2. Displacement-Based Approaches for the Removal of PBUTs
2.1. Infusion of Binding Competitors
HSA is the most abundant protein in blood plasma and, because of its extraordinary ligand binding capacity, is responsible for the transport of a wide variety of pharmaceutical drugs throughout the body [
47,
48]. As mentioned before, the HSA primary binding sites for the PBUTs IS, pCS, IAA and HA is Sudlow’s site II, whereas for CMPF is Sudlow’s site I [
3,
29]. The HSA-PBUT association constants (K
a) for IS, pCS, IAA, HA, and CMPF are 0.98 × 10
5 M
−1, 1.0 × 10
5 M
−1, 2.1 × 10
5 M
−1, 0.1 × 10
5 M
−1, and 130.5 × 10
5 M
−1, respectively [
49,
50].
Considering that certain pharmaceutical drugs circulate in the blood bound to the same HSA primary binding sites as PBUTs and hence, when present in the same environment as PBUTs, compete for the same binding site, they are often referred to as competitive binding molecules or albumin displacers. When the association constant between the drug and HSA is larger than the value of K
a between HSA and the PBUT, the latter will tend to release itself from HSA and be replaced by the binding competitor. This in turn increases the percentage of the free (unbound) form of the PBUT which is readily removed by conventional HD membranes. A parallel phenomenon which should be considered is the allosteric mechanism, where a molecule binds to other binding sites of HSA causing the protein molecule to undergo modifications of its conformation which can lead to the release of the PBUTs [
51,
52]. This was first seen by Loor et al. [
53] when trying to overcome the limitations of different methods available for the quantification of toxins in the plasma and using sodium octanoate as a binding competitor to separate IS and pCS from HSA.
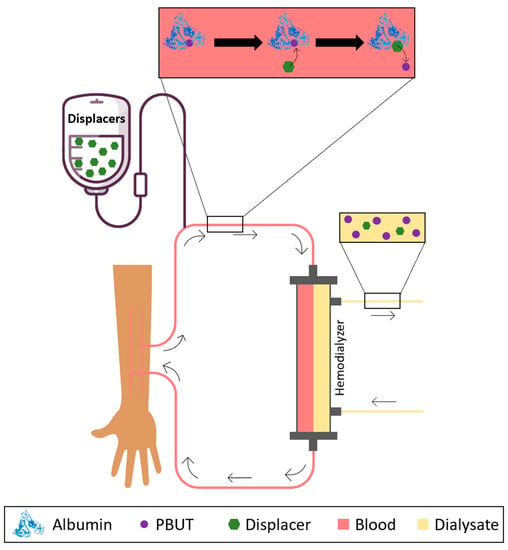
Having this in mind, researchers have considered the possibility of infusing binding competitors or albumin displacers into the blood or plasma as a way of promoting the release of PBUTs from albumin and increasing the concentration of the free fraction which is then easily removed by HD membranes. Figure 1 schematically shows the concept behind the infusion of albumin displacers into the bloodstream of patients undergoing HD.
Figure 1. Schematical representation of the removal of PBUTs by HD after infusion of HSA displacers or binding competitors into the bloodstream.
Tao et al. [
54] studied the in vitro removal of IS using spiked HSA with IS (21.3 mg/L) under static and dynamic conditions in the presence of two different binding competitors: L-tryptophan (TRP) (weak displacer of IS, K
a = 1 × 10
4 M
−1) and docosahexaenoic acid (DHA) (strong displacer, K
a = 6.9 × 10
8 M
−1) [
54]. In the experiments under static conditions, the spiked HSA-IS solutions were incubated with TRP and DHA, separately, for 4 h, in order to calculate the amount of IS displaced by each of the drugs. In experiments performed under dynamic conditions, the HSA-IS solution was circulated through a commercial HF hemodialyzer (F41S by Fresenius Medical Care; Waltham, MA, USA) for 10 min, after which the solution containing either TRP or DHA was administered to the reservoir containing the spiked plasma until a final concentration of 1 mmol/L was achieved. TRP or DHA was continuously infused into the blood flow circuit between the reservoir and the dialyzer until the end of the 4 h experiment at a rate of 50 µmol/min for TRP or 60 µmol/min for DHA. The feed and dialysate flow rates were 100 and 150 mL/min (counter-current, without recirculation) and the TMP was 0 mmHg (no forced convection). Results show that, under static conditions, the initial unbound fraction of IS was 8% and after being incubated for 4 h with TRP and DHA had increased to 11 and 25%, respectively [
54]. Results from the study performed under dynamic conditions showed that the removal of IS was 19 and 28% for TRP and DHA, respectively [
54]. DHA showed better results, confirming that is a stronger binding competitor compared to TRP and can successfully displace IS from HSA. As a negative control, phosphate-buffered saline (PBS), which does not contain displacers, was infused through the same hemodialyzer and the removal was only 10% which is approximately the same amount of the unbound fraction present at the beginning of the experiment [
54].
Tao et al. [
51], performed, ex vivo experiments, using human plasma and whole blood spiked with IS, IAA, HA, and pCS, for the evaluation of different binding competitors: ibuprofen (IBF), furosemide (FUR), and TRP. The biological samples used were: (i) plasma from ESRD patients (ii) plasma from a healthy population, and (iii) whole blood from a healthy population. In the experiments under static conditions, the uremic plasma was incubated with IBF (1 mmol/L), FUR (0.18 mmol/L), and TRP (1 mmol/L), separately, for 4 h at 37 °C, to evaluate the displacement of IS, pCS, and HA [
51]. The authors also evaluated under static conditions and using healthy plasma spiked with IS at a concentration of 32 mg/L, the dose-response to IBF (0 to 931 µmol/L) as well as the effect of using a mixture of IBF and FUR ([IBF] 0 to 931 µmol/L, and [FUR] fixed at 0 or 182 µmol/L) [
51]. The latter study (was carried out to evaluate if the infusion of FUR together with IBF would boost the IS displacement effect of IBF when given simultaneously, suggesting additive or synergetic effects of these two binding competitors. The authors also performed an in vitro study under dynamic conditions, using hollow fibers taken from the Optiflux
® F160NR hemodialyzer (Biotechnology Research Group, Dialyzer R&D; Ogden, UT, USA), with a total surface area of 0.039 m
2. In this study, the removal of IS (32 mg/L), IAA (2.6 mg/L), and HA (71.6 mg/L) from healthy whole blood was evaluated by a single-pass through the hemodialyzer (without recirculation), with blood and dialysate flow rates of 12.5 and 25 mL/min (counter-current), respectively, and with a TMP of 0 mmHg (no forced convection). A combination of IBF/FUR (IBF, 116 mmol/L / FUR, 23 mmol/L) or simply TRP (146 mmol/L) was infused at a rate of 70 μL/min and 138–150 μL/min, respectively [
51].
Results showed that under static conditions, the free fraction of IS and pCS increased in the presence of FUR, TRP, and IBF, by a factor of 1.3, 2.0, and 3.0, respectively, indicating that IBF has the highest binding affinity towards IS and pCS. For HA the highest increase of the free fraction was found in the presence of FUR, followed by IBF, and TRP did not show differences when compared with the control [
51]. The evaluation of the dose-response effect of IBF revealed that the increase in IBF concentration resulted in an increase in IS displacement and the addictive or synergistic effect of using IBF and FUR as binding competitors was verified [
51]. Under dynamic conditions, results showed that the combinations of IBF/FUR increased the removal of IS by a factor of 3 (from 6 to 18%) and IAA by a factor of 2 (from 17 to 35%). In the presence of TRP, the removal of IS increased from 6 to 11% (1.6×) and the removal of IAA increased from 17 to 27% (1.6×). None of the binding competitors showed a significant impact on the removal of HA. The authors concluded that this approach has the potential to be applied in current clinical HD sessions because all the binding competitors are FDA-approved and none were administered at toxic concentrations [
51]. However, it is important to note that TRP, which was used in these studies, is known to be metabolized into an indole, and ultimately converted to IS, which is exactly one of the target PBUTs and therefore should not be administrated into the bloodstream of ESRD patients.
In 2019, the first proof-of-concept clinical study was performed to explore the potential of using IBF as an albumin displacer to increase the depletion of IS and pCS in ESRD patients during HD sessions [
55]. The study was performed on 18 ESRD patients, with no residual urine production, during a 4 h-long therapy session to whom heparin was administrated prior to the beginning of the HD session. HFHD hemodialyzers Hemoflow F80A (Fresenius Medical Care; Waltham, MA, USA) were used and the blood and dialysate flow rates were set to 300 and 500 mL/min, respectively. The HD session was divided into three phases: (i) pre-infusion (0 to 20 min of HD session); (ii) IBF infusion (21 to 40 min of HD session); and (iii) post-infusion (41 to 240 min of HD session). The only intervention during the 4 h was during the infusion phase, where an IBF solution (3200 mg/L) was administered to the arterial blood line for 20 min at a rate of 12.5 mL/min (40 mg of IBF/min). The three phases were analyzed in terms of IS and pCS removal [
55] and the results showed that during the second phase (infusion of IBF) the clearance of IS and pCS increased from 6 to 20 mL/min and from 4 to 15 mL/min, respectively [
55]. During the post-IBF-infusion phase, the clearance of IS and pCS decreased to values similar to those seen in the pre-IBF-infusion. The authors conclude that IBF improves the removal of IS and pCS in ESRD patients undergoing a single HFHD session, but the competitive action of IBF occurs primarily during the infusion phase and that better results could be obtained if IBF is administered continuously during the entire HD session [
55]. IBF is a dialyzable compound, that easily crosses the hollow fiber membranes in the hemodialyzer. This means that, when not being infused, IBF is quickly eliminated during the HD session, reducing the possibility of the displacement of the HSA-PBUTs complex to occur. Hence, there is a need to infuse IBF throughout the HD session which could prove to be a limitation of this approach. Other compounds with competitive properties (on their own or mixed with others) and other displacer-based approaches should be explored [
55].
Instead of using pharmaceutical drugs, Li, et al. [
52] studied the competitive binding properties of natural herbal medicinal products, known to have standardized active ingredients, towards the enhanced removal of IS and pCS. Danhong (DHI) is a natural product commonly used intravenously in traditional Chinese medicine for the treatment of cardiovascular and cerebrovascular diseases and has also shown beneficial effects in confirmed cases of deterioration of kidney function [
56]. DHI is extracted from the rhizome of Salvia miltiorrhiza Bunge (
Labiatae) and the dry flower of
Carthamus tinctorius L. (
Asteraceae). The main bioactive compounds of DHI are salvianolic acid-based, including lithospermic acid (LA), salvianolic acid A (SaA), tanshinol (danshensu, DSS), caffeic acid (CA), salvianolic acid B (SaB), protocatechuic aldehyde (PA) and rosmarinic acid (RA) [
52]. RA, SaB, and LA are reported to be strong protein-binding ligands, with binding percentages for HSA between 92 and 99% [
57]. Li, et al. [
52] studied the potential of DHI and the salvianolic acid LA as binding competitors and studied the removal of PBUTs in in vitro and in vivo rat models. In vitro studies were performed with healthy rat plasma spiked with IS (50 mg/L) and pCS (50 mg/L), followed by an infusion of DHI, LA, and IBF, at a later stage. During the first two hours, the spiked plasma was flown through a dialysis probe (Microbiotech/se AB; Stockholm, Sweden) with a feed flow rate of 2 µL/min (single-pass HD), and during the last two hours, the spiked plasma containing each of the binding competitors at different concentrations (50, 200 or 400 µM) was passed through the same hemodialyzer (single-pass). For comparison purposes, IBF was also studied at the same concentrations as well as 1000 µM, under the same circulation conditions. Results showed that DHI (400 µM) improved the removal of IS and pCS by 99 and 142%, respectively. The salvianolic acid, LA (400 µM), increased the removal of IS and pCS by 197 and 198%, respectively, and infusion of IBF (400 µM) did not affect IS or pCS removal. Finally, it was concluded that, because the primary binding site of IS and pCS is different from that of LA, the displacement of HSA from the PBUTs is due to allosteric mechanisms rather than direct binding competitions [
52]. In the in vivo rat model, rats with very limited or no kidney function were subjected to microdialysis for 4 h, using a dialysis probe (Microbiotech/se AB; Stockholm, Sweden) and a blood flow rate of 2 µL/min. Conventional HD was performed for two hours after which DHI or LA (24.7 mg/kg) was infused at concentrations of 4 and 25 mL/kg, respectively. HD was then continued for an additional 2 h [
52]. Results showed that DHI increased the removal of IS and pCS by 136 and 272%, respectively and LA increased the removal of IS and pCS by 120 and 128%, respectively. Higher removal rates of IS and pCS were found when LA was infused in comparison to the first two hours where no binding competitor was present [
52]. Improved results are obtained when a cocktail of molecules is administered, rather than used separately. This indicates an additive or synergistic effect of salvianolic acid-based compounds, which was also suggested by Tao et al. [
51] when using a mixture of IBF and FUR simultaneously as binding competitors.
Intravenous lipid emulsions (ILEs) have been used in various clinical fields ranging from parental nutrition to resuscitation from drug overdoses. After being injected, the free fatty acids (FFAs) in the emulsion are released and can bind to nine different binding sites of HSA (FA1 to FA9). Shi et al. [
58] studied the binding of ILEs compounds to HSA in the presence of PBUTs. In vitro binding and HD studies were performed using HSA solutions and uremic rat models were used for the in vivo experiments. Oleic acid (OA) and linoleic acid (LLA), which mimic conventional lipid emulsions, and eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) which mimic omega-3 fish oil lipid, were the ILEs used in the in vitro assays. HSA solutions (40 g/L) were spiked with CMPF (61.2 mg/L), pCS (37.6 mg/L), IS (32.0 mg/L) and IAA (2.6 mg/L), separately and the competitive binding effect of the four different FFAs was evaluated by adding them to the spiked HSA solutions at concentration: OA (0.01 to 4.8 mmol/L), LLA (0.01 to 4.8 mmol/L), EPA (0.01 to 10.0 mmol/L) and DHA (0.01 to 10.0 mmol/L). Results show that OA and LAA significantly increased the free fraction of all the PBUTs, and CMPF was the one where the effect was highest, as it was displaced by 95%, followed by pCS, IS, and IAA. It was also concluded that OA and LA were much stronger displacers when compared to EPA and DHA. The in vitro HD study was performed using a polysulfone-based hemodialyzer suitable for small animals. The feed and dialysate flow rates were 10 and 6 mL/min, respectively, (counter-current mode), and the TMP was adjusted to 0 mmHg (no forced convection). An HSA solution (40 g/L) was spiked with CMPF, pCS, IS, and IAA, at concentrations of 61.2, 37.6, and 32.0 mg/L, respectively, and was circulated through the dialyzer for 10 min. Then, a mixture of LLA and OA (LLA:OA ratio 2:1) was infused continuously in the feed inlet to a final concentration of 1 mmol/L. As a negative control, the same procedure was performed using PBS (without FFAs). Results show superior removal of PBUTs when FFAs were infused. The removal of CMPF increased from 0 to 14%; pCS increased from 8 to 28%; IS increased from 12 to 35% and IAA increased from 15 to 40% [
58]. In the first in vivo study, a rat model (with ESRD) was used, and the commercial ILE Intralipid
TM was infused into the bloodstream of rats at a concentration of 3 mL/kg (per animal weight). A control group composed of rats with ESRD was infused with PBS. Blood samples were collected before and after the administration of ILE, and the binding profile between PBUTs and HSA was registered over time. Results show that after 30 min of administration of Intralipid
TM, the concentrations of the free forms of CMPF, pCS, IS and IAA present in the rat’s blood had significantly increased, reaching the highest value after 60 min of administration. However, after 210 min of infusion, the concentrations of the free forms were lower than the initial values. The authors concluded that FFA has the potential to displace PBUTs from HSA, but that the effect is reversible after a short period [
58]. The second in vivo study was performed for 3 h using the same polysulfone-based hemodialyzer, the blood and dialysate flow rates were 1 and 5 mL/min, respectively (counter-current mode, without recirculation), and the TMP was adjusted to 0 mmHg (no forced convection) [
58]. After 10 min of HD, Intralipid
TM (3 mL/kg of animal weight) was infused continuously until the end of the HD session while PBS was infused into the control group (rats with ESRD). The reduction ratio of pCS, IS and IAA was 3.3-, 2.1- and 1.7 times higher for the rats infused with Intralipid
TM than for the control group. The authors concluded that infusion of lipid solutions can lead to superior removal of PBUTs during HD sessions but advise that further studies are required to understand the potential side effects of long-term treatment with Intralipid
TM [
58].
2.2. Competitive Binding Membranes
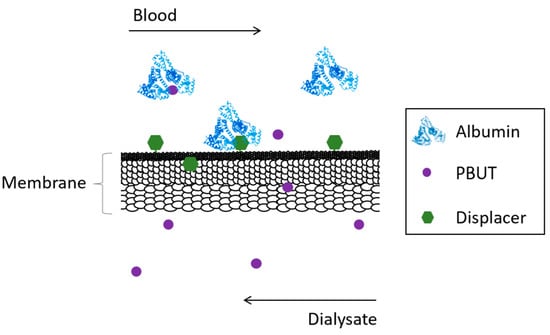
Competitive membranes are membranes which combine the characteristic filtration properties of the pure polymer membrane coupled with the ability to displace albumin from bound toxins by competitive binding. The competitive binding membranes are described as monophasic hybrid cellulose acetate/silica-based (CA/SiO2-based) membranes where the displacer is covalently bound to the polymer matrix of the membrane reducing drastically the risk of having it be leached into the blood as was seen for the adsorptive MMMs. Figure 2 schematically shows the concept behind competitive binding membranes.
Figure 2. Schematical representation of the removal of PBUTs by competitive binding membranes.
Mendes et al. [
59] were the first to develop CA/SiO
2 monophasic hybrid membranes (MHMs) characterized by the covalent bonding between the inorganic (silica) and organic (cellulose acetate) elements, by an innovative method which couples phase inversion and sol-gel techniques. In contrast to the adsorptive MMMs described in
Section 2, where the physical incorporation of inorganic particles into the polymer matrix renders membranes with two distinct phases (clusters of particles dispersed in a continuous polymer matrix) [
37,
38,
39,
40,
41,
42,
43], the MHMs exhibit only one phase where silica and CA are covalently bonded eliminating the risk of particles leaching from the membranes and consequent release into the bloodstream of the patients.
Membranes containing up to 40 mol% SiO
2 covalently bonded to CA were fabricated by reacting the silica precursor tetraethyl orthosilicate (TEOS) with CA under acidic conditions [
59]. Six integral asymmetric membranes containing 0, 10, 15, 20, 30, and 40 mol% were prepared and the covalent bonding between SiO
2 and CA was confirmed by Fourier transform infrared spectroscopy in attenuated total reflection mode (ATR-FTIR) with identification of the peak corresponding to the Si-O-C bond. The permeation performance of the MHMs was evaluated in terms of Lp and MWCO and results showed that the incorporation of 15 mol% silica increased the Lp to 59 kg/h/m
2/bar, a value much higher than was found for the pristine CA membrane, 32 kg/h/m
2/bar. Furthermore, the MWCO also increased from 54 kDa to 111 kDa for the pristine CA and 15 mol% silica membranes, respectively. For higher contents of SiO
2, the Lp value decreased to lower values than was seen for the pure CA membrane [
59].
Faria et al. [
60], performed further studies on CA/SiO
2 membranes containing 0, 5, 11, and 18 wt.% of SiO
2 in terms of hemocompatibility and permeation performance. Hemocompatibility assays revealed that all the membranes were non-hemolytic and that the CA/SiO
2 membranes presented lower thrombosis degrees and levels of platelet adhesion and activation when compared to the pristine CA membrane, indicating that the incorporation of silica enhances hemocompatibility making MHMs good candidates for blood-contacting applications. In terms of permeation performance, the average Lp of the membranes containing silica was 49 L/h/m
2/bar which is higher than pristine CA membrane by a factor of 2. Furthermore, all monophasic hybrid CA/SiO
2 membranes fully permeated urea (a surrogate marker of free water-soluble LMWMs) and retained ~100% albumin [
60].
Considering the chemical versatility and high reactivity of primary amines (-NH
2), Andrade et. al. [
61] developed a new group of MHMs, named CA/SiO
2/SiO
1.5-(CH
2)
3NH
2 membranes, by incorporation of a second silica precursor, (3-aminopropyl)-triethoxysilane (APTES), into the CA/SiO
2-based membranes [
61]. This functionalization can have the potential for chemical, pharmaceutical, and biomedical applications [
61]. MHM with a fixed content of 5 wt.% SiO
2 (derived from TEOS) and contents of SiO
1.5-(CH
2)
3NH
2 (derived from APTES) varying between 0 and 50 mol% were prepared and characterized in terms of permeation performance [
61]. The incorporation of 10 mol% SiO
1.5-(CH
2)
3NH
2 increased the Lp values from 23 to 69 kg/h/m
2/bar and for membranes with the content of SiO
1.5-(CH
2)
3NH
2 higher than 20 mol% the Lp values were lower than the Lp of the pure CA membrane [
61].
Janeca et al. [
62], studied the permeation performance of the CA/SiO
2/SiO
1.5-(CH
2)
3NH
2 membrane containing 5 wt.% SiO
2 and a molar ratio of SiO
2/SiO
1.5-(CH
2)
3NH
2 of 80:20 under dynamic conditions in a lab-scale hemodialysis circuit. For comparison purposes, a pure CA membrane was characterized in the same set-up under the same conditions. Results showed that the incorporation of SiO
2 and SiO
1.5-(CH
2)
3NH
2 increased the Lp from 28 (pure CA membrane) to 51 kg/h/m
2/bar and the MWCO from 18 (pure CA membrane) to 25 kDa. Additionally, the CA/SiO
2/SiO
1.5-(CH
2)
3NH
2 membrane fully permeated urea, creatinine, and uric acid (LMWMs) and retained 100% albumin [
62].
The possibility to covalently bond two different silica precursors, TEOS and APTES, to the CA matrix membrane presents an advantage as it eliminates the risk of leaching the inorganic materials into the bloodstream of the patients [
59,
60,
61,
62]. Moreover, it introduces a novel and highly efficient method of covalently incorporating a wide range of different compounds into CA membranes. Silica precursors can be functionalized with other molecules, such as polyureas and azo dyes [
63,
64,
65] prior to being reacted with CA by the sol-gel reaction. Very recent studies show the successful modification of TEOS and APTES with IBF a well-known HSA displacer [
66] and it is envisioned that both precursors can be functionalized with other drugs such as FUR and TRP. This opens the door for a huge number of opportunities namely the fabrication of new competitive binding membranes where the albumin displacer is covalently bonded to the polymer matrix of the membrane. Lopes et al. [
66], recently reported the synthesis of two new silica precursors, TEOS-IBF and APTES-IBF as well as the fabrication of integral asymmetric CA/SiO
2/IBF MHMs containing up to 15 wt.% of IBF and in which the silica precursors are covalently bonded to the CA polymer [
66]. It is expected that when the blood of uremic patients reaches the hemodialyzer and encounters the membranes, the displacers will compete with PBUTs for the same HSA binding site resulting in an increase in free PBUTs, which are easily dialyzed by the CA/SiO
2/IBF membranes. If this approach has the success that the authors expect, competitive membranes may be more advantageous than the infusion of large doses of drugs into the bloodstream and will present much fewer risks to ESRD patients.