One of the critical aspects in designing nanocomposite membrane is the selection of a well-matched pair of nanomaterials and a polymer matrix that suits their intended application. By making use of the fascinating flexibility of nanoscale materials, the functionalities of the resultant nanocomposite membranes can be tailored. The unique features demonstrated by nanomaterials are closely related to their dimensions, hence a greater attention is deserved for this critical aspect.
- nanomaterial dimensions
- nanocomposite membranes
- membrane separations
1. Introduction
Membrane-based separation has earned its place in a wide spectrum of commercial applications. Over the last few decades, progressive growth has been witnessed in the application of membrane technology in wastewater treatment, desalination, gas separation and energy generation [1][2][3]. With its commercial attractiveness based on advantages such as high energy efficiency, low carbon footprint and operational simplicity, attaining a reliable and sustainable separation process based on membrane technology is one of the greatest areas of interest in this field [4][5]. The membrane is known as the heart of the entire separation process. The separation performance and efficiencies are closely related to the intrinsic properties of membranes. A highly selective membrane material ensures high purity; a highly permeable membrane material allows sufficiently high productivity for large-scale application; a fouling resistant membrane material extends the shelf lifespan of the liquid separation membrane module which leads to cost saving. Although polymeric membranes are currently dominating the membrane market, there are intensive efforts to develop high-performance membranes with multifunctional physicochemical properties [6][7].
Nanotechnology has been dynamically adapted to a broad range of modern applications, including membrane development. The emergence of nanoscience is also concerned with the production of new or enhanced materials. Nanomaterials are the subject of intense research and are known to be highly versatile materials, in which their physical and chemical properties can be flexibly tailored [8]. At the atomic level, this can be accomplished by tuning the elemental composition, atomic/molecular arrangement and nanomaterial dimension via a bottom-up approach [9]. At a macroscopic level, post-synthesis modifications can be performed on the device or system to create new functionalities and capabilities. The interdisciplinary research of material chemistry and engineering applications hold the key to heightened material performance. Tapping these new opportunities, innovations have been constantly made to resolve the pertinent issues of membrane processes, including addressing the trade-off between selectivity and productivity as well as membrane fouling, membrane aging and plasticization [10]. One such innovation which has created new horizons in membrane-based separation processes is the development of nanocomposite membranes—an emerging generation of membrane which amalgamates polymers and inorganic materials as one entity [11]. The application of nanocomposite membranes has been extended in various separation processes and has made great strides in performance enhancement. The interplay between the two entities uniquely combine the benefits of each component and diminish their inherent limitations [12].
In the so-called nanocomposite membranes, the nanomaterials can be integrated with polymer matrix in several ways, including (i) the most common direct blending of nanomaterials with polymers to form mixed matrix membranes (MMMs) [13] and (ii) the post-incorporation of nanomaterials onto the preformed membrane surface through surface assembly, coating or grafting [14]. For thin-film composite membranes used for gas separation, nanofiltration (NF), reverse osmosis (RO) and forward osmosis (FO), the nanocomposite membranes can be prepared in more flexible manners, i.e., by incorporating the nanofillers into the substrate and/or the selective layer and by surface architecture [15]. Regardless of the structures and configurations, nanocomposite membranes have been hailed as one of the game changers in membrane technology. One of the most critical aspects in the development of nanocomposite membrane is a proper selection of both nanomaterials (dispersed phase) and polymer (continuous phase). Defining a set of questions related to material selection, such as what is the intended use and what the specific properties of the membrane are needed for that use can provide fruitful guidelines to visualize the outcome of the function-led membranes. Understanding the properties of materials is particularly crucial, not solely from a fundamental point of view, but also because knowledge in this aspect forms a strong basis for the development of nanocomposite membranes and their resultant performance. It is also of paramount interest for tailoring the membrane properties. The advent of characterization techniques provides adequate information about a newly discovered nanomaterial, hence helping to establish function-structure relationship.
Nanomaterials can be characterized by multiple parameters including the dimension, surface chemistry and crystal structures. A broad classification based on their geometry and dimension allow all nanomaterials to be classified into four groups i.e., zero-dimension (0D), one-dimensional (1D), 2-dimensional (2D) and three-dimensional (3D) [16][17][18]. Accordingly, 0D nanomaterials are mostly spherical or quasi-spherical, dots and clustered nanoparticles with all the dimension confined within nanoscale below 100 nm; 1D nanomaterials are commonly featured as nanotubes, nanorods and nanowires where one of their dimensions is beyond 100 nm. The typical examples of 2D nanomaterials are sheet-like graphene and hexagonal boron nitride with only one dimension falls within single or few atomic thicknesses. In 3D nanomaterial such as polycrystal and microporous framework assemblies, all dimensions of the structure are in the microscale range. In the context of nanocomposite membranes, the surface chemistry of the nanomaterials used as a nanofiller or surface modifier of membranes such as their surface charges, hydrophilicity, functional groups are critical aspects that affects the physicochemical properties of the resultant membranes [19][20]. On the other hand, the dimension of nanostructures imparts more significant influence on the nanomaterial distribution patterns, the transport behavior across the nanofiller, as well as the interactions and the accessibility of the nanostructures to the surrounding matrix or species. Understanding of the structural properties and uniqueness is an important tool for the development of a nanocomposite membrane that serves that right purpose. While the surface chemistry aspect, particularly that related to surface functionalization or modification of nanomaterials, has been comprehensively covered in considerable review articles, the important roles of nanostructure dimension has not been given equal emphasis.
2. Dimension Plays Its Role: An Overview
Although the chemical composition remains unchanged, the structural and chemical parameters are significantly influenced by their dimensions, i.e., geometries and structures [21]. The most classical example is carbon element which shows unique allotropy- the existence of a range of distinctive molecular structures from the same element [22]. The allotropes exist in multidimensions, including 0D fullerence, 1D carbon nanotube (CNT), 2D graphene and 3D graphite. These carbon allotropes, especially the low-dimensional CNT and graphene have been extensively applied for the development of nanocomposite membrane owing to their outstanding novel features [23]. Due to the variation in their structural geometries as well as the chemical bonds and interactions within the structure, these allotropes are characterized by drastically different chemical properties. Nanostructured titanium dioxide or titania also can also be synthesized in several dimensions, the 0D spherical nanoparticle, 1D titania nanotubes and 2D titania nanofilm as well as the more complex hierarchical 3D titania with interconnected networks [24]. Compared to the commercially used 0D spherical nanoparticles, titania nanostructures with higher dimensions are known to feature high accessible surface areas. Figure 1 and Figure 2 present the structural illustrations and transmission electron microscope (TEM) images, respectively, of some representative dimensionally different nanomaterials applied for gas separation and liquid separation nanocomposite membrane.
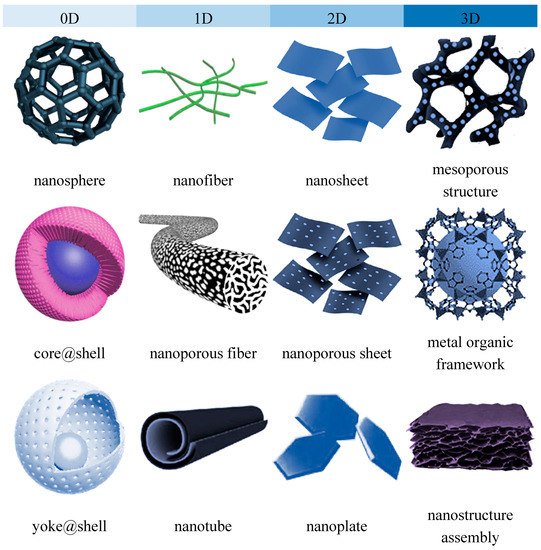
Figure 1. Representative illustrations of dimensionally different nanomaterials applied for gas separation and liquid separation nanocomposite membrane.

Figure 2. Transmission electron microscope (TEM) images of (a) 0D platinum loaded N-doped mesoporous carbon nanosphere [25], (b) 1D iron oxide nanoparticle filled carbon nanotube [26], (c) 2D metal organic framework nanosheet [27] and (d) 3D platinum loaded in between a MIL-101(Cr) core and MIL-101(Fe) shell [28].
The performance of nanocomposite membranes in liquid- and gas-separation applications depends critically upon the roles of the incorporated nanofillers. Together with many other interrelated factors, the geometry of the nanomaterial is important to dictate the ultimate performance of a nanocomposite membrane. The discrepancy in the performance of nanocomposite membranes incorporated with structurally different but with identical chemical composition highlights the importance of material architecture. Due to this reason, the structural features should be carefully identified. These features, such as pore size and distribution, interconnectivity of pores, and interlayer spacing, play critical roles in maximizing the desired performances. The different dimensions of these nanomaterials open rooms to develop intriguing functionalities. The tunable anisotropy in transport properties and sieving properties are two aspects closely related to membrane-separation mechanisms. The understanding on the role of nanomaterials associated to their dimension and geometry should be a subject of high interest from the material science point of view. With the advances in bottom-up and top-down synthesis, nanostructures can be feasibly synthesized in various dimensions without altering their chemical composition. In this section, the classification of the nanostructures is primarily based on their most commonly known and synthesized dimensions for nanocomposite membrane applications.
3. Tailoring the Dimensions of Nanostructures to the Separation Processes
The applications of various nanostructures have progressed from multiple aspects, including the fundamental understanding of water transport and molecule/ion-filler interaction through computational studies, separation performance evaluation through experimental approaches, modifications and functionalizations of nanofillers for performance enhancement. Due to their respective uniqueness and advantages originated from their structural properties and chemical composition, various nanomaterials of different dimensions have received equal attention in nanocomposite membranes. Just as the choice of the type polymer is largely dependent on the nature of the separation process, the selection of nanomaterials used for the preparation of nanocomposite membranes should also be made on the basis of their intended applications [29][30]. Tremendous efforts have been made in the preparation and performance evaluations of various forms of nanocomposite membranes. In this section, the discussion is made based on some recent exemplary work which aims at harnessing the benefits arisen from the uniqueness of the nanostructure dimensions, instead of the benefits rendered by their chemical compositions or surface properties. Out of the vast applications of membrane processes, two niche areas where this technology has been massively explored, i.e., gas separation and liquid separation are focused.
4. Synergy of Multidimensional Hybrid Nanostructures
To further enhance the performance, more than one type of nanostructure has been simultaneously used for the construction of the membrane structure. When nanostructures with different dimensions are used, the difference in the geometric structures allows for their distinct roles in the nanocomposite membrane design. The integration of two or more nanostructures with different geometrical structures can also potentially diminish the limitation of individual component. For instances, the decoration of 1D nanoparticles on 2D materials such as graphene and MXene, which serve as the backbone, alleviate the agglomeration issues [31][32]. The interlayer of 2D materials can also be integrated with 1D nanomaterials which act as spacer to reduce the restacking tendency of nanosheets. There are two ways that two or more nanomaterials can be introduced into the nanocomposite membranes, i.e., hybridization of two or more nanomaterials as a new single entity prior to their integration with the membrane or simultaneous introduction of two or more individual nanomaterials during the membrane preparation.
The incorporation of dual nanostructure or hybrid composed of two of more dimensionally different nanostructures into gas separation membranes have been ventured by numerous studies [33][34][35][36][37][38]. Wong et al. investigated the synergistic effects of nanotubular and nanosheet structures on the formation of an interfacially polymerized polyamide layer [39]. When the dual-fillers were dispersed in their common solvent, GO nanosheets with high dispersibility served as a dispersant for the CNTs in aqueous solution hence preventing the latter from aggregating (Figure 3a,b). The deposition of nanotubes onto the basal plane of GO reduced the tendency of nanotube agglomeration. The combination of these 1D and 2D nanostructures led to the formation of a thicker and rougher polyamide layer compared to that of incorporated with single-filler. In terms of the gas separation performance, the nanocomposite membranes harnessed the high selectivity offered by CNT and the high permeability contributed by GO, hence exhibiting the optimal CO2 permeability, CO2/N2 and CO2/CH4 selectivity that which are 29.7%, 63.5% and 54.1% higher than that of pristine TFC membranes. By coupling 2D MXene and GO with silica microsphere and HNT, Shi et al. demonstrated the importance of identifying the right pair of nanofillers to achieve the desired synergistic dual-filler effects [40]. The Pebax-based nanocomposite membrane incorporated with GO/HNTs dual-fillers exhibited much higher CO2/N2 selectivity than the nanocomposite membrane embedded with MXene/HNTs at the same loading. The findings stem from the different rigidity of GO and MXene where HNTs were expected to be better wrapped by the flexible GO sheets to promote their dispersion. The preferential horizontal orientation of GO and HNTs improved the tortuosity of gas transport and hence increased the diffusivity selectivity of CO2/N2. In contrast to GO, MXene/SiO2 dual fillers demonstrated synergic effect that could not be observed in MXene/HNT. The dispersion of spherical SiO2 was much better than that of HNT, hence can effectively prevent the stacking of MXene.
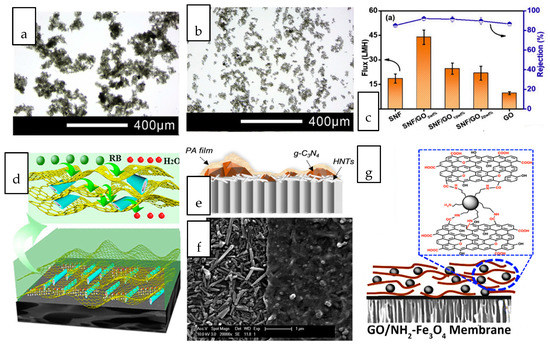
Figure 3. Optical images of (a) carbon nanotube (CNT) and (b) CNT/GO dual nanofiller dispersed in aqueous-based solution. (c) nanocomposite membranes incorporated with SNF/GOx exhibited drastically improved Na2SO4 rejection and flux for the SNF, GO, and membranes [41] (d) conceptual illustration of water transport path and nanocapillary network jointly created by porous reduced graphene oxide/halloysite nanotubes (PRGO/HNTs) (RB: Reactive Black) [42] (e) Schematic illustration of cross section and (f) surface image of TFN membrane incorporated with g-C3N4/HNT. The left part with the PA scraped off revealed the orientation of HNT [43], (g) Schematic illustration of NH2-Fe2O3 intercalated GO nanosheets [44].
Various interesting combinations of nanostructures with different dimensions, including the coupling of 0D/2D nanostructures [45][46][47][48][49][50][51][52][53][54], 1D/3D nanostructures [55], 1D/2D nanostructure [56][57][58], 3D/2D nanostructures [59][60][61][62][63] have been attempted for liquid separation nanocomposite membranes. A glimpse into these works revealed the huge potential of high surface area 2D nanosheets to serve as a versatile platform for the deposition of other nanostructures while their restacking issue can be simultaneously overcome through the insertion of foreign nanostructures. The synergistic advantages of 1D silk nanofiber (SNF) and 2D GO in the nanocomposite membranes over their single counterparts when used for salt and dye separation has been demonstrated [41]. Supported on a hydrolyzed polyacrylonitrile support, the silk nanofibers intercalated between GO layers, forming organic-inorganic stackings. The flux and salt rejections of the nanocomposite membranes incorporated with SNF/GO were increased by up to 80% compared to those of incorporated with individual SNF and GO (Figure 3c). The 1D silk nanofiber interspersed between the 2D GO layers inhibited the extension of GO nanosheets thus maintaining the salt rejection capability. The additional space created by the SNF interspersed between the GO nanosheet layers led to an optimal increase in the membrane free volume, creating more flow path for water transport. Sheet-like porous reduced graphene oxide (PRGO) and tubular halloysite nanotubes (HNT) were used to create a continuous sandwich-like water channel for efficient dye removal [42]. The driving force induced during solvent-evaporation coating facilitated the movement of HNT to the interlayer spacing of two adjacent PRGO sheets, forming a network of nanocapillaries as illustrated in Figure 3d. 2D graphitic carbon nitride (g-C3N4) and 1D HNTs were co-deposited onto a substrate through vacuum filtration prior to interfacial polymerization of polyamide selective layer [43]. Due to the distinct dimension of these nanostructures, the tubular HNTs inclined to horizontally oriented on the substrate surface to act as an interlayer, while g-C3N4 particles scattered within the PA layer as a porous nanofiller, as revealed in the surface morphology shown in Figure 3e,f. The intrinsic nanopores across the lamellar g-C3N4 and nanotubular structure of HNTs provided additional water transport pathways, which eventually led to elevated water permeability up to 20.5 L·m−2·h−1·bar−1 while maintaining the salt rejection capability.
Zero-dimensional nanoparticles are favourably used as intercalating agent for sheet-like nanostructures due to their good dispersibility throughout the interlayer spacing. Amine functionalized Fe3O4 nanospheres were intercalated within the interlayer of GO (Figure 3g) to enlarge the interlayer spacing and improve the water stability of the GO-coated membranes [44]. The nanocomposite membranes augmented by Fe2O3/GO exhibited a water flux of up to 78 L·m−2·h−1 in dye/salt separation, which was nearly 5 times greater than that with single GO. Three-dimensional nanostructures have been increasingly used for intercalation of nanosheets. The precisely controlled framework and cage-like structures of many 3D nanomaterials can offer additional sieving capabilities without hindering the passage of water molecules. Unlike impermeable nanostructures, the opening of nanoporous crystals with 3D channels can increase the porosity and introduce more nanofluidic channels [64]. By incorporating UiO-66 nanoporous crystals with sub-nano aperture size into the reduced graphene oxide (rGO) laminates, the result exhibited 15 times higher water permeability than the nanocomposite membrane incorporated with rGO without compromising the dye removal efficiency [65]. The improvement was attributed to the abundant adsorption sites and extra water paths rendered by the 3D/2D hybrid.
5. Anisotropy and Orientations of Nanostructures
Nanostructured materials with high aspect ratios or extended lateral dimensions are endowed with exciting physicochemical properties that are radically different from those isotropic counterparts. However, these unique direction-dependent properties can only be harnessed if the orientation of these anisotropic nanostructures is taken into consideration. The preferable orientation of the nanomaterials not only may exhibit properties superior to the disordered counterparts, but also maximize the anisotropic properties of the nanomaterials to approach their ideal performance [66][67]. As the alignment of 1D and 2D nanostructures in their preferable direction is important to fit their purpose, the directional alignment of anisotropic nanomaterials in the nanocomposite membranes should be given particular attention. In the majority of cases, the incorporated nanomaterials are assumed to be randomly orientated and uniformly dispersed within the polymer matrix. The enhancement in separation properties are generally discussed based on the overall physicochemical properties contributed by the nanomaterials, rather than the advantages arisen from the structure or dimension of the selected materials.
Tubular and sheet-like nanomaterials, when oriented in different directions within the polymer matrix, result in different gas transport behaviors. As depicted in Figure 4a, for composite membranes with a relatively thick selective layer, GO nanosheets aligned perpendicular to the membrane surface facilitated the diffusion of gas molecules through the interlayer spacing of nanosheets while that aligned parallel to the membrane surface lengthens the pathway for the passage of gas molecules although the gas molecules still preferably transported through the GO interlayer spacing [68]. However, despite this ideal orientation, the practicability of the membrane should also be taken into consideration. Formation of thin (~1 µm) selectively layer that is preferable for a practical separation process may not favour the vertically or randomly oriented GO nanosheets considering the defects and voids created. Zhang et al. aligned GO horizontally in the thin PEBAX selective layer (Figure 4b) by taking the advantage of shear effects during dip-coating process [69]. Although the tortuosity of the gas transport pathway has been increased upon the addition of GO, the parallel-aligned GO laminates provide size-selective and fast gas transport channels without creating defects within the selective layer. Compared to the randomly oriented and neat polymeric membrane, the nanocomposite membrane with parallel-aligned GO demonstrated improved CO2 permeance without compromising the CO2/N2 selectivity.
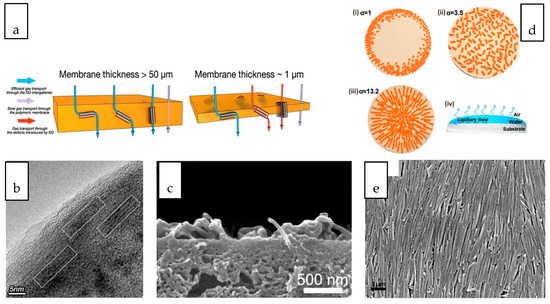
Figure 4. (a) Schematic illustration of gas transport pathway across membrane with different thickness [69] (b) Cross-sectional image of GO nanosheets aligned at parallel direction with membrane surface [69] (c) Cross-sectional images of nanocomposite membranes showing the protrusion of the tubes [70]. (d) Schematic illustration of nanomaterials with different aspect ratio (α) (i) coffee-ring effect observed in isotropic nanostructures, (ii) uniform distribution of ellipsoid nanostructures (iii) orientation of nanotubular nanostructure, (iv) evaporation deposition of nanomaterials on a substrate [71]. (e) Surface image of oriented HNT [71].
When 1D CNT emerged as one of the most studied nanofillers for nanocomposite membranes, the astonishing transport behaviour modelled from vertically aligned CNT has evoked several breakthrough efforts in preparing nanocomposite membranes with vertically-aligned CNT. The vertical alignment has been realized through an infiltration technique which involves the wetting and infiltration of the vertically aligned CNT array by the chosen polymer solution to fill up the inter-tube gaps [72]. The approach may not be favourable for large-scale preparation but it has stimulated more interest in exploring other scalable technique to accomplish the alignment of CNT within nanocomposite membranes. The magnetic and electrical responsiveness of CNT makes the application of a unidirectionally magnetic and electrical field a promising technique for facile nanotube alignment [73]. Polyamide NF TFC membrane with electrically-aligned CNTs that were vertically spanning from polysulfone substrate to polyamide selective layer demonstrated a several-fold increase in the water flux [70]. However, by considering the thickness of membrane and the length of CNT which normally ranges from a few microns to a few centimeters, the length of vertically aligned CNT must be carefully controlled to avoid protrusion of the tubes, as shown in Figure 4c [70]. In another study, the orientation of aluminogermanate imogolite nanotube to the direction of the water flow path within the polyamide layer of TFC membrane was evidenced as the primary factor contributing to the increased specific water flux [74]. The improvement was attributed to the reduction in the length of the water passage across the thin film.
Solvent evaporation is a common technique employed for the formation of a thin film embedded with nanofillers [75]. The deposition and distribution of nanostructures during evaporation is a dimension-dependent process. The coffee-ring effect refers to the deposition of the suspended particulate in a ring-like fashion, mainly observed for the deposition of isotropic materials, such as the nanosphere [76]. Figure 4d compares the deposition patterns of nanostructures of different aspect ratios [71]. It is observed that the deposition of nanostructures with anisotropic geometry through evaporation can significantly deform the interfaces and produce strong interparticle capillary interactions. These deformations are responsible for the elimination of the coffee-ring effect where uniform deposition of the nanostructures can be achieved. The uniform distribution and alignment is more significant with the increasing aspect ratio of the nanostructure, as evidenced in the orientation of HNT on the polyacrylonitrile substrate through evaluation (Figure 4e) [71].
6. Conclusions
Here, we have discussed the roles of nanostructure dimensions, specifically those based on their applications in nanocomposite membranes dedicated to gas separation and water reclamation. Despite the undeniable benefits offered by nanomaterials in the resultant nanocomposite membrane, there are many fundamental aspects which can be looked into. One of these is the role of the material dimensions. The classifications of nanomaterials based on their dimension serves as a useful guideline to screen a wide range of nanomaterials based on their structural properties. Nevertheless, proper inspection of their unique dimensional features is of utmost importance to avoid the mismatch between the nanomaterials and the purpose they serve when incorporated into the nanocomposite membranes. The discussions based on the exemplary works in gas and liquid nanocomposite membranes provided further insights into this aspect. The research gaps and perspectives on these lines have also been identified, some of which are a great challenge and remain to be further explored. The insights reached in the article can be extended beyond the materials that have been explored thus far. Nanocomposite membranes used for other purposes, for instance fuel cell and biomedical applications, will also become relevant if the roles of nanofillers are clearly defined. With many successful attempts acting as a driving force, it can be envisaged that the research and development of nanocomposite membranes in the field of separation will continue to expand in the coming decade.
This entry is adapted from the peer-reviewed paper 10.3390/membranes10100297
References
- Iulianelli, A.; Drioli, E. Membrane engineering: Latest advancements in gas separation and pre-treatment processes, petrochemical industry and refinery, and future perspectives in emerging applications. Fuel Process. Technol. 2020, 206, 106464, doi:10.1016/j.fuproc.2020.106464.
- Yusuf, A.; Sodiq, A.; Giwa, A.; Eke, J.; Pikuda, O.; De Luca, G.; Di Salvo, J.L.; Chakraborty, S. A review of emerging trends in membrane science and technology for sustainable water treatment. J. Clean. Prod. 2020, 266, 121867, doi:10.1016/j.jclepro.2020.121867.
- Wang, Y.; Seo, B.; Wang, B.; Zamel, N.; Jiao, K.; Adroher, X.C. Fundamentals, materials, and machine learning of polymer electrolyte membrane fuel cell technology. Energy AI 2020, 1, 100014, doi:10.1016/j.egyai.2020.100014.
- Zheng, X.; Zhang, Z.; Yu, D.; Chen, X.; Cheng, R.; Min, S.; Wang, J.; Xiao, Q.; Wang, J. Overview of membrane technology applications for industrial wastewater treatment in China to increase water supply. Resour. Conserv. Recycl. 2015, 105, 1–10, doi:10.1016/j.resconrec.2015.09.012.
- Matteucci, S.; Chakraborty, D. Risk management for industrial adoption of membrane technology. Curr. Opin. Chem. Eng. 2020, 28, 112–117, doi:10.1016/j.coche.2020.03.002.
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297, doi:10.1038/nmat4805.
- Cheng, X.Q.; Wang, Z.X.; Jiang, X.; Li, T.; Lau, C.H.; Guo, Z.; Ma, J.; Shao, L. Towards sustainable ultrafast molecular-separation membranes: From conventional polymers to emerging materials. Prog. Mater. Sci. 2018, 92, 258–283, doi:10.1016/j.pmatsci.2017.10.006.
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M. Remediation of wastewater using various nano-materials. Arab. J. Chem. 2019, 12, 4897–4919, doi:10.1016/j.arabjc.2016.10.004.
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon Nanotubes and Related Nanomaterials: Critical Advances and Challenges for Synthesis toward Mainstream Commercial Applications. ACS Nano 2018, 12, 11756–11784, doi:10.1021/acsnano.8b06511.
- Saleem, H.; Zaidi, S.J. Nanoparticles in reverse osmosis membranes for desalination: A state of the art review. Desalination 2020, 475, 114171, doi:10.1016/j.desal.2019.114171.
- Nasir, A.; Masood, F.; Yasin, T.; Hameed, A. Progress in polymeric nanocomposite membranes for wastewater treatment: Preparation, properties and applications. J. Ind. Eng. Chem. 2019, 79, 29–40, doi:10.1016/j.jiec.2019.06.052.
- Zhang, J.; Xin, Q.; Li, X.; Yun, M.; Xu, R.; Wang, S.; Li, Y.; Lin, L.; Ding, X.; Ye, H.; et al. Mixed matrix membranes comprising aminosilane-functionalized graphene oxide for enhanced CO2 separation. J. Membr. Sci. 2019, 343–354, doi:10.1016/j.memsci.2018.10.075.
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507, doi:10.1016/j.progpolymsci.2007.01.008.
- Xu, G.-R.; Wang, S.-H.; Zhao, H.-L.; Wu, S.-B.; Xu, J.-M.; Li, L.; Liu, X.-Y. Layer-by-layer (LBL) assembly technology as promising strategy for tailoring pressure-driven desalination membranes. J. Membr. Sci. 2015, 493, 428–443, doi:10.1016/j.memsci.2015.06.038.
- Zhao, D.L.; Japip, S.; Zhang, Y.; Weber, M.; Maletzko, C.; Chung, T.-S. Emerging thin-film nanocomposite (TFN) membranes for reverse osmosis: A review. Water Res. 2020, 173, 115557, doi:10.1016/j.watres.2020.115557.
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067, doi:10.1016/j.eti.2020.101067.
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803, doi:10.1016/j.pmatsci.2011.08.003.
- Khan, W.S.; Asmatulu, R. Nanotechnology Emerging Trends, Markets, and Concerns. Nanotechnology Safety 2013, 1–16, doi:10.1016/b978-0-444-59438-9.00001-1.
- Zhang, N.; Qi, W.; Huang, L.; Jiang, E.; Bao, J.; Zhang, X.; An, B.; He, G. Review on structural control and modification of graphene oxide-based membranes in water treatment: From separation performance to robust operation. Chin. J. Chem. Eng. 2019, 27, 1348–1360, doi:10.1016/j.cjche.2019.01.001.
- Ali, S.; Rehman, S.A.U.; Luan, H.-Y.; Farid, M.U.; Huang, H. Challenges and opportunities in functional carbon nanotubes for membrane-based water treatment and desalination. Sci. Total. Environ. 2019, 646, 1126–1139, doi:10.1016/j.scitotenv.2018.07.348.
- Fu, S.-Y.; Sun, Z.; Huang, P.; Li, Y.-Q.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30, doi:10.1016/j.nanoms.2019.02.006.
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Mater. 2018, 11, 295, doi:10.3390/ma11020295.
- Tiwari, S.K.; Kumar, V.; Huczko, A.; Oraon, R.; De Adhikari, A.; Nayak, G.C. Magical Allotropes of Carbon: Prospects and Applications. Crit. Rev. Solid State Mater. Sci. 2016, 41, 257–317, doi:10.1080/10408436.2015.1127206.
- Fattakhova-Rohlfing, D.; Zaleska, A.; Bein, T. Three-Dimensional Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9487–9558, doi:10.1021/cr500201c.
- Yang, T.; Ling, H.; Lamonier, J.-F.; Jaroniec, M.; Huang, J.; Monteiro, M.J.; Liu, J. A synthetic strategy for carbon nanospheres impregnated with highly monodispersed metal nanoparticles. NPG Asia Mater. 2016, 8, e240, doi:10.1038/am.2015.145.
- Baaziz, W.; Liu, X.; Florea, I.; Bégin-Colin, S.; Pichon, B.; Ulhaq, C.; Ersen, O.; Soria-Sánchez, M.; Zafeiratos, S.; Janowska, I.; et al. Carbon nanotube channels selectively filled with monodispersed Fe3-xO4 nanoparticles. J. Mater. Chem. A 2013, 1, 13853, doi:10.1039/c3ta12734f.
- Zhang, B.; Luo, Y.; Kanyuck, K.; Saenz, N.; Reed, K.; Zavalij, P.; Mowery, J.D.; Bauchan, G. Facile and template-free solvothermal synthesis of mesoporous/macroporous metal–organic framework nanosheets. RSC Adv. 2018, 8, 33059–33064, doi:10.1039/c8ra06576d.
- Zhao, M.; Yuan, K.; Wang, Y.; Li, G.; Guo, J.; Gu, L.; Hu, W.; Zhao, H.; Tang, Z. Metal–organic frameworks as selectivity regulators for hydrogenation reactions. Nat. Cell Biol. 2016, 539, 76–80, doi:10.1038/nature19763.
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: a review. Environ. Chem. Lett. 2019, 17, 1539–1551, doi:10.1007/s10311-019-00895-9.
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275, doi:10.1016/j.memsci.2014.11.019.
- Yinab, J.; Zhanb, F.; Jiao, T.; Dengb, H.; Zoua, G.; Baic, Z.; Zhangb, Q.; Peng, Q. Highly efficient catalytic performances of nitro compounds via hierarchical PdNPs-loaded MXene/polymer nanocomposites synthesized through electrospinning strategy for wastewater treatment. Chin. Chem. Lett. 2020, 31, 992–995, doi:10.1016/j.cclet.2019.08.047.
- Yanga, W.; Hua, W.; Zhanga, J.; Wanga, W.; Caib, R.; Pana, M.; Huanga, C.; Chenb, X.; Yanc, B.; Zenga, H. Tannic acid/Fe3+ functionalized magnetic graphene oxide nanocomposite with high loading of silver nanoparticles as ultra-efficient catalyst and disinfectant for wastewater treatment. Chem. Eng. J. 2021, 405, 126629, doi:10.1016/j.cej.2020.126629.
- Li, X.; Ma, L.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H.; Cao, X.; Yang, J.; Wang, B. Synergistic effect of combining carbon nanotubes and graphene oxide in mixed matrix membranes for efficient CO2 separation. J. Membr. Sci. 2015, 479, 1–10, doi:10.1016/j.memsci.2015.01.014.
- Ahmad, N.; Noh, A.M.; Leo, C.; Ahmad, A. CO2 removal using membrane gas absorption with PVDF membrane incorporated with POSS and SAPO-34 zeolite. Chem. Eng. Res. Des. 2017, 118, 238–247, doi:10.1016/j.cherd.2016.12.019.
- Galve, A.; Sieffert, D.; Staudt, C.; Ferrando, M.; Güell, C.; Téllez, C.; Coronas, J. Combination of ordered mesoporous silica MCM-41 and layered titanosilicate JDF-L1 fillers for 6FDA-based copolyimide mixed matrix membranes. J. Membr. Sci. 2013, 431, 163–170, doi:10.1016/j.memsci.2012.12.046.
- Jamil, N.; Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Roslan, R.A.; Lau, W.J.; Ismail, A.F. Mixed matrix membranes incorporated with reduced graphene oxide (rGO) and zeolitic imidazole framework-8 (ZIF-8) nanofillers for gas separation. J. Solid State Chem. 2019, 270, 419–427, doi:10.1016/j.jssc.2018.11.028.
- Lia, X.; Yua, S.; Lia, K.; Mab, C.; Zhanga, J.; Lia, H.; Changa, X.; Zhua, L.; Xuea, Q. Enhanced gas separation performance of Pebax mixed matrix membranes by incorporating ZIF-8 in situ inserted by multiwalled carbon nanotubes. Sep. Purif. Technol. 2020, 248, 117080, doi:10.1016/j.seppur.2020.117080.
- Moghadam, F.; Lee, T.H.; Park, I.; Park, H.B. Thermally annealed polyimide-based mixed matrix membrane containing ZIF-67 decorated porous graphene oxide nanosheets with enhanced propylene/propane selectivity. J. Membr. Sci. 2020, 603, 118019, doi:10.1016/j.memsci.2020.118019.
- Wong, K.C.; Goh, P.; Taniguchi, T.; Ismail, A.F.; Zahri, K. The role of geometrically different carbon-based fillers on the formation and gas separation performance of nanocomposite membranes. Carbon 2019, 149, 33–44, doi:10.1016/j.carbon.2019.04.031.
- Shi, F.; Sun, J.; Wang, J.; Liu, M.; Wang, S.; Cao, X.; Yan, Z.; Li, Y.; Nunes, S.P. Exploration of the Synergy Between 2D Nanosheets and a Non-2D Filler in Mixed Matrix Membranes for Gas Separation. Front. Chem. 2020, 8, 1–12, doi:10.3389/fchem.2020.00058.
- Sivakumar, M.; Liu, D.-K.; Chiao, Y.-H.; Hung, W.-S. Synergistic effect of one-dimensional silk nanofiber and two-dimensional graphene oxide composite membrane for enhanced water purification. J. Membr. Sci. 2020, 606, 118142, doi:10.1016/j.memsci.2020.118142.
- Zhu, L.; Wang, H.; Bai, J.; Liu, J.; Zhang, Y. A porous graphene composite membrane intercalated by halloysite nanotubes for efficient dye desalination. Desalination 2017, 420, 145–157, doi:10.1016/j.desal.2017.07.008.
- Liu, Y.; Wang, X.; Gao, X.; Zheng, J.; Wang, J.; Volodin, A.; Xie, Y.F.; Huang, X.; Van Der Bruggen, B.; Zhu, J. High-performance thin film nanocomposite membranes enabled by nanomaterials with different dimensions for nanofiltration. J. Membr. Sci. 2020, 596, 117717, doi:10.1016/j.memsci.2019.117717.
- Dong, L.; Li, M.; Zhang, S.; Si, X.; Bai, Y.; Zhang, C. NH2-Fe3O4-regulated graphene oxide membranes with well-defined laminar nanochannels for desalination of dye solutions. Desalination 2020, 476, 114227, doi:10.1016/j.desal.2019.114227.
- Wang, J.; Wang, Y.; Zhu, J.; Zhang, Y.; Liu, J.; Van Der Bruggen, B. Construction of TiO2 @graphene oxide incorporated antifouling nanofiltration membrane with elevated filtration performance. J. Membr. Sci. 2017, 533, 279–288, doi:10.1016/j.memsci.2017.03.040.
- Zhu, C.; Liu, G.; Han, K.; Ye, H.; Wei, S.; Zhou, Y. One-step facile synthesis of graphene oxide/TiO2 composite as efficient photocatalytic membrane for water treatment: Crossflow filtration operation and membrane fouling analysis. Chem. Eng. Process. Process. Intensif. 2017, 120, 20–26, doi:10.1016/j.cep.2017.06.012.
- Abadikhah, H.; Kalali, E.N.; Khodi, S.; Xu, X.; Agathopoulos, S. Multifunctional Thin-Film Nanofiltration Membrane Incorporated with Reduced Graphene Oxide@TiO2@Ag Nanocomposites for High Desalination Performance, Dye Retention, and Antibacterial Properties. ACS Appl. Mater. Interfaces 2019, 11, 23535–23545, doi:10.1021/acsami.9b03557.
- Dadvar, E.; Kalantary, R.R.; Panahi, H.A.; Peyravi, M. Efficiency of Polymeric Membrane Graphene Oxide-TiO2for Removal of Azo Dye. J. Chem. 2017, 2017, 1–13, doi:10.1155/2017/6217987.
- Xu, Y.; Peng, G.; Liao, J.; Shen, J.; Gao, C. Preparation of molecular selective GO/DTiO2-PDA-PEI composite nanofiltration membrane for highly pure dye separation. J. Membr. Sci. 2020, 601, 601, doi:10.1016/j.memsci.2019.117727.
- Chung, Y.T.; Mahmoudi, E.; Mohammad, A.W.; Benamor, A.; Johnson, D.; Hilal, N. Development of polysulfone-nanohybrid membranes using ZnO-GO composite for enhanced antifouling and antibacterial control. Desalination 2017, 402, 123–132, doi:10.1016/j.desal.2016.09.030.
- Chen, L.; Li, N.; Wen, Z.; Zhang, L.; Chen, Q.; Chen, L.; Si, P.; Feng, J.; Li, Y.; Lou, J.; et al. Graphene oxide based membrane intercalated by nanoparticles for high performance nanofiltration application. Chem. Eng. J. 2018, 347, 12–18, doi:10.1016/j.cej.2018.04.069.
- Liu, G.-F.; Huang, L.-J.; Wang, Y.-X.; Tang, J.; Wang, Y.; Cheng, M.-M.; Zhang, Y.; Kipper, M.J.; Belfiore, L.A.; Ranil, W.S. Preparation of a graphene/silver hybrid membrane as a new nanofiltration membrane. RSC Adv. 2017, 7, 49159–49165, doi:10.1039/c7ra07904d.
- Ma, Z.; Liang, S.; Zhang, S.; Xiao, K.; Wang, X.; Li, M.; Huang, X. Surface functionalization via synergistic grafting of surface-modified silica nanoparticles and layered double hydroxide nanosheets for fabrication of superhydrophilic but relatively oleophobic antifouling membranes. Sep. Purif. Technol. 2020, 247, 116955, doi:10.1016/j.seppur.2020.116955.
- Modi, A.; Bellare, J.R. Efficient separation of biological macromolecular proteins by polyethersulfone hollow fiber ultrafiltration membranes modified with Fe3O4 nanoparticles-decorated carboxylated graphene oxide nanosheets. Int. J. Biol. Macromol. 2019, 135, 798–807, doi:10.1016/j.ijbiomac.2019.05.200.
- Lee, T.H.; Roh, J.S.; Yoo, S.Y.; Roh, J.M.; Choi, T.H.; Park, H.B. High-Performance Polyamide Thin-Film Nanocomposite Membranes Containing ZIF-8/CNT Hybrid Nanofillers for Reverse Osmosis Desalination. Ind. Eng. Chem. Res. 2020, 59, 5324–5332.
- Zeng, G.; He, Y.; Ye, Z.; Yang, X.; Chen, X.; Ma, J.; Li, F. Novel Halloysite Nanotubes Intercalated Graphene Oxide Based Composite Membranes for Multifunctional Applications: Oil/Water Separation and Dyes Removal. Ind. Eng. Chem. Res. 2017, 56, 10472–10481, doi:10.1021/acs.iecr.7b02723.
- Han, Y.; Jiang, Y.; Gao, C. High-Flux Graphene Oxide Nanofiltration Membrane Intercalated by Carbon Nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 8147–8155, doi:10.1021/acsami.5b00986.
- Gao, S.J.; Qin, H.; Liu, P.; Jin, J. SWCNT-intercalated GO ultrathin films for ultrafast separation of molecules. J. Mater. Chem. A 2015, 3, 6649–6654, doi:10.1039/c5ta00366k.
- Zengab, H.; YuABC, Z.; Shaoab, L.; Liab, X.; Zhuab, M.; Liuab, Y.; Fengab, X.; Zhuab, X. Ag2CO3@UiO-66-NH2 embedding graphene oxide sheets photocatalytic membrane for enhancing the removal performance of Cr(VI) and dyes based on filtration. Desalination 2020, 491, 114558, doi:10.1016/j.desal.2020.114558.
- Chen, L.; Wang, W.; Fang, Q.; Zuo, K.; Hou, G.; Ai, Q.; Li, Q.; Ci, L.; Lou, J. High performance hierarchically nanostructured graphene oxide/covalent organic framework hybrid membranes for stable organic solvent nanofiltration. Appl. Mater. Today 2020, 20, 100791, doi:10.1016/j.apmt.2020.100791.
- Xie, A.; Cui, J.; Yang, J.; Chen, Y.; Lang, J.; Li, C.; Yan, Y.; Dai, J. Graphene oxide/Fe(III)-based metal-organic framework membrane for enhanced water purification based on synergistic separation and photo-Fenton processes. Appl. Catal. B: Environ. 2020, 264, 118548, doi:10.1016/j.apcatb.2019.118548.
- Khan, N.A.; Yuan, J.; Wu, H.; Cao, L.; Zhang, R.; Liu, Y.-N.; Li, L.; Rahman, A.U.; Kasher, R.; Jiang, Z. Mixed Nanosheet Membranes Assembled from Chemically Grafted Graphene Oxide and Covalent Organic Frameworks for Ultra-high Water Flux. ACS Appl. Mater. Interfaces 2019, 11, 28978–28986, doi:10.1021/acsami.9b09945.
- Song, N.; Sun, Y.; Xie, X.; Wang, D.; Shao, F.; Yu, L.; Dong, J. Doping MIL-101(Cr)@GO in polyamide nanocomposite membranes with improved water flux. Desalination 2020, 492, 114601, doi:10.1016/j.desal.2020.114601.
- Zengab, H.; YuABC, Z.; Shaoab, L.; Liab, X.; Zhuab, M.; Liuab, Y.; Fengab, X.; Zhuab, X.; Protected], [Email A novel strategy for enhancing the performance of membranes for dyes separation: Embedding PAA@UiO-66-NH2 between graphene oxide sheets. Chem. Eng. J. 2021, 403, 126281, doi:10.1016/j.cej.2020.126281.
- Guan, K.; Zhao, D.; Zhang, M.; Shen, J.; Zhou, G.; Liu, G.; Jin, W. 3D nanoporous crystals enabled 2D channels in graphene membrane with enhanced water purification performance. J. Membr. Sci. 2017, 542, 41–51, doi:10.1016/j.memsci.2017.07.055.
- Su, B.; Wu, Y.; Jiang, L. The art of aligning one-dimensional (1D) nanostructures. Chem. Soc. Rev. 2012, 41, 7832–7856, doi:10.1039/c2cs35187k.
- Goh, P.; Ismail, A.; Ng, B. Directional alignment of carbon nanotubes in polymer matrices: Contemporary approaches and future advances. Compos. Part A: Appl. Sci. Manuf. 2014, 56, 103–126, doi:10.1016/j.compositesa.2013.10.001.
- Karunakaran, M.; Shevate, R.; Kumar, M.; Peinemann, K.V. CO2-selective PEO-PBT (PolyActiveTM)/graphene oxide composite membranes. Chem. Commun. 2015, 51, 14187–14190.
- Zhang, Y.; Shen, Q.; Hou, J.; Sutrisna, P.D.; Chen, V. Shear-aligned graphene oxide laminate/Pebax ultrathin composite hollow fiber membranes using a facile dip-coating approach. J. Mater. Chem. A 2017, 5, 7732–7737, doi:10.1039/c6ta10395b.
- Liu, C.; Wang, W.; Zhu, L.; Cui, F.; Xie, C.; Chen, X.; Li, N. High-performance nanofiltration membrane with structurally controlled PES substrate containing electrically aligned CNTs. J. Membr. Sci. 2020, 605, 118104, doi:10.1016/j.memsci.2020.118104.
- Qin, L.; Zhao, Y.; Liu, J.-D.; Hou, J.; Zhang, Y.; Wang, J.; Zhu, J.; Zhang, B.; Lvov, Y.; Van Der Bruggen, B. Oriented Clay Nanotube Membrane Assembled on Microporous Polymeric Substrates. ACS Appl. Mater. Interfaces 2016, 8, 34914–34923, doi:10.1021/acsami.6b12858.
- Hinds, B.J.; Chopra, N.; Rantell, T.; Andrews, R.; Gavalas, V.; Bachas, L.G. Aligned Multiwalled Carbon Nanotube Membranes. Science 2004, 303, 62–65, doi:10.1126/science.1092048.
- Samieirad, S.; Mousavi, S.M.; Saljoughi, E. Alignment of functionalized multiwalled carbon nanotubes in forward osmosis membrane support layer induced by electric and magnetic fields. Powder Technol. 2020, 364, 538–552, doi:10.1016/j.powtec.2020.02.017.
- Li, M.; Brant, J.A. Effects of aluminogermanate imogolite nanotube orientation on mass transport across polyamide nanocomposite membranes. J. Membr. Sci. 2019, 585, 38–51, doi:10.1016/j.memsci.2019.05.024.
- Xie, K.; Fu, Q.; Qiao, G.G.; Webley, P.A. Recent progress on fabrication methods of polymeric thin film gas separation membranes for CO2 capture. J. Membr. Sci. 2019, 572, 38–60, doi:10.1016/j.memsci.2018.10.049.
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A.G. Suppression of the coffee-ring effect by shape-dependent capillary interactions. Nat. Cell Biol. 2011, 476, 308–311, doi:10.1038/nature10344.
