included assays were microcystins and cylindrospermopsin, which was expected given their toxicity and wide distribution.tt
7. Conclusions
The research field of cyanobacterial toxicology has greatly benefited from the use of bioassays, as they have provided a more complete understanding of the diverse mechanisms of cyanotoxin action. Most studies concerned with the effects of cyanotoxins are conducted by observing changes occurring in a living organism after exposure to toxins in some form, usually a purified single toxin, although more complex crude extracts and bloom biomass are frequently analyzed. Exploring the effects of unknown or not sufficiently characterized substances produced by cyanobacteria is crucial, and bioassays are an important tool for achieving this. From molecular to organism levels, a wide range of bioassays are available. Some of them, such as the Artemia salina assay, offer a simple and inexpensive solution for rapid screening of a large number of samples containing potentially toxic compounds, without sacrificing reliability and sensitivity of the test, while others, such as the zebrafish embryo assay, can provide the means for a more in-depth analysis of toxicity on different biological levels. However, any single test is usually insufficient to fully characterize the toxicity of a cyanobacterial bloom; thus, a combination of the appropriate ones should be employed to achieve an accurate estimation. Bioassays can provide quick responses, but they require an understanding of the sensitivity limitations of the bioassays. Validation of the suitability of the chosen method is necessary, particularly when used to investigate critical samples such as raw or treated drinking water and complex samples such as cyanobacterial blooms. In vitro bioassays are useful in developing an understanding of the biochemical processes underlying toxicity, while in vivo studies, despite technical and ethical concerns, continue to play an important role in supporting risk assessment and guideline value derivation. In contrast to in vitro assays, which provide a simplified and isolated view of a toxicant’s impact, in vivo assays offer a more comprehensive representation of the complex interactions that occur in living organisms. Moving forward, there is a need for further research to refine and optimize vertebrate bioassays for cyanotoxicity testing. This includes developing standardized protocols for bioassays that can be used across different laboratories and regions, as well as identifying novel model organisms (including microorganisms and plants) that can provide new insights into the mechanisms of cyanotoxicity and which have fewer ethical concerns. Additionally, new technologies such as in vitro models and computational modeling can complement vertebrate bioassays and help reduce the number of animals used in research.
This entry is adapted from the peer-reviewed paper 10.3390/biology12050711
References
- Francis, G. Poisonous Australian Lake. Nature 1878, 18, 11–12. https://doi.org/10.1038/018011d0.
- Carmichael, W.W. Cyanobacteria Secondary Metabolites—The Cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. https://doi.org/10.1111/j.1365-2672.1992.tb01858.x.
- Simeunović, J.; Svirčev, Z.; Krstić, S.; Lazić, L. Occurrence of Cyanobacterial Blooms in Vojvodina Water Ecosystems. Geogr. Pannonica 2005, 9, 13–19.
- Wood, S.A.; Selwood, A.I.; Rueckert, A.; Holland, T.; Milne, J.R.; Smith, K.F.; Smits, B.; Watts, L.F.; Cary, C.S. First Report of Homoanatoxin-a and Associated Dog Neurotoxicosis in New Zealand. Toxicon 2007, 50, 292–301. https://doi.org/10.1016/j.toxicon.2007.03.025.
- Drobac, D.; Tokodi, N.; Simeunović, J.; Baltić, V.; Stanić, D.; Svirčev, Z. Human Exposure to Cyanotoxins and Their Effects on Health. Arh. Hig. Rada Toksikol. 2013, 64, 305–316. https://doi.org/10.2478/10004-1254-64-2013-2320.
- Svirčev, Z.; Baltić, V.; Gantar, M.; Juković, M.; Stojanović, D.; Baltić, M. Molecular Aspects of Microcystin-Induced Hepato-toxicity and Hepatocarcinogenesis. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2010, 28(1), 37–41. https://doi.org/10.1080/10590500903585382.
- Žegura, B.; Štraser, A.; Filipič, M. Genotoxicity and Potential Carcinogenicity of Cyanobacterial Toxins—A Review. Mutat. Res. Rev. Mutat. Res. 2011, 727, 16–41. https://doi.org/10.1016/j.mrrev.2011.01.002.
- Szlag, D.C.; Sinclair, J.L.; Southwell, B.; Westrick, J.A. Cyanobacteria and Cyanotoxins Occurrence and Removal from Five High-Risk Conventional Treatment Drinking Water Plants. Toxins 2015, 7, 2198–2220. https://doi.org/10.3390/toxins7062198.
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of Knowledge and Concerns on Cyanobacterial Blooms and Cyanotoxins. Environ. Int. 2013, 59, 303–327. https://doi.org/10.1016/j.envint.2013.06.013.
- Wiegand, C.; Pflugmacher, S. Ecotoxicological Effects of Selected Cyanobacterial Secondary Metabolites a Short Review. Toxi-col. Appl. Pharmacol. 2005, 203, 201–218. https://doi.org/10.1016/j.taap.2004.11.002.
- Meriluoto, J.; Spoof, L.; Codd, G.A. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; John Wiley & Sons:, Chich-ester, West Sussex, UK, 2017.
- Ferrão-filho, A.S.; Kozlowsky-suzuki, B. Cyanotoxins : Bioaccumulation and Effects on Aquatic Animals. Mar. Drugs 2011, 9, 2729–2772. https://doi.org/10.3390/md9122729.
- Sanseverino, I.; António, D.C.; Loos, R.; Lettieri, T. Cyanotoxins: Methods and Approaches for Their Analysis and Detection; 2017; EUR 28624; doi:10.2760/36186.
- Berry, J. Cyanobacterial Toxins in Food-Webs: Implications for Human and Environmental Health. In Current Topics in Public Health; Rodriguez-Morales, A.J., Ed.; 2013; pp. 531–589.
- Tencalla, F.G.; Dietrich, D.R.; Schlatter, C. Toxicity of Microcystis aeruginosa Peptide Toxin to Yearling Rainbow Trout (On-corhynchus Mykiss). Aquat. Toxicol. Elsevier B.V., Amsterdam, Netherlands, 1994, 30, 215–224. https://doi.org/10.1016/0166-445X(94)90059-0.
- Azevedo, S.M.F.O.; Evans, W.R.; Carmichael, W.W.; Namikoshi, M. First Report of Microcystins from a Brazilian Isolate of the Cyanobacterium Microcystis aeruginosa. J. Appl. Phycol. 1994, 6, 261–265. https://doi.org/10.1007/BF02181936.
- Vasconcelos, V.M.; Sivonen, K.; Evans, W.R.; Carmichael, W.W.; Namikoshi, M. Hepatotoxic Microcystin Diversity in Cya-nobacterial Blooms Collected in Portuguese Freshwaters. Water Res. 1996, 30, 2377–2384. https://doi.org/10.1016/0043-1354(96)00152-2.
- Vieira, J.M.D.S.; Azevedo, M.T.D.P.; De Oliveira Azevedo, S.M.F.; Honda, R.Y.; Corrêa, B. Toxic Cyanobacteria and Microcys-tin Concentrations in a Public Water Supply Reservoir in the Brazilian Amazonia Region. Toxicon 2005, 45, 901–909. https://doi.org/10.1016/j.toxicon.2005.02.008.
- Msagati, T.A.M.; Siame, B.A.; Shushu, D.D. Evaluation of Methods for the Isolation, Detection and Quantification of Cyano-bacterial Hepatotoxins. Aquat. Toxicol. 2006, 78, 382–397. https://doi.org/10.1016/j.aquatox.2006.03.011.
- Nicholson, B.; Burch, M. Evaluation of Analytical Methods for the Detection and Quantification of Cyanotoxins in Relation to Australian Drinking Water Guidelines; National Health and Medical Research Council of Australia: Canberra, Australia, 2001.
- Fawell, J.K.; Mitchell, R.E.; Hill, R.E.; Everett, D.J. The Toxicity of Cyanobacterial Toxins in the Mouse; II Anatoxin-A. Hum. Exp. Toxicol. 1999, 18, 168–173. https://doi.org/10.1177/096032719901800306.
- Robinson, N.A.; Miura, G.A.; Matson, C.F.; Dinterman, R.E.; Pace, J.G. Characterization of Chemically Tritiated Microcystin-LR and Its Distribution in Mice. Toxicon 1989, 27, 1035–1042.
- Chernoff, N.; Hill, D.; Lang, J.; Schmid, J.; Le, T.; Farthing, A.; Huang, H. The Comparative Toxicity of 10 Microcystin Con-geners Administered Orally to Mice: Clinical Effects and Organ Toxicity. Toxins 2020, 12, 403. https://doi.org/10.3390/toxins12060403.
- Lovell, R.A.; Schaeffer, D.J.; Hooser, S.B.; Haschek, W.M.; Dahlem, A.M.; Carmichael, W.W.; Beasley, V.R. Toxicity of Intra-peritoneal Doses of Microcystin-LR in Two Strains of Male Mice. J. Environ. Pathol. Toxicol. Oncol. Off. Organ Int. Soc. Environ. Toxicol. Cancer 1989, 9, 221–237.
- Kuiper-Goodman, T.; Falconer, I.; Fitzgerald, J. Human Health Aspects. In Toxic Cyanobacteria in Water; Chorus, I., Bartram, J., Eds.; 1999; pp. 133–174, ISBN 0419239308.
- Boaru, D.A.; Dragoş, N.; Schirmer, K. Microcystin-LR Induced Cellular Effects in Mammalian and Fish Primary Hepatocyte Cultures and Cell Lines: A Comparative Study. Toxicology 2006, 218, 134–148. https://doi.org/10.1016/j.tox.2005.10.005.
- Runnegar, M.T.; Falconer, I.R.; Silver, J. Deformation of Isolated Rat Hepatocytes by a Peptide Hepatotoxin from the Blue-Green Alga Microcystis aeruginosa. Naunyn. Schmiedebergs. Arch. Pharmacol. 1981, 317, 268–272. https://doi.org/10.1007/BF00503829.
- Gupta, N.; Pant, S.C.; Vijayaraghavan, R.; Rao, P.V.L. Comparative Toxicity Evaluation of Cyanobacterial Cyclic Peptide Tox-in Microcystin Variants (LR, RR, YR) in Mice. Toxicology 2003, 188, 285–296. https://doi.org/10.1016/S0300-483X(03)00112-4.
- Chorus, I.; Falconer, I.R.; Salas, H.J.; Bartram, J. Health Risks Caused by Freshwater Cyanobacteria in Recreational Waters. J. Toxicol. Environ. Health Part B Crit. Rev. 2000, 3, 323–347. https://doi.org/10.1080/109374000436364.
- Rinehart, K.L.; Namikoshi, M.; Choi, B.W. Structure and Biosynthesis of Toxins from Blue-Green Algae (Cyanobacteria). J. Appl. Phycol. 1994, 6, 159–176. https://doi.org/10.1007/BF02186070.
- Terao, K.; Ohmori, S.; Igarashi, K.; Ohtani, I.; Watanabe, M.F.; Harada, K.I.; Ito, E.; Watanabe, M. Electron Microscopic Stud-ies on Experimental Poisoning in Mice Induced by Cylindrospermopsin Isolated from Blue-Green Alga Umezakia Natans. Tox-icon 1994, 32, 833–843. https://doi.org/10.1016/0041-0101(94)90008-6.
- Runnegar, M.T.; Xie, C.; Snider, B.B.; Wallace, G.A.; Weinreb, S.M.; Kuhlenkamp, J. In Vitro Hepatotoxicity of the Cyanobac-terial Alkaloid Cyclindrospermopsin and Related Synthetic Analogues. Toxicol. Sci. 2002, 67, 81–87. https://doi.org/10.1093/toxsci/67.1.81.
- Ohtani, I.; Moore, R.E. Cylindrospermopsin: A Potent Hepatotoxin from the Blue-Green Alga Cylindrospermopsis raciborskii. Am. Chem. Soc. 1992, 114, 7941–7942.
- Falconer, I.R.; Hardy, S.J.; Humpage, A.R.; Froscio, S.M.; Tozer, G.J.; Hawkins, P.R. Hepatic and Renal Toxicity of the Blue-Green Alga (Cyanobacterium) Cylindrospermopsis raciborskii in Male Swiss Albino Mice. Environ. Toxicol. 1999, 14, 143–150. https://doi.org/10.1002/(SICI)1522-7278(199902)14:1<143::AID-TOX18>3.0.CO;2-H.
- Sivonen, K.; Jones, G.J. Cyanobacterial Toxins. In Toxic Cyanobacteria in Water: A Guide to their Public Health Consequences, Moni-toring and Management; Elsevier: Amsterdam, The Netherlands, 1999; pp. 43–112. https://doi.org/10.1016/B978-012373944-5.00005-5.
- Humpage, A.R.; Fenech, M.; Thomas, P.; Falconer, I.R. Micronucleus Induction and Chromosome Loss in Transformed Hu-man White Cells Indicate Clastogenic and Aneugenic Action of the Cyanobacterial Toxin, Cylindrospermopsin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 472, 155–161. https://doi.org/10.1016/S1383-5718(00)00144-3.
- Shaw, G.R.; Seawright, A.A.; Lam, P.S. Cylindrospermopsin, a Cyanobacterial Alkaloid: Evaluation of Its Toxicologic Activity. Ther. Drug Monit. 2000, 22, 89–92.
- Puddick, J.; van Ginkel, R.; Page, C.D.; Murray, J.S.; Greenhough, H.E.; Bowater, J.; Selwood, A.I.; Wood, S.A.; Prinsep, M.R.; Truman, P.; et al. Acute Toxicity of Dihydroanatoxin-a from Microcoleus autumnalis in Comparison to Anatoxin-A. Chemo-sphere 2021, 263, 127937. https://doi.org/10.1016/j.chemosphere.2020.127937.
- Carmichael, W.W.; Mahmood, N.A.; Hyde, E.G. Natural Toxins from Cyanobacteria (Blue-Green Algae). In Marine Toxins: Origin, Structure, and Molecular Pharmacology; ACS Publications: Washington, DC, USA, 1990; Volume 418, pp. 87–106.
- Schatz, D.; Keren, Y.; Vardi, A.; Sukenik, A.; Carmeli, S.; Börner, T.; Dittmann, E.; Kaplan, A. Towards Clarification of the Biological Role of Microcystins, a Family of Cyanobacterial Toxins. Environ. Microbiol. 2007, 9, 965–970. https://doi.org/10.1111/j.1462-2920.2006.01218.x.
- Lawrence, C.; Adatto, I.; Best, J.; James, A.; Maloney, K. Generation Time of Zebrafish (Danio rerio) and Medakas (Oryzias latipes) Housed in the Same Aquaculture Facility. Lab Anim. 2012, 41, 158–165. https://doi.org/10.1038/laban0612-158.
- Goldsmith, J.R.; Jobin, C. Think Small: Zebrafish as a Model System of Human Pathology. J. Biomed. Biotechnol. 2012, 2012, 817341. https://doi.org/10.1155/2012/817341.
- Botha, N.; Gehringer, M.M.; Downing, T.G.; Van De Venter, M.; Shephard, E.G. The Role of Microcystin-LR in the Induction of Apoptosis and Oxidative Stress in CaCo2 Cells. Toxicon 2004, 43, 85–92. https://doi.org/10.1016/j.toxicon.2003.10.025.
- Gupta, U.S.; Guha, S. Microcystin Toxicity in a Freshwater Fish, Heteropneustes fossilis (Bloch). Curr. Sci. 2006, 91, 1261–1271.
- Lin, W.; Hou, J.; Guo, H.; Li, L.; Wang, L.; Zhang, D. The Synergistic Effects of Waterborne Microcystin-LR and Nitrite on Hepatic Pathological Damage, Lipid Peroxidation and Antioxidant Responses of Male Zebrafish. Environ. Pollut. 2018, 235, 197–206. https://doi.org/10.1016/j.envpol.2017.12.059.
- Shahmohamadloo, R.S.; Poirier, D.G.; Ortiz Almirall, X.; Bhavsar, S.P.; Sibley, P.K. Assessing the Toxicity of Cell-Bound Microcystins on Freshwater Pelagic and Benthic Invertebrates. Ecotoxicol. Environ. Saf. 2020, 188, 109945. https://doi.org/10.1016/j.ecoenv.2019.109945.
- Arukwe, A.; Goksøyr, A. Eggshell and Egg Yolk Proteins in Fish: Hepatic Proteins for the next Generation: Oogenetic, Pop-ulation, and Evolutionary Implications of Endocrine Disruption. Comp. Hepatol. 2003, 2, 4. https://doi.org/10.1186/1476-5926-2-4.
- Michael, A.S.; Thomps, C.G.; Abraliov, M. Artemia salina as a Test Organism for Bioassay. Science 1956, 123, 464.
- Fournie, J.W.; Courtney, L.A. Histopathological Evidence of Regeneration Following Hepatotoxic Effects of the Cyanotoxin Microcystin-LR in the Hardhead Catfish and Gulf Killifish. J. Aquat. Anim. Health 2002, 14, 273–280. https://doi.org/10.1577/1548-8667(2002)014.
- Li, X.; Liu, Y.; Song, L. Cytological Alterations in Isolated Hepatocytes from Common Carp (Cyprinus carpio L.) Exposed to Microcystin-LR. Environ. Toxicol. 2001, 16, 517–522. https://doi.org/10.1002/tox.10012.
- Fischer, W.J.; Dietrich, D.R. Pathological and Biochemical Characterization of Microcystin-Induced Hepatopancreas and Kidney Damage in Carp (Cyprinus carpio). Toxicol. Appl. Pharmacol. 2000, 164, 73–81. https://doi.org/10.1006/taap.1999.8861.
- Arman T., Clarke J.D. Microcystin Toxicokinetics, Molecular Toxicology, and Pathophysiology in Preclinical Rodent Models and Humans. Toxins (Basel). 2021 Jul 29;13(8):537. doi: 10.3390/toxins13080537.
- Oberemm, A.; Fastner, J.; Steinberg, C.E.W. Effects of Microcystin-LR and Cyanobacterial Crude Extracts on Embryo-Larval Development of Zebrafish (Danio rerio). Water Res. 1997, 31, 2918–2921. https://doi.org/10.1016/S0043-1354(97)00120-6.
- Oberemm, A.; Becker, J.; Codd, G.A.; Steinberg, C. Effects of Cyanobacterial Toxins and Aqueous Crude Extracts of Cyanobac-teria on the Development of Fish and Amphibians. Environ. Toxicol. 1999, 14, 77–88. https://doi.org/10.1002/(SICI)1522-7278(199902)14:1<77::AID-TOX11>3.0.CO;2-F.
- Black, J.J.; Maccubbin, A.E.; Schiffert, M. A Reliable, Efficient, Microinjection Apparatus and Methodology for the in Vivo Exposure of Rainbow Trout and Salmon Embryos to Chemical Carcinogens. J. Natl. Cancer Inst. 1985, 6, 1123–1128.
- Achenbach, J.C.; Leggiadro, C.; Sperker, S.A.; Woodland, C.; Ellis, L.D. Comparison of the zebrafish embryo toxicity assay and the General and Behavioral Embryo Toxicity Chemical Screening. Toxics 2020, 8, 126.
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, J.D. A Microwell Cytotoxicity Assay Using Artemia salina (Brine Shrimp). Planta Med. 1993, 59, 250–252. https://doi.org/10.1055/s-2006-959661.
- Kiviranta, J.; Sivonen, K.; Niemelä, S.I.; Huovinen, K. Detection of Toxicity of Cyanobacteria by Artemia salina Bioassay. Envi-ron. Toxicol. Water Qual. 1991, 6, 423–436. https://doi.org/10.1002/tox.2530060407.
- Vezie, C.; Benoufella, F.; Sivonen, K.; Bertru, G.; Laplanche, A. Detection of Toxicity of Cyanobacterial Strains Using Artemia salina and Microtox ® Assays Compared with Mouse Bioassay Results. Phycologia 1996, 35, 198–202.
- Lee, T.-H.; Chen, Y.-M.; Chou, H.-N. Toxicity Assay of Cyanobacterial Strains Using Artemia salina in Comparison with the Mouse Bioassay. Acta Zool. Taiwanica 1999, 10, 1.
- Lopes, V.R.; Fernández, N.; Martins, R.F.; Vasconcelos, V. Primary Screening of the Bioactivity of Brackishwater Cyanobacte-ria: Toxicity of Crude Extracts to Artemia salina Larvae and Paracentrotus lividus Embryos. Mar. Drugs 2010, 8, 471–482. https://doi.org/10.3390/md8030471.
- Mayorga, P.; Perez, K.R.; Cruz, S.M.; Caceres, A. Comparison of Bioassays Using the Anostracan Crustaceans Artemia Salin a and Thamnocephalus platyurus for Plant Extract Toxicity Screening. Brazilian J. Pharmacogn. 2010, 20, 897–903. https://doi.org/10.1590/S0102-695X2010005000029.
- Dos Santos, H.D.; De Oliveira, F.F.; De Oliveira, R.A. Influence of Solubility of Ethanol Extracts in Artemia salina Tests. Rev. Virtual Quim. 2017, 9, 1535–1545. https://doi.org/10.21577/1984-6835.20170089.
- Malbrouck, C.; Kestemont, P. Effects of Microcystins on Fish. Environ. Toxicol. Chem. 2006, 25, 72–86. https://doi.org/10.1897/05-029R.1.
- Tessier, A.J.; Leibold, M.A.; Tsao, J. A Fundamental Trade-off in Resource Exploitation by Daphnia and Consequences to Plankton Communities. Ecology 2000, 81, 826–841. https://doi.org/10.2307/177380.
- OECD. Test No. 202: Daphnia Sp. Acute Immobilisation Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 2004; OECD Publishing, Paris, ISBN 9789264069947.
- OECD. Daphnia magna Reproduction Test, OECD Guidelines for the Testing of Chemicals, Test No. 211; OECD: Paris, France, 2012.
- Hebert, P.D.N.; Ward, R.D. Inheritance during Parthenogenesis in Daphnia magna. Genetics 1972, 71, 639–642.
- Colbourne, J.K.; Singan, V.R.; Gilbert, D.G. WFleaBase: The Daphnia Genome Database. BMC Bioinformatics 2005, 6, 45. https://doi.org/10.1186/1471-2105-6-45.
- Bownik, A. Physiological Endpoints in Daphnid Acute Toxicity Tests. Sci. Total Environ. 2020, 700, 134400. https://doi.org/10.1016/j.scitotenv.2019.134400.
- Asselman, J.; De Coninck, D.I.M.; Glaholt, S.; Colbourne, J.K.; Janssen, C.R.; Shaw, J.R.; De Schamphelaere, K.A.C. Identification of Pathways, Gene Networks, and Paralogous Gene Families in Daphnia Pulex Responding to Exposure to the Toxic Cyanobacterium Microcystis aeruginosa. Environ. Sci. Technol. 2012, 46, 8448–8457. https://doi.org/10.1021/es301100j.
- He, Z.; Chen, Y.; Huo, D.; Gao, J.; Xu, Y.; Yang, R.; Yang, Y.; Yu, G. Combined-Methods Elucidate the Multi-Organ Toxicity of Cylindrospermopsin (CYN) on Daphnia magna. Environ. Pollut. 2023, 324, 121250. https://doi.org/10.1016/j.envpol.2023.121250.
- Lyu, K.; Meng, Q.; Zhu, X.; Dai, D.; Zhang, L.; Huang, Y.; Yang, Z. Changes in ITRAQ-Based Proteomic Profiling of the Cladoceran Daphnia magna Exposed to Microcystin-Producing and Microcystin-Free Microcystis aeruginosa. Environ. Sci. Technol. 2016, 50, 4798–4807. https://doi.org/10.1021/acs.est.6b00101.
- Shahmohamadloo, R.S.; Simmons, D.B.D.; Sibley, P.K. Shotgun Proteomics Analysis Reveals Sub-Lethal Effects in Daphnia magna Exposed to Cell-Bound Microcystins Produced by Microcystis aeruginosa. Comp. Biochem. Physiol. Part D Genom. Proteom. 2020, 33, 100656. https://doi.org/10.1016/j.cbd.2020.100656.
- Schwarzenberger, A.; Sadler, T.; Motameny, S.; Ben-Khalifa, K.; Frommolt, P.; Altmüller, J.; Konrad, K.; von Elert, E. Deciphering the Genetic Basis of Microcystin Tolerance. BMC Genom. 2014, 15, 776. https://doi.org/10.1186/1471-2164-15-776.
- Kozma Törökné, A.; László, E.; Chorus, I.; Fastner, J.; Heinze, R.; Padisák, J.; Barbosa, F.A. Water Quality Monitoring by Thamnotoxkit F(TM) Including Cyanobacterial Blooms. Water Sci. Technol. 2000, 42, 381–385. https://doi.org/10.2166/wst.2000.0342.
- ISO (the International Organization for Standardization). Water Quality—Determination of the Acute Toxicity to Thamnocephalus platyurus (Crustacea, Anostraca) Qualité; ISO (the International Organization for Standardization), Geneva, Switzerland: 2011; Volume 2011.
- Torokne, A. The Potential of the Thamnotoxkit Microbiotest for Routine Detection of Cyanobacterial Toxins. In New Microbi-otests for Routine Toxicity Screening and Biomonitoring; Persoone, G., Janssen, C.R., De Coen, W.M., Eds.; Springer Science: New York, NY, USA, 2000; pp. 533–540.
- Tarczynska, M.; Nalecz-Jawecki, G.; Brzychcy, M.; Zalewski, M.; Sawicki, J. The Toxicity of Cyanobacterial Blooms as Deten-nined by Microbiotests and Mouse Assays. In New Microbiotests for Routine Toxicity Screening and Biomonitoring; Persoone, G., Janssen, C.R., De Coen, W.M., Eds.; Springer Science: New York, NY, USA, 2000; Volume 4, pp. 527–532, ISBN 9781461369240.
- OECD. Test No. 235: Chironomus Sp., Acute Immobilisation Test; OECD Publishing, Paris: 2011; pp. 1–4.
- OECD. Test No. 233: Sediment-Water Chironomid Life-Cycle Toxicity Test Using Spiked Water or Spiked Sediment; OECD Publishing, Paris: 2010; p. 29.
- OECD. Test No. 218: Sediment-Water Chironomid Toxicity Using Spiked Water, OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing, Paris: 2004; pp. 1–21.
- OECD. Test No. 219: Sediment- Water Chironomid Toxicity Test Using Siked Water; OECD Publishing, Paris: 2004; pp. 1–21.
- Armitage, P.D.; Cranston, P.S.; Pinder, L.C.V. The Chironomidae. Biology and ecology of non-biting midges, 1st ed.; Chapman & Hall, London, UK: 1995; ISBN 9789401043083.
- Schirmer, K. Proposal to Improve Vertebrate Cell Cultures to Establish Them as Substitutes for the Regulatory Testing of Chemicals and Effluents Using Fish. Toxicology 2006, 224, 163–183. https://doi.org/10.1016/j.tox.2006.04.042.
- Verma, A.; Verma, M.; Singh, A. Animal Tissue Culture Principles and Applications. In Animal Biotechnology; Verma, A.S., Singh, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 2, pp. 1–12, ISBN 9788578110796.
- Li, Z. In Vitro Micro-Tissue and -Organ Models for Toxicity Testing. Compr. Biotechnol. Second Ed. 2011, 5, 551–563. https://doi.org/10.1016/B978-0-08-088504-9.00503-1.
- Moysidou, C.M.; Barberio, C.; Owens, R.M. Advances in Engineering Human Tissue Models. Front. Bioeng. Biotechnol. 2021, 8, 620962. https://doi.org/10.3389/fbioe.2020.620962.
- Yu, H.; Cook, T.J.; Sinko, P.J. Evidence for Diminished Functional Expression of Intestinal Transporters in Caco-2 Cell Monolayers at High Passages. Pharm. Res. 1997, 14, 757–762. https://doi.org/10.1023/A:1012150405949.
- Kaur, G.; Dufour, J.M. Cell Lines. Spermatogenesis 2012, 2, 44–52. https://doi.org/10.4161/spmg.19885.
- Dietrich, D.; Hoeger, S. Guidance Values for Microcystins in Water and Cyanobacterial Supplement Products (Blue-Green Algal Supplements): A Reasonable or Misguided Approach? Toxicol. Appl. Pharmacol. 2005, 203, 273–289. https://doi.org/10.1016/j.taap.2004.09.005.
- Van Apeldoorn, M.E.; Van Egmond, H.P.; Speijers, G.J.A.; Bakker, G.J.I. Toxins of Cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. https://doi.org/10.1002/mnfr.200600185.
- Gutiérrez-Praena, D.; Guzmán-Guillén, R.; Pichardo, S.; Moreno, F.J.; Vasconcelos, V.; Jos, Á.; Cameán, A.M. Cytotoxic and Morphological Effects of Microcystin-LR, Cylindrospermopsin, and Their Combinations on the Human Hepatic Cell Line HepG2. Environ. Toxicol. 2019, 34, 240–251. https://doi.org/10.1002/tox.22679.
- Neumann, C.; Bain, P.; Shaw, G. Studies of the Comparative in Vitro Toxicology of the Cyanobacterial Metabolite DeoxyCylindrospermopsin. J. Toxicol. Environ. Health Part A 2007, 70, 1679–1686.
- Štraser, A.; Filipič, M.; Žegura, B. Genotoxic Effects of the Cyanobacterial Hepatotoxin Cylindrospermopsin in the HepG2 Cell Line. Arch. Toxicol. 2011, 85, 1617–1626. https://doi.org/10.1007/s00204-011-0716-z.
- Chong, M.W.K.; Gu, K.D.; Lam, P.K.S.; Yang, M.; Fong, W.F. Study on the Cytotoxicity of Microcystin-LR on Cultured Cells. Chemosphere 2000, 41, 143–147.
- Senousy, H.H.; Abd Ellatif, S.; Ali, S. Assessment of the Antioxidant and Anticancer Potential of Different Isolated Strains of Cyanobacteria and Microalgae from Soil and Agriculture Drain Water. Environ. Sci. Pollut. Res. 2020, 27, 18463–18474. https://doi.org/10.1007/s11356-020-08332-z.
- Batsalova, T.; Basheva, D.; Bardarov, K.; Bardarov, V.; Dzhambazov, B.; Teneva, I. Assessment of the Cytotoxicity, Antioxidant Activity and Chemical Composition of Extracts from the Cyanobacterium Fischerella Major Gomont. Chemosphere 2019, 218, 93–103. https://doi.org/10.1016/j.chemosphere.2018.11.097.
- Sazdova, I.; Keremidarska-Markova, M.; Chichova, M.; Uzunov, B.; Nikolaev, G.; Mladenov, M.; Schubert, R.; Stoyneva-Gärtner, M.; Gagov, H.S. Review of Cyanotoxicity Studies Based on Cell Cultures. J. Toxicol. 2022, 2022, 5647178. https://doi.org/10.1155/2022/5647178.
- Davidović, P.G.; Blagojević, D.J.; Lazić, G.G.; Simeunović, J.B. Gene Expression Changes in Daphnia magna Following Waterborne Exposure to Cyanobacterial Strains from the Genus Nostoc. Harmful Algae 2022, 115, 102232. https://doi.org/10.1016/j.hal.2022.102232.
- Porazinski, S.R.; Wang, H.; Furutani-Seiki, M. Essential Techniques for Introducing Medaka to a Zebrafish Laboratory—Towards the Combined Use of Medaka and Zebrafish for Further Genetic Dissection of the Function of the Vertebrate Genome. In Practical Approaches for Implementing Forward Genetic Strategies in Zebrafish; Nair, S., Pelegri, F.J., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA; Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; London, UK, 2011; pp. 211–241.
- Lyu, K.; Gu, L.; Wang, H.; Zhu, X.; Zhang, L.; Sun, Y.; Huang, Y.; Yang, Z. Transcriptomic Analysis Dissects the Mechanistic Insight into the Daphnia Clonal Variation in Tolerance to Toxic Microcystis. Limnol. Oceanogr. 2019, 64, 272–283. https://doi.org/10.1002/lno.11038.
- Andersen, M.L.; Winter, L.M.F. Animal Models in Biological and Biomedical Research—Experimental and Ethical Concerns. An. Acad. Bras. Cienc. 2019, 91, e20170238. https://doi.org/10.1590/0001-3765201720170238.1.
- Greek, R.; Menache, A. Systematic Reviews of Animal Models: Methodology versus Epistemology. Int. J. Med. Sci. 2013, 10, 206–221. https://doi.org/10.7150/ijms.5529.
- Bédard, P.; Gauvin, S.; Ferland, K.; Caneparo, C.; Pellerin, È.; Chabaud, S.; Bolduc, S. Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering 2020, 7, 115. https://doi.org/10.3390/bioengineering7030115.
- Escher, B.; Leusch, F. Bioanalytical Tools in Water Quality Assessment; IWA Publishing: London, UK, 2011; Volume 10, ISBN 9781789061970.
- Tousova, Z.; Oswald, P.; Slobodnik, J.; Blaha, L.; Muz, M.; Hu, M.; Brack, W.; Krauss, M.; Di Paolo, C.; Tarcai, Z.; et al. European Demonstration Program on the Effect-Based and Chemical Identification and Monitoring of Organic Pollutants in European Surface Waters. Sci. Total Environ. 2017, 601–602, 1849–1868. https://doi.org/10.1016/j.scitotenv.2017.06.032.
- Codd, G.A.; Jefferies, T.M.; Keevil, C.W.; Potter, E. Detection Methods for Cyanobacterial Toxins; Woodhead Publishing, Sawston, United Kingdom, 1994.
