Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
The limited ability of mammals to regenerate has garnered significant attention, particularly in regard to skin wound healing (WH), which is a critical step for regeneration. In human adults, skin WH results in the formation of scars following injury or trauma, regardless of severity. This differs significantly from the scarless WH observed in the fetal skin of mammals or anamniotes.
- skin repair
- regeneration
- mammals
- wound healing
- scarring
- Anterior Gradient proteins
- anamniotes
1. Introduction
Wound healing is a complex and coordinated cascade of events that can be subdivided into four overlapping phases: hemostasis, inflammation, proliferation, and remodeling. This process is controlled by the interaction of numerous factors, which can simultaneously affect several phases at once.
In the first phase vasoconstriction, platelet aggregation, degranulation and blood clot formation occur. Then, the recruitment and differentiation of macrophages take place in the inflammatory stage. Next steps are re-epithelialization, angiogenesis, collagen synthesis and ECM formation, which occur during the proliferation stage. Finally, during the last stage, in addition to collagen remodeling, the final formation of the vasculature takes place. The following sections concern the main processes of WH from the point of view of those key molecular events, which have possible AGR influence.
2. Hemostasis
The coagulation process initiates immediately after injury. The early regeneration processes are activated by growth factors, cytokines released from damaged blood vessels and degranulated platelets. Destruction of blood vessels results in the formation of a blood clot composed of fibrin, fibrinogen and fibronectin with accumulated platelets. A blood clot normally forms in the first few hours after injury, stops blood loss, prevents infection penetration and makes a matrix for cell accumulation and a reservoir of growth factors needed in the later stages of the healing process [47,48]. Blood clot platelets secrete PDGF, which was shown to be chemotactic for cells recruited in WH processes such as monocytes and fibroblasts. Further, PDGF increases fibroblast proliferation and correspondingly ECM production. Furthermore, PDGF promotes fibroblasts to assume the functional characteristics of myofibroblasts [49]. During the initial stages of skin wound healing, both TGF-β and TNF-α are actively secreted (Table 1) [50].
Table 1. The list of factors involved in WH that can interact with AGR2. From right to left: the name of each factor, an overview of its role in wound repair, the important difference in expression in embryonic tissues, and the type of interaction with iAGR and eAGR.
| Factors | WH Function | Embryo Important Differencies |
Interaction with AGR2 |
|---|---|---|---|
| PDGF | monocytes recruitment, stimulation of fibroblasts proliferation and migration, promotion of fibroblast-to-myofibroblast differentiation [49,51,52] | lower levels in serum compared with adult serum [53] | eAGR2 promotes fibroblast-to-myofibroblast differentiation [24,54] |
| TGF-β | neutrophils, macrophages, and fibroblasts recruitment; stimulation of angiogenesis and fibroplasia; initiation of the proliferative phase; stimulation of fibroblasts and pericytes; promotion of contraction of the wound bed; fibroblast-to-myofibroblast differentiation and ECM remodeling [50,51,52,55] | lower levels in serum compared with adult serum and no in intact skin [24,53] | TGF-β inhibits eAGR2 expression; eAGR2 promotes fibroblast-to-myofibroblast differentiation; endoplasmic reticulum stress activates iAGR2 expression with TGF-β1 and AGR2 secretion; iAGR2 is activated in fetal post-wound skin [24,50,54,56] |
| TNF-α | Promotion of inflammation, cell proliferation and angiogenesis [51] | no significant differences were found | TNF-α inhibit eAGR2 expression in combination with epithelial permeability [56,57,58,59,60] |
| HIF-1α | Stimulation of angiogenesis, VEGF expression; control of endothelial cells migration, fibroblast growth and collagen synthesis; recruitment of MSCs [61,62,63,64,65] | constitutive presence in intact skin [66] | eAGR2 induces lactate production, glucose uptake and HIF-1α expression; eAGR2 expression is upregulated by HIF-1α [15,67,68] |
| VEGF | Stimulation of angiogenesis, activation of endothelial cells, proteolytic enzymes secretion, releasing of MMPs for further proliferation and migration [69,70] | lower levels [71] | eAGR2 enhances VEGF homodimerization, thus controlling angiogenesis, endothelial cells and fibroblasts invasion [45,72] |
| FGFs | control cell proliferation, differentiation and migration; FGF 1, 2, 5, 7, 10 upregulation during cutaneous WH; in scarless WH FGF signaling downregulates; FGF2 expression increases under the hypoxia [51,64,65,73,74] | constitutive presence in intact skin as morphogens; FGF7 and FGF10 downregulate in fetal scarless wounds; FGF2 expression decrease in both scarless and scarring fetal wounds [73,74] | eAGR2 enhances FGF2 homodimerization, thus controlling angiogenesis, endothelial cells and fibroblasts invasion [45] |
| MMPs | Regulation of matrix stiffness, lysing surrounding tissues, control of ECM remodeling [70,75] | no significant differences were found | nAG activates MMP, increase collagen degradation [76,77] |
| Collagen I, III | collagen III (proliferation phase)-thin fibrils, part of the provisional matrix replaced by the collagen I, orientating along the epidermal surface; low ratio of type III (10%) to type I (20%) collagen [78,79] | higher ratio of collagen III (60%) to collagen I (30%) [79] | nAG suppresses collagen I and III synthesis, increases collagen degradation [76,77] |
| Cell capabilities | keratinocytes-proliferation, migration along the fibrin of the blood clot, closure of the wound surface [7,80,81,82]; fibroblasts-recruitment to the wound bed, transformation to myofibroblast, secretion ECM [52,55,75,83]; M1-macrophages migration, secrete pro-inflammatory mediators, switch to M2 form [84]; M2-macrophages secrete TGF-β1, PDGF [85,86,87] | lower number of M1, M2-macrophages, higher ratio of M2-macrophages to M1, reduced presentation time [88] | eAGR2 accelerates fibroblasts and keratinocytes migration; eAGR2 promotes epithelial morphogenesis, disrupts cell–cell contact and basal laminin; iAGR2 prevents EMT induction [24,89,90,91,92,93] |
In certain tumor systems, both TGF-β and TNF-α have been shown to inhibit eAGR2 expression (Figure 3A) [56].
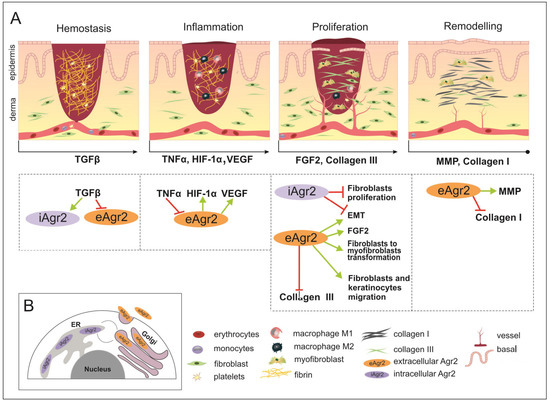
Figure 3. The wound healing (WH) process with appropriate key factors, which might be affected by intracellular AGR2 (iAGR2) or extracellular AGR2 (eAGR2). (A) A schematic illustration of four overlapping stages of WH. Arrows located below each of the pictures indicate the start of a specific signature of the WH stage, such as the factor action. This is considered to be due to the connection with AGR2 or the ability to be influenced by AGR2 being superimposed on the skin WH. In the hemostasis stage, platelets give rise to a blood clot formation, in which molecules of fibronectin, vitronectin and thrombospondins form the temporal scaffold structure for the migration of leukocytes and the invasion of keratinocytes, fibroblasts and neutrophils. AGR2 expression is induced upon injury. The inflammation phase starts with the release of mediators such as TNF-α, IL-1β and IL-6, which further amplify the proinflammatory response. The eAGR2 involves [see the boxes below the scheme marked by arrows] negative regulation from TNF-α and activation of VEGF, influencing the range of the proinflammatory reactions. Then, platelets, leukocytes, macrophages and other inflammatory cells activate the following phase, in which angiogenesis, epithelization and fibroblast activation take place. During the proliferation stage, after the appearance of granulation tissue, the wound begins to contract. During the remodeling stage, there is granulation tissue and angiogenesis reduction, the ECM is reorganized by extra collagen deposition, the increase in wound tensile strength occurs from the beginning of the wound healing process to the end of closure. eAGR2 influences collagen III and collagen I deposition, positively regulating keratinocyte migration and fibroblast-to-myofibroblast transformation. The role of AGR2 in EMT is dependent on intra- or extracellular protein localization. The increasing ratio of eAGR/iAGR seems to be responsible for a set of different impacts on cancer cell migration, invasiveness and the progress of metastasis. However, the precise mechanism(s) regulating the levels of AGR2 and/or their balance remain to be further investigated. (B) The scheme of AGR2 cellular localization. The scheme presents the ER-resident iAGR localization, which is connected with PDI function and the (UPR) signaling pathway. The AGR2 proteins undergoing the secretory route are present in the Golgi complex, from which they can be returned back to the ER due to KTEL-receptor signaling or secreted to the extracellular space as eAGR.
It is important to note that fetal serum has significantly lower levels of PDGF-AB and TGF-β1 compared with adult serum [53]. However, TGF-β is not present in the fetal dermis, but upon wounding, the expression of iAGR2 is activated in fetal post-wound skin of mice [24,50]. However, the relationship between AGR2 and TGF-β may be complex and context dependent, and further research is necessary to fully understand its implications for cancer progression and profibrotic changes.
3. Inflammation
Blood clot platelets with secretion of both TGF-β and PDGF take part in the massive cell death at the site of injury and produce TSLP, IL-25, IL-33, which in common activate IL-4/ IL-13-producing cells and recruit macrophages to the wound area [51]. Th2 cytokines IL-4/ IL-13 have a pleiotropic effect on macrophages: they promote macrophages to activate wound healing when colocalized along with apoptotic cells and induce macrophages to produce IL-10 with anti-inflammatory activity, and tissue remodeling growth factors such as TGF-β and matrix metalloproteinases (MMPs) [94]. M2 macrophages with reparative function in wounds express the markers Arginase1 and RELMα (Retnla), which in turn promote matrix deposition [85]. At the beginning of the inflammation phase, macrophages that have migrated to the wound area are characterized by secretion of the pro-inflammatory mediators, such as IL-1, IL-6, IL-12, TNFα, and iNOS, in contrast to IL-10 at the low level of secretion, which corresponds to the M1-polarized macrophages [84]. The macrophage phenotype switches gradually to an alternatively activated form known as M2, which is characterized by the secretion of TGF-β1 in common with PDGF, while macrophage recruitment to the damaged area continues [85,86,87]. Macrophages participating in wound healing also produce VEGF, which stimulates multiple components of the angiogenic cascade [69]. In addition to secretory activity, macrophages phagocytize remaining debris [95]. At the end of the inflammatory phase, T-lymphocytes and B-lymphocytes migrate to the wound area, and although the role of B-lymphocytes in this process is not completely clear, it is believed that T-lymphocytes take part in the cross-linking of collagens in the wound matrix [96]. Genetically modified mouse models with deficient TNF-α exhibited accelerated wound closure. The lack of polymorphonuclear leukocytes and enhancement in angiogenesis and collagen content were shown [57]. In this case, negative regulation of eAGR2 by TNF-α could decline wound closure (Figure 3A). Mice experimental models with a lack of key inflammatory factors for skin repair showed a change in the rate of healing, which resulted in increased scarring [58]. Nuclear factor erythroid 2-related factor 2 (Nrf2) knockout mice, with a deficiency of transcription factor that regulates keratinocyte growth factor, manifest positively regulated prolonged inflammatory response and protracted expression of IL-1β and TNF-α (Table 1) [59]. NRF2 specifically activates the HIF-1α promoter in normoxic conditions and maintains constitutive HIF-1-α expression. NRF2 is downregulated in hypoxia and restored following reoxygenation; this feedback regulation was demonstrated by in vitro studies in murine renal/kidney tubular epithelial cells [97]. This complex regulatory network participating in wound healing possibly includes the AGR2 factor, which was specifically upregulated in response to the depletion of oxygen (Figure 3A) [67]. In comparison with wound healing of adult skin tissue, the fetal healing process is characterized by reduced inflammation. Fetal neutrophils express lower levels of adhesion molecules compared to adult neutrophils and display a decreased ability to recruit to the injury site in comparison with such processes in an adult skin injury. The study of human second-trimester fetal skin demonstrated low levels of immune cells and leukocyte chemokines compared to adult skin. The most important deficiency was found in the CD45(+) leukocyte population. The discrepancy between fetal and mature skin was demonstrated in murine models [88]. The overall number of macrophage is lower in fetal wounds than that in adults, with a high ratio of anti-inflammatory (M2) to M1 pro-inflammatory (M1) macrophages recruited in the wound bed in early gestation steps during mouse development (Table 1). In addition, the presentation time of inflammatory cells is reduced compared to an adult wound.
The role of AGR2 in maintaining intestinal homeostasis and pathogenesis of the inflammatory bowel disease was studied on the model of intestinal epithelial cells culture Caco-2 [60]. After cell exposure to TNF-α, the levels of both mRNA and the protein AGR2 decreased, which was accompanied by an increase in epithelial permeability, which was estimated by transepithelial electrical resistance. The overexpression of AGR2 in Caco-2 had a protective role, as the decrease in epithelial barrier hyperpermeability was demonstrated [60]. The increased expression of Tight Junction proteins and stabilization of intracellular cytoskeletal structures was shown in the Caco-2 epithelial cell line with AGR2. Moreover, AGR2 induced inhibition of NF-κB p65 translocation into the nucleus, thus mediating the negative regulator of the NF-κB-dependent axis [37]. It seems that AGR2 has no potential involvement in the first stages of wound healing. However, it is important to mention that both TGF-β and TNF-α are actively secreted during the inflammation stage and have been shown to inhibit AGR2 expression in tumor systems (Table 1). So, it can lead to a lower contribution at the beginning of the proliferation stage.
4. Proliferation
In the proliferative phase, the wound surface closes, granulation tissue forms, and the vascular network restores. The secretion of TGF-β1, in common with PDGF, is partly responsible for initiating the proliferative phase and the initial migration of fibroblasts and perivascular progenitor cells (also known as pericytes) into the developing granulation tissue matrix. PDGF destabilizes perivascular progenitors that are associated with blood vessels, allowing them to migrate into the wound bed (Table 1) [52].
In vitro experiments on mouse fibroblast cultures revealed that extracellular recombinant AGR2 controls the migration velocity and directional persistence of cells. The mechanism of action is presumably through the FAK pathway (focal adhesion) and the JNK pathway, since their inhibition results in a significant decline in cell migration [24].
Growth factors such as VEGF, PDGF, bFGF bind to endothelial cells receptors, thereby activating intracellular signaling cascades. Activated endothelial cells secrete proteolytic enzymes that lyse BM, so endothelial cells can divide and migrate into the wound. During proliferation, endothelial cells release MMPs, which lyse surrounding tissues for further endothelial proliferation [70].
The AGR2 protein was revealed to be a chaperone-like enhancer of VEGF and FGF2. AGR2 can directly bind to these molecules and enhance their homodimerization, thus controlling angiogenesis, endothelial cells and fibroblast invasion (Table 1) [45]. In the prostate cancer cell line PC-3, extracellular AGR2 was shown to enhance VEGF receptor activity through the formation of disulfide bonds and activate the NF-κB pathway [72].
Tissue hypoxia stimulates angiogenesis through hypoxia-inducible factor 1 alpha (HIF-1α), which induces the expression of VEGF and endothelial cells to migrate towards low-oxygen areas. It has been shown that in the middle of the wound, the partial pressure of oxygen can be lower than 10 mmHg compared to normal tissue levels of 45–50 mmHg [61,62]. Multiple cell types express HIF-1α in response to injury, including keratinocytes, fibroblasts, and infiltrating immune cells. Fibroblast growth and collagen synthesis require hypoxic conditions; for example, collagen synthesis begins at a pO2 of 10 to 20 mmHg, and at 25 mmHg it becomes maximum. Additionally, overexpression of HIF-1α during wound healing recruits MSCs and stimulates regeneration (Table 1) [63].
During neovascularization, hypoxia decreases, which leads to a fall in the lactate level in tissues and a decrease in the new vessel growth, but collagen synthesis continues. Further, endothelial cells express FGF2 under the hypoxia conditions and hypoxia induced the expression of both FGF1 and FGF2 in macrophages [64,65].
FGF family members control cell proliferation, differentiation and migration. FGF1, 2, 5, 7, 10 are upregulated during cutaneous wound healing. Different FGFs are expressed throughout embryogenesis, where they act as morphogens [73]. In the mouse model, it was shown that after wounding, expression of FGF7 and FGF10 was downregulated in fetal scarless wounds. FGF2 expression decreased in both scarless and scarred fetal wounds [74]. On average, in scarless wound healing and in the fetus, FGF signaling was found to be downregulated (Table 1).
Unlike adult skin, HIF-1α expression was detected in intact fetal skin, which means that HIF-1a constitutive presence in skin before injury can be involved in scarless embryonic wound healing [66]. AGR2 induces lactate production and glucose uptake and was shown to induce the expression of several important enzymes such as lactate dehydrogenase A (LDHA), phosphoglycerate kinase 1 (PGK1), kallikrein 2 (HK2), and enolase 1-α (ENO1), which participate in glucose uptake and lactate production. Moreover, AGR2 induces HIF-1α (Table 1) [15]. An investigation of glioblastoma multiforme tumor (GBM) cells showed that AGR2 expression was regulated by HIF-1α and directed glioblastoma cell growth and the vascularity of tumors (Figure 3A) [68].
Re-epithelialization is provided by keratinocytes recruited from the wound edge and the hair follicle stem cells (SCs) (bulge and gland) [80]. The process is activated by signaling pathways triggered by wound edge cells secreting EGF, KGF, IGF-1, and NGF, a variety of different cytokines and growth factors, in response to injury [81]. Cell migration proceeds due to the weakening of intercellular contacts and the cytoskeleton’s reorganization. After basement membrane damage, the contact inhibition and physical tension of cell contacts decrease, the SRC kinases activate, and the membrane permeability increases, resulting in a rearrangement of the cell tonofilaments and the start of migration [7]. In addition, collagenase and elastase weaken the cell contacts, and as a result, keratinocytes can migrate along the fibrin of the blood clot in the upper layers of granulation tissue along the chemotactic gradient formed by cytokines [82]. Rho GTPases regulate cell migration, during which cytoskeletal actin fibers are polymerized and new focal contacts with the ECM are formed due to the activation of integrins [98]. When GTPases stop working, the cytoskeleton is reorganized, actin fibers in filopodia are degraded, and a new epithelial layer with contacts is formed that closes the wound [7].
In vivo experiments on mice demonstrated that recombinant AGR2 accelerated the migration of epithelial cells to form elongated migrating tongues. Additional confirmation was obtained using mouse cell cultures (fibroblasts and keratinocytes) treated with mitomycin C, which excluded the proliferation influence (Table 1) [24]. AGR2’s ability to control cell migration, e.g., induce accelerated re-epithelialization, looks very promising in terms of regeneration capacity improvement.
The last step in the proliferation phase is the formation of granulation tissue mass. Being a temporary tissue, it replaces the provisional wound matrix, the main components of which are fibrin and fibronectin, and during this process it can form a scar [52,83]. Additionally, the tissue is characterized by a large number of fibroblasts, granulocytes, macrophages, capillaries and poorly organized collagen bundles. In addition, since angiogenesis is not yet fully complete, this tissue is highly vascularized.
As fibroblasts migrate into the wound bed and interact with the components of the newly synthesized wound ECM, they undergo phenotypic changes triggered by TGF-β1, becoming myofibroblasts expressing α-SMA [55]. Myofibroblasts are rapidly migrating cells that contribute to wound margin contraction. Moreover, myofibroblasts secrete collagens and components of the ECM, for example, fibronectin, glycosaminoglycans, proteoglycans and hyaluronic acid, as well as MMPs that regulate matrix stiffness. The ECM provides a scaffold for cell adhesion and regulates cell growth, movement and differentiation through its stiffness, which means it regulates the activity of fibroblasts in the wound healing process. At the end of this phase, the number of mature fibroblasts decreases due to myofibroblast differentiation and apoptosis [75]. Human fetal skin samples being xenotransplanted in nude mice either subcutaneously (a) or cutaneously (b) demonstrated scarfree (a) and scar (b) wound healing [99]. Scars in cutaneous fetal grafts could be the result of the infiltration of the murine granulation tissue with disposing collagen. It was shown that during wound healing, adipocytes are regenerated from myofibroblasts, cells that are considered differentiated and non-adipogenic. The reprogramming of myofibroblasts involves newly formed hair follicles that trigger BMP signaling and activation of the expression of adipocyte transcription factors [100].
When examining the differences between fetal and adult wound healing, it is important to note that multiple factors are at play and can affect various stages of the process. Although myofibroblasts secrete the same TGF-β1 in both cases, its impact on the healing process differs between the two [101]. Different signaling pathways are activated in early human fetal skin cells in comparison with mature fibroblasts, with a prevalence of short-lived forms or phosphorylation of Smad2/3 and c-Jun N-terminal kinase components of TGF-β1 cascade. This altered response to TGF-β1 stimuli might be partly responsible for transition between non-scarring fetal regeneration and scarring postnatal processes.
TGF-β indirectly mediates AGR2 downregulation through Smad signaling controlled by activated MAPKs, as shown in studies on A549 and PANC-1 cells (Figure 3A) [89]. On the other hand, endoplasmic reticulum stress induction in HT-29 epithelial cells activates intracellular expression of AGR2, as well as TGF-β1 and leads to AGR2 secretion in the supernatant. Moreover, fibroblast-to-myofibroblast differentiation can be stimulated by recombinant AGR2 added to the culture medium (Figure 3A) [54]. It is noteworthy that apparently myofibroblast number increases in recombinant AGR2 presence take place without abnormal homeostasis, according to post-wound healing analysis [24].
Judging by studies conducted on human cancer cell lines, the AGR2 effect on EMT, migration and invasion abilities depends on the form of the protein, intracellular or extracellular (Figure 3B) [89,90]. It was shown that extracellular AGR2 promotes epithelial morphogenesis and tumorigenesis by disrupting cell–cell contact, disrupting basal laminin and activating fibroblast-associated cancer invasion (Table 1) [91,92]. On the other hand, intracellular AGR2 protects the epithelial cellular phenotype by preventing EMT induction (Figure 3A) [93].
5. Remodeling
In the last phase of wound healing, the number of cells that make up the granulation tissue decreases by apoptosis, and the provisional matrix is reorganized. Thus, a mature wound is characterized by a reduced number of both vessels and cells [102]. During wound formation, the components of the extracellular matrix undergo certain changes. Collagen III, which was produced during the proliferation phase, is replaced at this time by collagen I. Type III collagen fibrils are thinner than type I fibrils and oriented in small parallel bundles (Table 1). It should be noticed that the collagen I fibrils formed after injury are oriented along the epidermal surface, while the intact skin fibrils are arranged in a stronger intertwined network. Subsequently, myofibroblasts induce wound contraction through multiple collagen contacts and help reduce the surface of the scar [78]. Finally, the blood circulation processes of angiogenesis are slowed down. At this stage, the foundation is laid for the formation of a scar on the wound [7].
Fetal skin is characterized by a higher ratio of type III (60%) to type I (30%) collagen than the same ratio in the adult skin (only 10% to 20%) (Table 1). During embryogenesis, the ratio of collagens is declining, but final remodeling takes place only at the postnatal period [79].
In vitro experiments on human fibroblasts transfected with plasmid encoding recombinant newt AG (nAG). nAG expression inhibits fibroblasts proliferation and significantly suppresses collagen I and III in intact cell cultures and after TGF-β treatment, as shown by the BRDU test of proliferation activity. In addition, nAG expression activates MMP, which leads to an increase in collagen degradation [76]. The results were confirmed in a rabbit ear injury model, where a recombinant nAG protein solution was injected into the wound (Table 1) [77]. Lower levels of collagen I and collagen III and higher levels of MMP1 led to a higher degree of scar maturation in experimental wounds compared to controls (Figure 3A).
This entry is adapted from the peer-reviewed paper 10.3390/ijms24097895
This entry is offline, you can click here to edit this entry!
