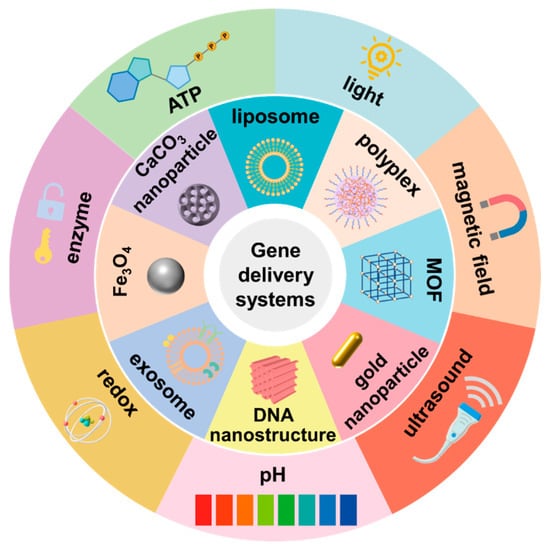
1. Introduction
For the past few decades, a variety of nanocarriers have been employed in the gene delivery field, which encompasses various organic nanoparticles, inorganic nanoparticles and biomimetic nanoparticles, such as liposomes [
59], polymeric nanoparticles [
60,
61,
62,
63], gold-based nanoparticles [
64,
65,
66], calcium carbonate nanoparticles [
67,
68], metal organic frameworks (MOFs) [
69,
70] and exosomes [
71,
72,
73]. As the most typical gene delivery systems, PEI-based polymeric nanoparticles and cationic lipids-based liposomes have been commercialized and extensively employed to transfect various cells. However, unsatisfactory transfection efficiency and cytotoxicity remain the obstacles for their in vivo use. To solve these problems, researchers have made tremendous efforts to engineer the polymers, and readers may refer to the review by Yang et al. that discussed in detail the different strategies to modify the polymers [
55]. Generally speaking, stimulus-responsive nanocarriers can respond to external or internal stimuli to more precisely and smartly control nanocarriers’ in vivo behaviors. Considering the differences between tumor and normal tissues in pH, redox condition and expression of enzymes, these internal signals are of great use in fabricating nanocarriers that are capable of regulating the gene release in a tumor-specific manner. Moreover, some external stimuli, including light, magnetic field and ultrasound, have also been utilized to spatiotemporally control the transport of nanocarriers (
Figure 1).
Figure 1. Overview of the main stimuli-responsive nanoparticles in gene therapy.
2. pH-Responsive Nanocarriers
The pH is highly heterogeneous and varies with the physiological environment. For example, the extracellular environment of tumors tends to be more acidic (pH ≈ 6.5) than that in normal tissues (pH 7.4), and the pH of endosome/lysosome is even lowered to 5. Therefore, the heterogeneity of pH has been broadly harnessed to enhance cellular uptake and control drug release, etc.
2.1. pH-Responsive Detachment of Outlayer Coating
PEGylation is a common strategy used in gene delivery system to prolong the circulation time. However, coating of PEG shields the positive charges of the polyethylenimine/DNA complex and may limit the cellular uptake, endosomal escape and subsequent transfection efficiency. To overcome the dilemma of PEG coating, Guan et al. designed a pH-responsive, detachable, polyethyleneglycol (PEG) shielding gene delivery system [
27]. The aldehyde-modified mPEG was coated onto PEI/DNA via pH-responsive Schiff base bonds. The PEG-shielded nanoparticles were stable in normal tissue during the transport process, while at the tumor site, the PEG was detached due to the cleavage of Schiff base bonds by the slightly acidic tumor extracellular pH. The re-exposed PEI/DNA could effectively cross the cellular membranes and result in gene expression. Similarly, using a pH-sensitive imine bond to conjugate the PEG derivative O’-methylpolyethyleneglycol (omPEG) into lipid, a PEG-detachable nanoparticle was designed for the tumor-directed delivery of chemo- and gene therapies [
74]. The nanoparticles were coated with multifunctional peptides and a PEG derivative to decrease noncancerous cellular uptake, while in the acidic tumor environment, the detachment of PEG rendered the exposure of peptides for active tumor targeting via specific ligand–receptor binding. The pH-detachable nanoparticles demonstrated improved cellular uptake in HNC human tongue squamous carcinoma (SAS) cells, and the released miR-200 and irinotecan synergistically improved the therapeutic efficacy in a SAS tumor-bearing mouse model.
Apart from pH-sensitive PEG detachment, pH-responsive outlayer detachment has also been reported [
75]. In this study, miR146a was conjugated to the polyethylenimine-(4-(bromomethyl) phenylboronic (PEI-PBA) via the ester linkage, and the formed inner core was further shielded by an outer-layer polyplex PEI−DMA-C225 through the electrostatic interactions. The outer-layer polyplex of nanoparticles exposed positively charged amino groups again in the endo/lysosomes, and thus detached from the nanoparticles. Afterwards, the intracellular ATP triggered the release of miR146a by competitively binding with the PEI-PBA. This pH/ATP-activated complex significantly suppressed invasion, colony formation and migration in DU145 cells and inhibited tumor growth in vivo, which demonstrated the potential in the treatment of androgen-independent prostate cancer.
2.2. pH-Responsive Gene Release
Various nanomaterials, including the metal–organic framework [
69], micelle [
76], CaP-phospholipid complex [
77], calcium carbonate nanoparticles [
78], quantum dot [
79], black phosphorus nanosheet [
80] and polymer [
81], have been used for pH-triggered gene release. For example, taking advantage of the pH sensitivity of CaP, an As
2O
3 and HER2-siRNA coloaded CaP-phospholipid complex nanocarrier (AH RNP) was constructed to control drug release in an acidic lysosome environment [
77]. Unlike the free Cy5siRNA, which showed a burst release profile, regardless of the pH, the Cy5siRNA in AH RNPs demonstrated a pH-dependent release profile: its release rate was faster at pH 5.5 than that at pH 7.4 due to the dissociation of CaP in the acidic environment. Moreover, the degradation of nanomaterials could facilitate their clearance from the body to minimize the toxicity [
82,
83].
Lipid Nanoparticles (LNPs) have been widely used in the gene delivery field, and some LNP-based drugs have been approved (e.g., Onpattro for the treatment of amyloidosis) [
9,
84]. LNPs are composed of ionizable lipid, PEGylated lipid, phospholipid and cholesterol. As a critical component of LNP, ionizable lipid plays a key role in LNP potency [
9]. Unlike permanently charged cationic lipids, ionizable lipids are uncharged at the physiological pH and become positively charged at an acidic pH. This feature renders the LNP the characteristic of pH-responsive gene release [
85]. In the acidic environment of an endosome, the ionizable lipids become protonated and interact with the negatively charged endosomal membrane, which leads to the destabilization of the endosomal membrane and facilitates the endosomal escape of nucleic acids [
86,
87].
Considering the heterogenicity of different tumors, multimodal therapeutic strategies should be adopted to achieve effective anti-tumor efficacy. Black phosphorus (BP), a dimensional nanomaterial with a high surface-to-volume ratio, has attracted interest in biomedical research recently. Using PEG and PEI dual-functionalized BP nanosheets (PPBP), Chen et al. constructed a degradable gene delivery system for cancer-targeted synergistic therapy [
80]. The PPBP demonstrated high drug loading of human telomerase reverse transcriptase (hTERT) siRNA and exhibited potent photodynamic therapy/photothermal therapy (PDT/PTT) activities. In a low pH and reactive oxygen species (ROS)-rich environment, the PPBP gradually degraded and endowed the release of hTERT siRNA to fulfill its biological functions. Combination of PTT, PDT and siRNA-mediated hTERT knockdown synergistically suppressed tumor growth and metastasis in the mouse model.
2.3. pH-Responsive Charge Reversal
Tissue factor (TF), a critical initiator of blood coagulation, is upregulated in a variety of tumors and plays a vital role in tumor progression and metastasis [
28]. Targeting tumor-associated TF has shown efficacy in anticancer metastasis and the prevention of cancer-associated abnormal hypercoagulability. To overcome the side effects of TF silence in normal tissues, such as tissue bleeding complications, strategies to specifically deplete TF in tumors are highly desired. In this context, Liu et al. developed a peptide-based gene delivery system that specifically reduced TF expression in tumors [
28]. An amphipathic peptide was used as the scaffold to complex with TF siRNA, and to improve the delivery efficiency, a cyclic RGD (cRGD) peptide that shows high affinity to the overexpressed αvβ3 on most tumors and a polyhistidine sequence that is responsive to the slightly acidic microenvironment in tumor tissues were introduced. The pH-sensitive sPNPs were sensitive to the acidity in the tumor microenvironment and reverted their potentials from negatively charged to positively charged. In combination with the recognition of cRGD-αvβ3, the sPNPs efficiently facilitated cellular uptake. In the lysosomes, the sPNPs were depolymerized and released siRNA by virtue of the protonation effect of polyhistidine. In a 4T1-breast-tumor-bearing mouse model, sPNPs suppressed the expression of tumor-associated TF by approximately 75% and significantly inhibited lung metastasis, outperforming pH-insensitive PNPs.
3. ROS-Responsive Nanocarriers
Many diseases, including tumor, are characterized by a high level of reactive oxygen species (ROS). The reprogramming of redox metabolism induces abnormal accumulation of ROS in cancer cells [
88], making ROS levels much higher in cancer cells (up to 100 μM) than in normal tissue (≈0.02 μM) [
89,
90]. In turn, to maintain the balance between the generation and depletion of ROS, tumor cells change antioxidant defense systems by upregulating the reducing substances. For example, glutathione (GSH), the main reducing substance in the tumor cytosol, maintains a concentration of about 2–10 mM, 100–1000 times higher than that in normal tissues [
91]. Therefore, taking advantage of the differences in GSH expression, various redox-responsive gene delivery systems have been fabricated for targeted tumor therapy [
82,
92,
93,
94,
95]. Among these designs, the most common strategy is to introduce a disulfide bond to endow the nanocarriers with the redox-sensitive characteristics, as the disulfide bond is prone to leakage in the presence of reducing substances, such as GSH.
In addition to disulfide bonds, boric acid ester linkage has been included in fabricating redox-responsive nanomaterials. In the pioneering work reported by Shen, they devised a boric acid ester-based, charge-switchable, cationic polymer B-PDEAEA, whose phenylboronic acid group was prone to oxidation by the elevated intracellular ROS levels and converted to being negatively charged for ROS-controlled DNA release. Considering the unsatisfactory transfection efficiency of B-PDEAEA in the presence of serum, they further coated B-PDEAEA/DNA with a PEGylated fusogenic lipid to improve its stability, but the delivery efficiency was slightly enhanced [
97]. Therefore, they replaced the fusogenic lipid with cationic lipids and developed a lipopolyplex-based gene carrier in a subsequent study [
98]. The lipid coating improved the stability of the lipopolyplex, enhanced cellular uptake and promoted nuclear localization. Moreover, the ROS generated by the cationic liposome could in turn accelerate the charge reversal of the B-PDEAEA polymer and release DNA. Inspired by their work, some other boric acid ester-based gene delivery systems have also been reported [
89,
99].
4. Enzyme-Responsive Nanocarriers
Enzymes are bioactive substances that are highly specific and efficient in catalyzing their substrates, which play an essential role in various biological processes. Along with the tumor progression, the profiles of enzymes in tumors have also evolved. Taking advantage of the differences in enzyme expression between normal tissues and tumors, researchers have fabricated enzyme-sensitive nanocarriers for tumor-targeted gene therapy, including enzyme-triggered gene release, enzyme-controlled carrier dissociation, enzyme-enhanced cellular uptake, etc.
Esterases are a class of enzyme with the ability to hydrolyze ester bonds. Qiu et al. synthesized an esterase-responsive cationic polymer, PQDEA, which contains an acetyloxybenzyl ester [
102]. This acetyloxybenzyl ester could be cleaved by the high intracellular esterase, leading to the elimination of p-hydroxymethylphenol and thus the charge reversal of the cationic polymer. Owing to the weaker interactions between the anionic polymer and the plasmid, the plasmid was released for TRAIL suicide gene expression. The LPQDEA demonstrated higher transfection efficiency than PEI 25K and lipo, and effectively promoted the apoptosis of tumor cells in the HeLa tumor model.
Matrix metalloproteinases are overexpressed in many tumors and are associated with tumor progression, invasion and metastasis [
103]. Researches have indicated that matrix metalloproteinase-9 (MMP9), a type-IV collagenase/gelatinase, is overexpressed across various tumors, including colon, breast and gastric tumors [
104,
105,
106]. Considering the high levels of MMP9 in the tumor tissues, Boehnke et al. constructed MMP9-sensitive nanotheranostics using layer-by-layer (LPL) technology. The liposome acted as an inner core, and poly-l-arginine (PLR), siRNA, and propargyl-modified polyl-aspartate (pPLD) were subsequently adsorbed onto it to improve colloidal stability and enhance the gene delivery efficiency. The MMP9-sensitive biosensor peptides were further conjugated to the LPL-coated liposome using copper(I)-catalyzed click conjugation chemistry. In the tumor microenvironment, biosensor peptides could be specifically cleaved and release the reporter fragments, which provided valuable information about the disease progression. In three mouse models including pancreatic, colorectal and ovarian cancer, the biosensor LbL nanoparticles demonstrated potent diagnostic capabilities and higher than 50% knockdown efficiency [
107].
Cell-penetrating peptide (CPP) is a short peptide, usually composed of no more than 30 amino acids [
108,
109]. Due to its outstanding transduction ability and biocompatibility, CPP has been commonly utilized in the nanomedicine field [
110]. However, the CPP-mediated cellular penetration is non-specific and may lead to undesired side effects for normal tissues. To address this issue, tumor microenvironment-sensitive polypeptides (TMSP) have been developed to overcome the shortcomings of traditional CPP. Generally, TMSP is composed of three segments: the cell-penetrating peptides (CPP, oligoarginine), the shielding peptides (EGGEGGEGGEGG) and a matrix metalloproteinases-2/9 (MMP-2/9)-cleavable peptide linker (PVGLIG, etc.) [
111,
112]. In the normal physiological environment, the CPP is shielded by negatively charged peptides and remains inert. While in the tumor microenvironment, the MMP-2/9-cleavable peptide linker is cleaved and re-exposes the CPP to enhance cellular uptake. Modification of TMSP onto nanoparticles has demonstrated great potential in promoting the cellular uptake of therapeutic agents, such as chemotherapeutics drug, siRNA and even plasmid [
111,
112,
113,
114,
115]. For instance, researchers designed an amphiphilic dendrimer-engineered nanocarrier system (ADENS) for co-delivering paclitaxel and siRNA [
114]. Due to its hollow core/shell structure, siRNA was encapsulated in the hydrophilic cavity, while paclitaxel was loaded in the hydrophobic interlayer. Compared with the unmodified ADENS, the TMSP-modified ADENS showed enhanced cellular uptake, tumor penetration and accumulation via an MMP-2/9-dependent mechanism. Moreover, researchers utilized the TMSP grafting strategy to construct a TMSP-responsive gene delivery system for CRISPR-based gene therapy [
115]. The fluorinated polyethylenimine (PF) was chosen to condense the CRISPR plasmid owing to its superiority in improving cellular uptake, the endosomal escape of polymer/DNA polyplexes and intracellular DNA disassociation from the polymer [
55,
116,
117]. A haluronic acid (HA)-PEG-TMSP (HPT) module was further introduced via electrostatic interactions. In the cellular uptake experiments, pretreating the cells with MMP inhibitor (GM6001) inhibited the cellular uptake of HPT-PFs, while adding MMP further increased the cellular uptake. These results suggested the modification of TMSP indeed promoted the cellular uptake. Moreover, it was found that HPT-modified nanoparticles demonstrated significantly higher transfection efficiency than unmodified nanoparticles, suggesting the dual modification of HA and TMSP-facilitated gene delivery. In addition to TMSP-mediated cellular uptake, MMP-responsive PEG detachment has also been reported for efficient gene therapy [
118].
Hyaluronidases, the enzymes to degrade HA, are expressed in many tissues [
119]. Most members of the hyaluronidase family participate in tumor progression [
120]. Studies have indicated the high level of hyaluronidase in some tumors, such as breast and prostate cancer [
121,
122]. Therefore, hyaluronidases could be used to trigger HA degradation for gene release [
123,
124,
125,
126]. Choi et al. developed a versatile RNA interference nanoplatform capable of delivering both siRNA and miRNA [
127]. This HA-based nanoplatform can actively target tumor cells by virtue of the high affinity of HA to CD44 receptors (usually highly expressed on tumor cells), and after the CD44 receptor-mediated endocytosis, its CaP layer dissolved in the acid lysosome, and the nanoplatform was degraded by intracellular hyaluronidase, leading to the release of all payloads. Apart from the hyaluronidase-triggered degradation of a HA-based nanocarrier, a novel hyaluronidase-triggered charge reversal polymetformin (PMet)-based nanocarrier has also been devised for co-delivering doxorubicin (DOX) and plasmid encoding the IL-12 gene (pIL-12) to treat metastatic breast cancer [
125]. PMet-based polycationic micelles incorporated DOX in the inner part and absorbed pIL-12 on the surface. To minimize the interference of proteins in blood and improve the nanocarrier’s stability, the polycationic micelles were further coated by thiolated hyaluronic acid (HA-SH) via electrostatic interaction and a thiol crosslink. In the presence of hyaluronidase, HA-SH was deshielded, which rendered the exposure of cationic PMet. The subsequent protonation of PMet in endo/lysosome conditions promoted the release of pIL-12 and DOX. This nanocarrier exhibited superior antitumor efficacy and anti-metastasis efficacy in in the 4T1 tumor model mice.
5. ATP-Responsive Nanocarriers
Adenosine triphosphate (ATP) is one of the most abundant molecules in the cell and also the direct energy source of cellular metabolism. In tumor cells, it is usually at a high level. Its intracellular concentration is above 10 mM, nearly thousands of times higher than that in the extracellular environment (<5 μM) [
128,
129,
130], making it a promising stimulus for constructing an intelligent nanocarrier. The phenylboronic acid (PBA) group and its derivatives can selectively form ester bonds with diol compounds in aqueous solutions, and this process was reversed under conditions of lower pH and a lower concentration of diol compounds. As ATP contains diol moieties in its ribose ring, it can interact with PBA to form ester bonds. Therefore, a PBA-modified nanocarrier has been widely used in ATP-responsive gene delivery. In an example, two block catiomers were crosslinked by phenylboronate ester formed between their PBA moieties and polyol (D-gluconamide (GlcAm)) moieties, and complexed with the plasmid to form the nanoparticle. In the intracellular compartment, the high levels of ATP competitively bound with PBA and replaced GlcAm, leading to the dissociation of nanoparticles and the acceleration of plasmid release [
131]. This ATP-responsive nanoparticle exhibited higher transfection efficiency in HuH-7 cells than its non-sensitive counterpart, laying the foundation for the application of PBA-modulated nanoparticles in the ATP-responsive gene delivery field. Similarly, PBA-grafted PEI has been reported to be an efficient carrier for targeted gene silencing [
132]. The borate ester bond of PBA-dopamine in this carrier can be replaced by the intracellular ATP, triggering the siRNA release from the carrier. In A375 cell-bearing BALB/c nude mice, the TXPPBA/VEDA/siEGFR complexes demonstrated the best tumor inhibiting efficacy and EGFR protein knockdown among these formulations. Further, in another study, PBA-modified PEI was crosslinked with alginate via the PBA group and diol group to improve the siRNA loading ability [
133]. After treatment with 5 mM ATP, both PEI-PBA and CrossPPA groups released almost all of their siRNA within 5 min, while nearly no release of siRNA was observed in 25k PEI/siRNA or 1.8k PEI/siRNA groups. The mechanism revealed that ATP-mediated CrossPPA decrosslinking and charge reversal were essential for RNA release. Apart from these mentioned polymers, this PBA-grafting strategy still works in DNA nanostructures [
134].
6. External Stimulus-Responsive Nanocarriers
Besides the above nanocarriers that take advantage of the difference between tumor tissue and normal tissue, some external stimulus-responsive nanocarriers have drawn attention recently. Compared with the internal stimulus-controlled drug delivery, whose sensitivity is highly dependent on the difference between targeted and untargeted parts, external stimulus-responsive nanocarriers are less dependent on the internal signal and mainly respond to the external stimulus, such as light, the magnetic field and ultrasound.
7. Magnetic Field-Controlled Gene Delivery System
Iron oxide nanoparticles have been widely used in the drug delivery field due to their unique magnetic properties, controllable size and ease of scalable production. Extensive studies have employed surface-engineered iron oxide nanoparticles for magnetic-guided drug delivery, as the iron oxide nanoparticles could actively accumulate at the desired site under the magnetic fields [
135,
136,
137,
138,
139]. In a study, two differently shaped magnetic mesoporous silica nanoparticles (M-MSNs) were developed for magnetically mediated suicide gene therapy [
138]. The herpes simplex virus thymidine kinase/ganciclovir (HSV-TK/GCV) gene was adsorbed onto M-MSNs, whose surface was modified by PEG-g-PLL to offer a positive charge for plasmid loading. Due to the unique magnetofection properties of these two M-MSNs, the TK gene expression was enhanced under the magnetic field. Moreover, biodistribution study revealed that the tumor accumulation of both M-MSNs was significantly increased by the magnetic field, suggesting their potential of magnetically guided tumor targeting. The Rod-like M-MSNs exhibited better tumor targeting properties than sphere-like M-MSNs regardless of the magnetic field, highlighting the critical role of shape in the preferential tumor accumulation of M-MSNs. Consistent with the tumor targeting capability, the superior antitumor efficacy was also seen in the Rod-like M-MSNs under the magnetic field in nude mice bearing HepG2 tumor xenografts. Additionally, these two M-MSNs could serve as MRI contrast agent to monitor therapeutic efficacy by MRI. Another magnetic field-guided tumor-targeted multimodal nanoplatform has been reported by Wang et al. recently [
140]. They encapsulated doxorubicin (DOX) into the exosomes by electroporation, and further modified the exosomes with polydopamine (PDA) coated magnetic Fe
3O
4 nanoparticles (Fe
3O
4@PDA). A robust fluorescence signal was detected at the tumor site in mice treated by Exo-DOX-Fe
3O
4@PDA-MB with magnetic field while nearly no fluorescence signal was observed in mice without magnetic field, verifying the excellent magnetically targeted properties of this platform. Furthermore, the platform could achieve cooperative gene/chemo/photothermal cancer therapy by virtue of the potent photothermal properties of PDA, the cytotoxicity of DOX and miR-21-responsive molecular imaging. However, use of permanent magnetic field in this study may interfere with the interaction of magnetic nanoparticles and induce the aggregation of magnetic nanoparticles. The author reasoned that use of low-frequency pulsed magnetic fields or alternating magnetic fields may be a reasonable solution to reduce the aggregation of magnetic nanoparticles.
Inspired by the movement of natural motile bacteria E. Coli, various magnetic micro-/nanoswimmers of helical shapes have been developed such as artificial bacterial flagella (ABFs) [
141]. ABFs can move under the control of low-strength rotating magnetic fields. In a study, ABFs were functionalized with lipoplex, which was formed by lipofectamine and plasmid, for targeted gene delivery in human embryonic kidney (HEK 293) cells. The functionalized ABFs were guided by the magnetic field and targeted to the HEK 293 cells for gene expression.
8. Light-Responsive Gene Delivery System
Many nanoparticles including organic or inorganic nanoparticles possess extraordinary photothermal or photodynamic characteristics [
100,
142,
143,
144], making light an attractive stimulus to control the gene delivery. Light of various wavelengths has been used in light-controlled nanocarriers, including ultraviolet light [
145], visible light [
146,
147] and near-infrared (NIR) light [
148]. Taking the coumarin-anchored polyamidoamine (PAMAM) dendrimer as an example, it can self-assemble in aqueous solution via hydrophobic interactions because of its unique amphiphilic properties and improve the binding affinity with nucleic acids because of the hydrophobic substitute coumarin. More importantly, coumarin can cyclodimerize when exposed to light of a wavelength above 300 nm while the crosslinked coumarin can be degraded into monomers under a shorter wavelength UV light. Therefore, this nanocarrier can control the drug release by UV light and minimize the side effect on normal tissues [
145]. However, the limited penetrating ability of UV light may hinder their clinical application [
149]. Therefore, in another example, Wang et al. developed a far-red light-mediated nanocarrier which could be triggered by far-red light (661 nm) at low optical power density [
150]. Under irradiation, the incorporated photosensitizer produced non-cytotoxic levels of ROS, which could enhance endosomal escape and promote p53 gene release via degradation of thioketal-crosslinked polyethylenimine (TK-PEI). The light-responsive characteristics of this nanocarrier were verified both in vitro and in vivo. Similarly, a photolabile spherical nucleic acid (PSNA) has also been reported that was self-assembled by siRNA conjugated peptide nucleic acid-based ASO (pASO) via a ROS-sensitive linker [
151]. The NIR light-triggered ROS can break the
1O
2-cleavable linker, leading to the disassembly of PSNA and in turn facilitate the siRNA release. The released siRNA and pASO efficiently inhibited the expression of HIF-1α and Bcl-2 under irradiation and potently inhibit tumor growth. Other photo-responsive units such as the photosensitive platinum(IV) complexes (Pt(IV)) [
148] has also been applied in constructing light-controllable gene release.
Photo-chemical internalization (PCI), a unique mechanism to escape from the endosome by destroying the endosome membranes via the produced ROS under irradiation, have shown great promise in recent years, especially in the gene delivery field [
147,
152,
153,
154]. Even plasmid of a much larger size (e.g., CRISPR-based plasmid, ≈10,000 bp) has been successfully delivered via PCI [
155]. Generally, to make use of the PCI effect, the nanocarrier contains a sort of photosensitizer that can produce ROS, and this process relies on both light and oxygen. Nevertheless, the efficiency of ROS generation by photosensitizers is relatively limited due to the hypoxic tumor microenvironment. To overcome this dilemma, Zhang et al. developed a ROS-independent photoactivatable nanoparticle named CNP
PtCP/si(c-fos) [
147]. Rather than incorporating a photosensitizer for ROS production, they synthesized a polymer containing the photoactivatable Pt(IV) prodrugs that can generate azidyl radicals (N3
•) to facilitate N3
•-mediated PCI. Compared with the ROS, the N3
• possess milder oxidation energy and are less dependent on oxygen [
156], thus minimizing the damage to the co-loaded drugs and exhibiting application potential in wider field.
In addition to facilitating drug release or promoting PCI via light, strategies to modulate the gene expression under the control of light has also been applied. By incorporating a light-sensitive transcription factor (GAVPO) and a specific promoter, researchers developed a light switchable transactivator [
157,
158], which have the capability to initiate the gene transcription under irradiation. The PEI-based targeted nanoparticles can actively target the tumor tissues and overcome the intracellular delivery barriers, and the released plasmid can encode diphtheria toxin A to induce cell apoptosis under the irradiation of blue light [
159]. Likewise, by replacing the original promoter with a heat-inducible promoter, Chen et al. devised an optogenetically activatable CRISPR-Cas9 nanosystem [
64]. This nanosystem consisted of cationic polymer-coated Au nanorod and a transformed CRISPR plasmid driven by a heat-inducible promoter. Taking advantage of the excellent photothermal properties of Au nanorod, this nanosystem transformed the light into heat to trigger the expression of CRISPR plasmid, achieving spatiotemporally controllable gene editing and reducing the off-target effect. On the basis of the potent performance of this nanosystem, in their subsequent study, this nanosystem was used for cancer immunotherapy by combination of photothermal therapy-triggered immunogenic cell death (ICD) and CRISPR-mediated PD-L1 knockout for immune checkpoint blockade [
66].
9. Ultrasound-Targeted Gene Delivery System
Ultrasound-targeted gene delivery (UTGD), also termed as ultrasound-targeted microbubble destruction or ultrasound mediated gene delivery, allows specifically directing the gene to the target site via ultrasound-focused techniques. Moreover, UTGD can facilitate cellular uptake by enhancing the membrane permeability when exposed to ultrasound [
160,
161]. Up to now, varieties of genes have been successfully delivered by UTGD. Basically, genes or nucleic acids are loaded onto ultrasonic nanoparticles via two ways: (1) direct conjugation onto the surface or (2) complexed with the cationic polymers first and then modified onto the nanoparticle [
162]. Use of cationic lipids such as DPPC, DSPC and DOTAP or ionizable lipids to condense nucleic acids has been reported for UTGD. For example, Un et al. reported an ultrasound (US)-responsive gene carriers for treating metastatic and relapsed melanoma, using DSTAP, DSPC and NH
2-PEG
2000-DSPE or mannose-modified PEG
2000-DSPE [
163]. This mannose-modified bubble lipoplexes achieved efficient DNA vaccination under US exposure and elicited potent antitumor efficacy. In another study, Tayier et al. developed a kind of novel nanobubble for targeted gene delivery under focused ultrasound [
164]. The nanobubbles were extracted from Halobacterium NRC-1 (Halo) and could produce stable ultrasound contrast signals in vitro and in vivo [
165]. Further modification of PEI endowed the nanobubbles with the DNA condensation ability. Compared with the conventional microbubbles whose particle size are over 1 μm, this biosynthetic nanobubble has a much smaller size of around 200 nm, making it easier to enter the target cells. Notably, this nanobubble DNA/CBNBs significantly improved the gene transfection efficiency under ultrasound as revealed by the increased number of EGFP-positive cells. Moreover, nearly threefold of fluorescent signals were observed in the DNA/CBNBs group under ultrasound in comparison with the DNA/PEI group in a mouse model, further demonstrating the potential of ultrasound-enhanced gene delivery. Ultrasound-enhanced ROS nanocarrier has been constructed to control the gene release as well [
166]. This nanocarrier was decorated with IR780, a sonosensitizer that can generate ROS under ultrasound stimulation. The generated ROS can revert the charge of the ROS-sensitive polymer, causing gene release as a result of charge repulsion for ultrasound-triggered gene delivery. Despite the advancement of ultrasound in gene delivery, special attention should be paid to the safety issues of using ultrasound due to ultrasound-associated tissue heating [
162]. The non-homogeneity of sound transmission may offset the delivery efficiency and the therapeutic outcomes.
10. Multi-Stimulus Responsive Gene Delivery System
Various delivery systems that have been exploited for stimuli-responsive nucleic acids delivery are summarized in Table 1. Instead of using a single stimulus to control the gene delivery, several stimuli have been applied simultaneously in fabricating multi-stimuli responsive gene delivery systems, which has gained more and more attention recently owing to their superiority in accuracy and specificity.
Table 1. Summary of stimuli-responsive delivery systems.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15051450