Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
Within cells, there are numerous compartments called ‘organelles’ that perform a range of specialised functions required to support life. Organelles are constantly adapting to their environment, changing shape and cooperating with each other depending on the cellular needs, which is essential for cell health as defects in these processes lead to human diseases. Organelles within eukaryotic cells are not isolated static compartments, instead being morphologically diverse and highly dynamic in order to respond to cellular needs and carry out their diverse and cooperative functions.
- organelles
- peroxisomes
- mitochondria
- membrane protrusion
- organelle interaction
1. Introduction
The view of membrane-bound organelles in eukaryotic cells as individual, static entities is outdated. The development of organelle-specific fluorescent markers in combination with advanced live cell microscopy approaches has allowed unprecedented insights into subcellular organelle dynamics, including movement, tubulation, fusion and division. An interesting phenomenon, which now regains attention, is the ability of several organelles, including plastids, peroxisomes and mitochondria, to extend and retract thin membrane tubules (also referred to as protuberances, extensions, or protrusions) of approx. 80–200 nm in diameter and up to 30 µm or more in length. Although such organelle extensions were initially reported in early morphological studies (e.g., [1,2]), their dynamic nature first became apparent in plant cells, where they were named stromules [3,4], peroxules [5,6,7] and matrixules [7,8], respectively (reviewed in [9]). Other organelles in plant cells, e.g., the vacuoles or nuclei, have also been reported to extend tubules but are not well studied [10,11].
Membrane protrusions have gained attention in modern cell biology as they contribute to the communication and connection of organisms, cells and organelles. They include bacterial tubule-like structures, which allow bacteria to exchange cellular molecules (e.g., proteins) with each other [21], as well as cell-to-cell membrane protrusions such as cytonemes and tunnelling nanotubes, which connect individual mammalian cells to enable the transfer of signals or even organelles [22,23]. The widespread appearance of membrane protrusions in biological systems underlines their significance.
2. Possible Functions of Organelle Membrane Extensions
2.1. A Role for Membrane Protrusions in Organelle Biogenesis and Dynamics
Peroxisomal membrane extensions are linked to the growth and division process of peroxisomes for multiplication/proliferation (Figure 1). PEX11β-mediated membrane deformation and protrusion formation can result in the generation of tubular peroxisomes, which subsequently constrict and divide involving MFF, FIS1 and DRP1. The initial thin membrane protrusions appear to be an intermediate between the spherical and tubular states, as they accumulate in cells with a defect in peroxisome division (e.g., in MFF deficiency). This is also observed in plant cells [8,9] and in yeast. In yeast, loss of the fission GTPases Dnm1 and/or Vps1 results in enlarged peroxisomes, which can form long protrusions [52,53]. The latter emanates towards the budding site in an attempt to deliver and inherit peroxisomes to the daughter cell, which is occasionally successful in the mutants. Protrusion formation under those conditions depends on Pex11 as Dnm1/Pex11 mutants are unable to generate peroxisomal protrusions [53].
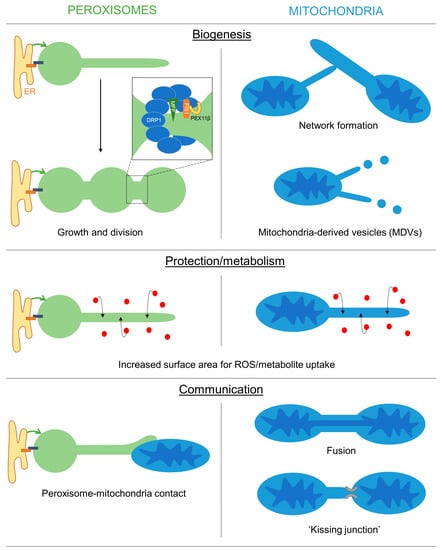
Figure 1. Overview of the possible functions of peroxisomal and mitochondrial membrane extensions. Schematic showing the potential roles of peroxisomal/mitochondrial membranes extensions in mediating organelle biogenesis, protection/metabolism and inter-organelle communication.
One function of membrane extensions is therefore linked to peroxisome formation. The latter is influenced by environmental conditions (e.g., the presence of peroxisomal substrates such as VLCFA) involving cellular signalling processes (e.g., via PPARs). However, the signalling pathways that regulate peroxisome multiplication/proliferation in humans are not well understood [71]. The research modelling approach revealed that the presence and frequency of peroxisomal protrusions and tubules are largely dependent on the lipid flow, elongation and division rates in different cell types (see above) [12,18,46]. For example, in human skin fibroblasts, peroxisomes are mainly spherical but can massively hyper-elongate when division is blocked. This indicates that the lipid flow rate, elongation and division rates must be high in fibroblasts and protrusions, or elongated peroxisomes are rarely captured due to the fast dynamics/turnover.
Similarly, membrane extensions of mitochondria contribute to mitochondrial dynamics and the formation of the mitochondrial network in the peripheral zones of mammalian cells. The tubular membrane bridges that form, quickly thicken and become part of the mitochondrial network, indicating they represent an intermediate during network formation. Furthermore, mitochondrial extensions have been linked to incomplete DRP1-mediated mitochondrial fission [39]. Finally, mitochondrial protrusions contribute to the biogenesis of MDVs [36].
2.2. A Protective Role for Organelle Membrane Protrusions
In plant cells, the formation of peroxisomal membrane protrusions (peroxules) is promoted by high levels of reactive oxygen species (ROS), e.g., during exposure to high-intensity light [20,49,72,73,74]. Remarkably, thin peroxules form within seconds following exposure to ROS and are suggested to contribute to a rapid uptake and neutralization of these damaging radicals [20,72,73,75]. This may be achieved by the increased surface area to volume ratio [76,77], which could facilitate the ROS exchange efficiency between the cytosol and the organelle (Figure 1). Persistent ROS stress results in a complete tubulation of peroxisomes, which ultimately divide and multiply to increase peroxisome numbers in the plant cell [72]. Tubulation and division of peroxisomes in mammalian cells after exposure to H2O2 or UV irradiation have also been observed [78]. However, the generation of oxidative stress in mammalian peroxisomes, mitochondria or the cytosol, e.g., by expressing organelle-targeted KillerRed, did not result in morphological alterations of peroxisomes [79]. In MFF-deficient fibroblasts with hyper-elongated peroxisomes and mitochondria, no changes in the oxidation state were observed in the cytosol and mitochondria using an H2O2-responsive variant of roGFP2, whereas peroxisomes showed reduced levels of H2O2 when compared to control cells [18].
Similarly, stress conditions have been reported to promote the formation of mitochondrial extensions, e.g., through Ca2+ dysregulation, manganese exposure or complex III inhibition. The protective effect of those extensions may primarily lie in the increased interconnectivity of mitochondria. In addition, stress conditions that impair the function of mitochondrial membrane proteins/complexes promote membrane extension and subsequent MDV formation to facilitate lysosomal delivery and degradation of those mitochondrial proteins [36].
2.3. Organelle Protrusions, Communication and Metabolic Exchange
Interestingly, in plant cells, peroxules also interact with mitochondria, presumably to prevent damage to those ROS-distressed organelles [20,74]. An interaction between peroxisomal protrusions and mitochondria was also observed in mammalian cells after overexpression of PEX11β, which promotes membrane expansion [19]. Interactions of elongated peroxisomes with mitochondria were more frequent than those of spherical organelles, but both interactions were long-lasting. Interestingly, in a large-scale mapping approach, PEX11β was found to be co-regulated with proteins of the mitochondrial ATP synthase complex, suggesting coordination of peroxisomal and mitochondrial functions. MIRO1, a membrane adaptor for the microtubule-dependent motors kinesin and dynein, was also co-regulated with PEX11β. In yeast, potential peroxisome-mitochondria tether proteins (e.g., Pex11-Mdm34, Pex34, Fzo1) have been identified [80,81], and contacts between both organelles appear to facilitate the transfer of acetyl-CoA from peroxisomes to mitochondria for efficient fatty acid degradation and energy generation [81].
An additional function of peroxisomal membrane protrusions may therefore be to facilitate organelle interaction and communication (Figure 1). Peroxisomes and mitochondria are both oxidative organelles, which contribute to cellular redox balance, but also cooperate in the β-oxidation of fatty acids in mammalian cells. This requires the transfer of chain-shortened fatty acids from peroxisomes to mitochondria and the exchange of cofactors. Peroxisomal protrusions, which interact with mitochondria, may therefore facilitate metabolic exchange as well as contribute to redox homeostasis. Similarly, in yeast, peroxisomes and lipid droplets can interact through peroxisomal extensions (called pexopodia) that extend into lipid droplets to facilitate the diffusion of fatty acids for β-oxidation in peroxisomes [82,83]. In plant seeds, peroxisomal extensions are also reported to deliver the Arabidopsis SDP1 lipase to oil bodies for triacylglycerol degradation and fatty acid mobilization [84].
As in mammalian cells, 70–80% of the peroxisomes can be engaged in close contact with the ER, which often wraps around peroxisomes, this causes a potential problem in terms of reconciling this immobilization with the need for direct interaction and simultaneous cooperation with mitochondria. The peroxisome-ER contacts are important for ether lipid synthesis and lipid transfer, whereas their interaction with mitochondria is important for fatty acid- and co-factor exchange [85]. Peroxisome membrane expansion may overcome this problem and allow the peroxisomes to stay tethered to the ER while also interacting with mitochondria (or other organelles). The membrane protrusions may therefore represent an alternative, more dynamic form of organelle contact site, which supports simultaneous interaction and communication with a third organelle without changing position.
Mitochondrial extensions can connect individual mitochondria, either by fusion or by kissing junctions, which allow the exchange of proteins and metabolites. Although peroxisomes share components of the division machinery with mitochondria, they do not share fusion proteins such as MFN1/2 or OPA1 and have not been reported to fuse or exchange fluorescent matrix proteins through a mechanism similar to mitochondrial fusion [86,87]. However, they do show “kiss and run” behaviour and self-interaction [86] and can form reticular-like structures [88]. Nevertheless, it is likely that peroxisome extensions, which contact other organelles, do not result in fusion, but instead form kissing junctions or contact sites. If specific tether proteins are involved in these contacts and if they are identical to the ones already described at mammalian peroxisomes (e.g., ACBD5, ACBD4 involved in peroxisome-ER interaction) is currently unknown. Organelle interactions by membrane extensions may increase the surface area, creating a membrane interface that would facilitate the exchange of metabolites through organelle-specific transport mechanisms [89].
It is also intriguing that membrane extensions are more frequently formed when organelles are immobilized, e.g., by physical constraints, as observed for mitochondria in skeletal and cardiac muscle cells that are densely packed with myofibrils. Tethering of peroxisomes to the ER also results in immobilization and is linked to protrusion formation. Low numbers of peroxisomes, as observed in PEX5-deficient or MFF-deficient cells, limit the frequency of potential contact events with other organelles. The research propose that these conditions also promote protrusion formation to compensate for reduced numbers and maintain the interaction and metabolic cooperation with other subcellular organelles, such as mitochondria and lipid droplets, which all contribute to cellular lipid metabolism. Overall, membrane protrusions appear to accomplish long-range interactions to maintain cellular homeostasis.
This entry is adapted from the peer-reviewed paper 10.3390/biology12050664
This entry is offline, you can click here to edit this entry!
