This review highlights the advantages to use nanosized structures for medical applications in the prevention and treatments of HIV infection. We briefly evaluated the most recent developments associated with different polymeric nanosystems.
- nanosystems, prevention HIV , treatments HIV
Dear author, the following contents are excerpts from your papers. They are editable.
(The entry will be online immediately after you submit it. The entry will better spread your research and findings and readers are able to access your paper directly through DOI number at the end of the entry. No worry about the format, we'll correct it after your submission)
1. Introduction
Sexually-transmitted infections (STIs) are a global health concern worldwide as they cause acute diseases, infertility, and significant mortality. Among the bacterial, viral, and parasitic pathogens that can be sexually transmitted, human immunodeficiency virus (HIV) has caused one of the most important pandemic diseases, which is acquired immune deficiency syndrome (AIDS). 32.7 million people have died from AIDS-related illnesses since the start of the epidemic. Moreover, in 2019, 38 million people were living with HIV worldwide. The need to deal with this viral infection becomes more obvious, because it represents not only a problem for public health, but also a substantial economic problem. In this context, it is necessary to focus efforts on developing methods for prevention, detection and treatment of HIV infections that significantly reduce the number of newly infected people and provide a better quality of life for patients. For several decades, biomedical research has been developed allowing quick solutions through the contribution of effective tools. One of them is the use of polymers as vehicles, drug carrier agents, or as macromolecular prodrugs. Moreover, nanosystems (NSs) play an especially important role in the diagnosis, prevention, and therapy against HIV infection.
2. Nanotechnology Approaches for Prevention of HIV Infection
Preventive strategies are the most effective actions to fight global infections. Vaccines have been successful at controlling other major infectious diseases such as measles, mumps, rubella, and polio, with smallpox completely eradicated [40]. In the absence of a preventive vaccine for HIV/AIDS, enormous efforts are made by researchers worldwide to achieve an effective formulation that passes the clinical trials.
Since sexual transmission is the major route of infection of HIV, another preventive strategy is the development of effective topical pre-exposure prophylaxis such as microbicides, which can be defined as medical products intended to be administered into the vagina and/or rectum in order to avoid early steps of viral transmission upon sexual intercourse [41]. The principle for dealing with new HIV infections relies on the inhibition of the virus at the mucosal level by one or several compounds, which have specific antiviral activity [41]. Despite the progress made in microbicide technology, the world is still awaiting approval of the first microbicide product, indicating the need for more research and development to design better systems.
2.1. Vaccines
As stated by Prof. Burton of the Scripps Research Institute in La Jolla, California, in the US, “of any pathogen, HIV provides perhaps the greatest challenge to successful vaccine development” [42]. An effective vaccine will contribute to elimination of HIV infection worldwide, however, candidate vaccines evaluated to date have failed to demonstrate efficacy [43]. A vaccine is an invention that certainly involves an empirical trial-and-error step that inherently differs from a rational design approach; in the case of HIV vaccines, the so-called rational design approach failed because the immunogenicity and antigenicity were confused [44]. The major barriers for HIV vaccine development are the failure to produce adequate vaccine immunogens and the inability of conventional delivery approaches to produce the needed immune response [45]. Therefore, significant efforts are still made to generate an efficacious vaccine for the prevention of HIV infection. In this context, NSs have exhibited outstanding properties as carriers for the improvement of solubility and pharmacokinetics of vaccine agents such as nucleic acids and therapeutic proteins [46]
A promising approach for developing HIV vaccines is the use of NPs as delivery agents for HIV antigenic peptides. As an example, nanovaccine formulation for HIV prevention was prepared by using chitosan/dextran sulfate NPs with the peptide antigen entrapped by ionic interactions [47]. Dacoba et al. engineered different polysaccharide NPs loaded with an HIV peptide antigen candidate, which is a sequence around the protease cleavage site 5 (PCS5) [48,49]. To form the NPs, PCS5 was first conjugated to two different polysaccharides (chitosan and hyaluronic acid) through either a stable or a cleavable bond and then, associated with an oppositely charged polymer (dextran sulfate and chitosan) and an immunomodulatory molecule, polyinosinic: polycytidylic acid (poly(I:C)). The results showed that different factors such as the attachment of the antigen (ionic interactions, and cleavable or noncleavable conjugations), the presence of immunomodulatory molecules such as poly(I:C), or the nature of the polysaccharides could importantly influence the type of elicited immune response. Regarding the delivery agents for HIV antigenic peptides, Martín-Moreno et al. used cationic nanocompounds, G4-70/30 dendrimer and the β-cyclodextrin derivative AMC6 to introduce HIV-1 peptides into human dendritic cells (DCs) [50]. Afterwards, the authors studied their maturation that makes them HIV-1-specific antigen-presenting cells to generate a T cell response when introduced back to the patient. Recently, HIV envelope glycoprotein (Env) was incorporated into different lipid assemblies. Micelles and nanodiscs with various lipid compositions were used. The authors used this methodology for studying Env in membranous environments, but it can also be adapted for vaccine engineering [51].
An immune-active nanovaccine delivery system to target DCs was designed using inulin acetate, which is a novel immune-active polymeric material (InAc-NPs) that targeted the TLR4 signaling on DCs for their activation and maturation [52]. Safety of this nanovaccine was demonstrated by their quick clearance from the injection site and the absence of skin toxicity. Additionally, in vivo InAc-NPs generated efficient humoral responses, demonstrating great potential in cancer immunotherapy and against various infectious diseases such as HIV.
An hybrid delivery system based on polyethylene glycol-graft-polyethylenimine (PEG-g-PEI)/DNA polyplexes formulated into poly(lactic-co-glycolic acid) (PLGA) microspheres was evaluated for DNA vaccine delivery [53]. Intramuscular injection of this DNA vaccine delivery system induces immune responses at a low dose of DNA in big animals such as guinea pigs and rhesus macaques. Therefore, this technology holds promise for use in human beings.
Table 1. Nanotechnology approaches for HIV vaccines.
| Prevention Approach | Goal | Nanoformulation | References |
|---|---|---|---|
| Vaccines | Transport of HIV antigens to targeted immune cells | Chitosan/dextran sulfate NPs with HIV antigenic peptides | [48,49] |
| G4-70/30 dendrimer and the β-cyclodextrin derivative AMC6 for peptide delivery | [50] | ||
| Complex based on fourth- generation poly(amidoamine) dendrimers (G4-PAMAM) and peptide epitopes | [54] | ||
| PLGA NPs with HIV antigenic peptide conjugated to an adjuvant |
[55] | ||
| Inulin acetate NPs with encapsulated antigen (ovalbumin) | [52] | ||
| DNA vaccine delivery | (PEG-g-PEI)/DNA polyplexes formulated into PLGA microspheres | [53] |
2.2. Topical Microbicides
Microbicides are defined as topical prophylactic agents in the form of gels, creams, foams, impregnated sponges, suppositories, intravaginal rings, or films for self-administration into the vagina or rectum before intercourse to protect against HIV and other sexually-transmitted pathogens such as genital herpes, gonorrhea, and chlamydia [56]. In a general way, the chemical/physical action of these formulations protects the uninfected person, male or female, from infectious agents that might be present in the genital secretions of his/her sexual partner. One of the ways that sexual transmission of the virus can occur is through cervicovaginal or colorectal mucous membranes of receptive individuals upon contact with semen containing HIV.
Mechanisms of sexual transmission of cell-free HIV through the cervicovaginal route is schematized in Figure 2 [57,58]. After ejaculation, HIV present in semen or (a) produced by HIV-infected leukocytes from a donor requires first crossing the mucosal fluids. Then, viral particles can overcome the epithelial barrier by: (b) direct access to the lamina propria across gaps in the epithelium, (c) capture and transepithelial transport of virions by Langerhans cells, (d) partial penetration of the epithelium and infection of intraepithelial CD4+ T cells (or other leukocytes) that then migrate to the lamina propria, or (e) epithelial crossing through intercellular spaces or by transcytosis. Once in the lamina propria, (f) HIV particles can productively infect target cells such as macrophages, CD4+ T cells, or DCs. (g) DCs can also mediate trans-infection of other target cells, namely CD4+ T cells. Following initial infection of target cells, (h) local viral amplification occurs mainly in CD4+ T cells prior to migration of (i) free virus and/or (j) infected cells to regional lymph nodes. (k) HIV transfer to lymph nodes may also be mediated by non-productively infected DCs trans.
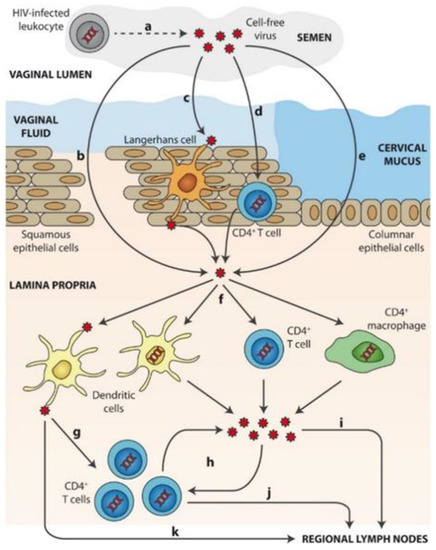
Figure 2. Mechanisms of sexual transmission of cell-free HIV through the cervicovaginal route. Reprinted with permission from [58].
“Microbicide” is the most used term in literature even though there are some topical prevention strategies that do not kill microorganisms but do prevent their transmission. In this text, we refer to all these strategies as microbicides.
There are different topical prevention strategies currently under development that consist of agents that inactivate HIV directly: detergents and agents that modify pH, those that target viral replication or viral entry, and those which target host-cell structures [59].
First, microbicides must display preventive activity against HIV infection for several hours over a broad pH range, though this is not the only requirement that they should satisfy. In addition, they should guarantee successful application, distribution, and retention of the agent where it is needed. Therefore, viscosity and other physical characteristics should be optimized to ensure the most favorable antiviral activity, good coverage of the mucosa surface, sufficient tissue penetration if necessary, and a product that is as undetectable as possible [59]. Longer-acting tolerated agents are attractive because they might allow less-frequent administration [59]. Furthermore, microbicides have to demonstrate long-term safety without causing adverse effects, damage to mucosal integrity, inflammation or immunogenicity, or disturbing the normal vaginal flora.
In illustrating the most recent and relevant examples of research works involving NSs for the development of microbicides, it is important to mention the contribution of dendrimers. Considering their impact, VivaGel® was the first dendrimer-based drug proposed as a new drug. VivaGel® has potent virucidal activity against HIV-1 but toxicity studies in tissue explants, in vitro, animal, and non-human primate models show the relative safety of this product. Consequently, the development of VivaGel® as a microbicide has been discontinued following safety issues in clinical trials [41]. Meanwhile, other candidates have been intensively investigated.
Extensive research has shown that polyanionic carbosilane dendrimers possess considerable anti-HIV-1 and anti-HIV-2 activity, namely due to their ability to bind to gp120 and CD4 and interfere with their interaction. Among different carbosilane dendrimers that have been studied, G2-S16 has emerged as one of the most promising candidates. In addition to its unquestionable microbicidal effect, it has demonstrated in vivo biocompatibility. Recent studies showed that G2-S16 dendrimer does not cause irritation or inflammation in the vaginal epithelium and does not alter the natural immunity of the vagina, which strongly supports the biosafety of this dendrimer for vaginal application to control viral transmission [60,61,62].
Moreover, polyanionic carbosilane dendrimers present synergistic effects when associated with antiretroviral (ARV) drugs. Regarding this, Sepúlveda–Crespo et al. studied the triple combination of anionic carbosilane dendrimers (G2-STE16, G2-S24P, and G2-S16) with tenofovir, maraviroc, or both against HIV-1 infection [63]. Combinations showed a greater broad-spectrum anti-HIV-1 activity than the single-drug, preserved this activity in acid environment or seminal fluid, and demonstrated strong synergistic interactions at high inhibitory concentrations. Finally, the treatment of vaginal epithelium in vivo (female BALB/c mice) showed no irritation. This result is a consequence of combining lower doses of different compounds that act synergistically, which leads to minimize systemic exposure and toxic side effects.
The development of prophylactic strategies with dual microbicide and contraceptive activity is also interesting. Platycodin D (PD), a promising contraceptive, was combined with G1-S2 or G2-S16 dendrimers to develop a prophylactic strategy with dual activity [64]. The results show that PD does not affect the antiviral activity of the dendrimers and they do not affect the spermicide activity of PD. The spermicide effect over human semen was achieved in less than 30 s.
Recently, anionic poly(alkylideneamine) dendrimers with carboxylate or sulfonate terminal groups were tested as microbicide agents against HIV-1 infection [65]. Dendrimers with eight carboxylate or sulfonate terminal groups (G1C and G1S dendrimers) showed important antiviral activity against infection both at acidic and basic pH values and, long term chemical stability in solid state and aqueous solution. In vivo assays using BALB/c mice revealed that G1C and G1S dendrimers did not cause noticeable irritation or inflammation in the vaginal epithelium.
Unlike dendrimers, there is little published data on nanogels as microbicidal agents. As example, poly(N-vinylcaprolactam) NGs demonstrated an inhibitory effect against HIV-1 infection by themselves [66].
Sánchez–López et al. prepared suitable delivery nanocarriers for releasing HIV-1 fusion inhibitor peptide in vaginal mucosa: polymeric NPs of PLGA and lipid large unilamellar vesicles loaded with the inhibitor peptide [67]. The authors comparatively studied both systems and found high entrapment efficiency of the inhibitor peptide in lipid vesicles, which was understood because of the hydrophobic nature of the peptide. PLGA NPs demonstrated an in vitro drug release similar to the free peptide whereas lipid vesicles demonstrated favorably prolonged release. Besides, none of the NSs were able to permeate across the vaginal tissue, thus probably avoiding adverse systemic effects in vivo. Lipid vesicles can deliver a sustained inhibitor peptide concentration in the vaginal tissue enhancing peptide penetration. Based on the results, lipid vesicles are a suitable formulation as a microbicide against HIV infection.
Bictegravir (BIC), a newly FDA-approved integrase strand transfer inhibitor, has proven efficacious in treating HIV-1. Recently, Mandal et al. investigated its prophylaxis effect [68]. PLGA-loaded BIC NPs demonstrated BIC therapeutic selectivity, intra-cellular delivery, retention, and sustained drug-release potency; improved BIC cytotoxicity; and enhanced HIV-1 protection compared to BIC in solution. Finally, polymeric protection increases the tolerability index of BIC compared to BIC solution. Furthermore, PLGA NPs loaded with two potent ARVs, griffithsin (GRFT) and dapivirine (DPV), were also evaluated as a potential long-acting microbicide product [69]. These drugs have different targets: the fusion and reverse transcription steps of HIV replication. Both were successfully encapsulated, GRFT (45% of initially added) and DPV (70%), and showed a biphasic release with initial burst phase followed by a sustained release phase. The combination of drugs in both unformulated and encapsulated in NPs showed strong synergistic drug activity. These findings showed that the co-delivery of GRFT and DPV promises to behave as a highly potent microbicide.
Table 2. Nanotechnology approaches for HIV microbicides.
| Type of NSs | NSs or Nanoformulation | Mechanism/Description | References |
| Carbosilane dendrimers | Anionic carbosilane dendrimers: G2-S16 and others |
Anti-HIV-1 and anti-HIV-2 activity due to their ability to bind to gp120 and CD4 and interfere with their interaction. | [60,61,62] [70,71] |
| associated with nanoparticles, fatty acids | [72,73,74] | ||
| associated with antiretroviral drugs, contraceptives | [63,64] | ||
| Anionic poly(alkylideneamine) dendrimers with carboxylate or sulfonate terminal groups | G1C and G1S dendrimers | Antiviral activity against infection at acidic and basic pH values, long term chemical stability. | [65] |
| Polymeric systems | Poly(N-vinylcaprolactam) NGs | Inhibitory effect against HIV-1 infection by themselves. | [66] |
| PLGA NPS and lipid large unilamellar vesicles loaded with the inhibitor peptide | Release of HIV-1 fusion inhibitor peptide in vaginal mucosa. | [67] | |
| PLGA-loaded Bictegravir NPs | Bictegravir, an integrase strand transfer inhibitor, tested for prophylaxis. | [68] | |
| PLGA NPs loaded with antiretrovirals: griffithsin and dapivirine | The combination of drugs showed strong synergistic drug activity. | [69] | |
| PCL fibers surrounding PEO fibers that incorporated mPEG-PLGA NPs loaded with griffithsin | Sustained release of griffithsin during 90-day period were achieved. | [75] | |
| PLGA NPs as carriers for efavirenz | For intrarectal administration. | [76] |
Recent work has studied a multilayered nanoparticle-electrospun fiber (NP-EF) composite for sustained-release of GRFT [75]. pH-responsive and surface-modified GRFT fibers provided in vitro long-term dual protection against herpes simplex virus type 2 (HSV-2) and HIV-1 infections. Composites were fabricated from polycaprolactone (PCL) fibers surrounding polyethylene oxide (PEO) fibers that incorporated methoxy poly(ethylene glycol)-b-poly(lactide-co-glycolide) (mPEG-PLGA) GRFT NPs. High loading of GRFT NPs and sustained release of GRFT during a 90-day period were achieved. Both NPs and NP-EF composites inhibited HIV-1 infection in vitro, and moreover, these vehicles demonstrated protection against a lethal dose of HSV-2 infection in a murine model. The data indicate the preliminary safety and biocompatibility of these delivery platforms.
In contrast to formulations for vagina, there is much less information about rectal anti-HIV microbicides. José das Neves and col. reported experimental work about the in vitro and in vivo performance of PLGA-based NPs as carriers for the model drug efavirenz (EFV) for intrarectal administration [76]. In particular, the effect of non-covalent PEG coating of PLGA NPs (PEG-PLGA NPs) on the pharmacokinetics of EFV following rectal administration to mice was assessed. Both drug-loaded PLGA-NPs and PEG-PLGA NPs improved the colorectal availability of EFV after rectal administration as compared to free drug. Nevertheless, prolonged drug residence at the lower colon was observed at higher concentrations when PEG modification was used. Thereby, this work gives evidence of the usefulness of mucus-diffusive nanocarriers in engineering effective and safe rectal microbicides.
Reference
- Mesquita, L.; Galante, J.; Nunes, R.; Sarmento, B.; Neves, J.D. Pharmaceutical vehicles for vaginal and rectal administration of anti-hivmicrobicide nanosystems. Pharmaceutics 2019, 11, 145.
- Burton, D.R. Advancing an HIV vaccine; advancing vaccinology. Nat. Rev. Immunol. 2019, 19, 77–78.
- Cao, S.; Woodrow, K.A. Nanotechnology approaches to eradicating HIV reservoirs. Eur. J. Pharm. Biopharm. 2019, 138, 48–63.
- Van Regenmortel, M.H. Structure-based reverse vaccinology failed in the case of HIV because it disregarded accepted immunological theory. Int. J. Mol. Sci. 2016, 17, 1591.
- Gao, Y.; Wijewardhana, C.; Mann, J.F.S. Virus-like particle, liposome, and polymeric particle-based vaccines against HIV-1. Front. Immunol. 2018, 9, 1–18.
- Grande, F.; Ioele, G.; Occhiuzzi, M.A.; De Luca, M.; Mazzota, E.; Ragno, G.; Garofalo, A.; Mazzalupo, R. Reverse Transcriptase Inhibitors Nanosystems Designed for Drug Stability and Controlled Delivery. Pharmaceutics 2019, 11, 197.
- Klein, M.; Menta, M.; Dacoba, T.G.; Crecente-Campo, J.; Alonso, M.J.; Dupin, D.; Loinaz, I.; Grassl, B.; Séby, F. Advanced nanomedicine characterization by DLS and AF4-UV-MALS: Application to a HIV nanovaccine. J. Pharm. Biomed. Anal. 2020, 179, 113017–113038.
- Dacoba, T.G.; Omange, R.W.; Li, H.; Crecente-Campo, J.; Luo, M.; Alonso, M.J. Polysaccharide Nanoparticles Can Efficiently Modulate the Immune Response against an HIV Peptide Antigen. ACS Nano 2019, 13, 4947–4959.
- Dacoba, T.G.; Ruiz-Gatón, L.; Benito, A.; Klein, M.; Dupin, D.; Luo, M.; Menta, M.; Teijeiro-Osorio, D.; Loinaz, I.; Alonso, M.J.; et al. Technological challenges in the preclinical development of an HIV nanovaccine candidate. Drug Deliv. Transl. Res. 2020, 10, 621–634.
- Martín-Moreno, A.; Jiménez Blanco, J.L.; Mosher, J.; Swanson, D.R.; García Fernández, J.M.; Sharma, A.; Ceña, V.; Muñoz-Fernández, M.A. Nanoparticle-delivered HIV peptides to dendritic cells a promising approach to generate a therapeutic vaccine. Pharmaceutics 2020, 12, 656.
- Rantalainen, K.; Berndsen, Z.T.; Antanasijevic, A.; Schiffner, T.; Zhang, X.; Lee, W.; Torres, J.L.; Zhang, L.; Irimia, A.; Copps, J.; et al. HIV-1 Envelope and MPER Antibody Structures in Lipid Assemblies. Cell Rep. 2020, 31, 107583.
- Rajput, M.K.S.; Kesharwani, S.S.; Kumar, S.; Muley, P.; Narisetty, S.; Tummala, H. Dendritic Cell-Targeted Nanovaccine Delivery System Prepared with an Immune-Active Polymer. ACS Appl. Mater. Interfaces 2018, 10, 27589–27602.
- Lu, Y.; Wu, F.; Duan, W.; Mu, X.; Fang, S.; Lu, N.; Zhou, X.; Kong, W. Engineering a ‘PEG-g-PEI/DNA nanoparticle-in- PLGA microsphere’ hybrid controlled release system to enhance immunogenicity of DNA vaccine. Mater. Sci. Eng. C 2020, 106, 110294.
- Rodríguez-Fonseca, R.A.; Bello, M.; Muñoz-Fernandez, M.A.; Jimenez, J.L.; Rojas-Hernandez, S.; Fragoso-Vázquez, M.J.; Gutierrez-Sanchez, M.; Rodrigues, J.; Cayetano-Castro, N.; Borja-Urby, R.; et al. In silico search, chemical characterization and immunogenic evaluation of amino-terminated G4-PAMAM-HIV peptide complexes using three-dimensional models of the HIV-1 gp120 protein. Colloids Surfaces B Biointerfaces 2019, 177, 77–93.
- Rostami, H.; Ebtekar, M.; Ardestani, M.S.; Yazdi, M.H.; Mahdavi, M. Co-utilization of a TLR5 agonist and nano-formulation of HIV-1 vaccine candidate leads to increased vaccine immunogenicity and decreased immunogenic dose: A preliminary study. Immunol. Lett. 2017, 187, 19–26.
- Stone, A. Microbicides: A new approach to preventing HIV and other sexually transmitted infections. Nat. Rev. Drug Discov. 2002, 1, 977–985.
- Shattock, R.J.; Moore, J.P. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 2003, 1, 25–34.
- Das Neves, J.; Nunes, R.; Rodrigues, F.; Sarmento, B. Nanomedicine in the development of anti-HIV microbicides. Adv. Drug Deliv. Rev. 2016, 103, 57–75.
- Lederman, M.M.; Offord, R.E.; Hartley, O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat. Rev. Immunol. 2006, 6, 371–382.
- Ceña-Diez, R.; García-Broncano, P.; de la Mata, F.J.; Gómez, R.; Resino, S.; Muñoz-Fernández, M.Á. G2-S16 dendrimer as a candidate for a microbicide to prevent HIV-1 infection in women. Nanoscale 2017, 9, 9732–9742.
- Martín-Moreno, A.; Sepúlveda-Crespo, D.; Serramía-Lobera, M.J.; Perisé-Barrios, A.J.; Muñoz-Fernández, M.A. G2-S16 dendrimer microbicide does not interfere with the vaginal immune system. J. Nanobiotechnol. 2019, 17, 1–14.
- Rodriguez-Izquierdo, I.; Gasco, S.; Muñoz-Fernández, M.A. High Preventive Effect of G2-S16 Anionic Carbosilane Dendrimer against Sexually Transmitted HSV-2 Infection. Molecules 2020, 25, 2965.
- Serramía, M.J.; Gómez, R.; la Mata, J.D.; Luis, J.; Ángeles, M. Triple combination of carbosilane dendrimers, tenofovir and maraviroc as potential microbicide to prevent HIV-1 sexual transmission. Nanomedicine 2015, 10, 899–914.
- Ceña-Diez, R.; Martin-Moreno, A.; De la Mata, F.J.; Gómez-Ramirez, R.; Muñoz, E.; Ardoy, M.; Muñoz-Fernandez, M.A. G1-S4 or G2-S16 carbosilane dendrimer in combination with Platycodin D as a promising vaginal microbicide candidate with contraceptive activity. Int. J. Nanomed. 2019, 14, 2371–2381.
- Maciel, D.; Guerrero-Beltrán, C.; Ceña-Diez, R.; Tomás, H.; Muñoz-Fernández, M.Á.; Rodrigues, J. New anionic poly(alkylideneamine) dendrimers as microbicide agents against HIV-1 infection. Nanoscale 2019, 11, 9679–9690.
- Macchione, M.A.; Guerrero-Beltrán, C.; Rosso, A.P.; Euti, E.M.; Martinelli, M.; Strumia, M.C.; Fernandez-Muñoz, M.A. Poly(N-vinylcaprolactam) Nanogels with Antiviral Behavior against HIV-1 Infection. Sci. Rep. 2019, 9, 5732.
- Sánchez-López, E.; Paús, A.; Pérez-Pomeda, I.; Calpena, A.; Haro, I.; Gómara, M.J. Lipid vesicles loaded with an HIV-1 fusion inhibitor peptide as a potential microbicide. Pharmaceutics 2020, 12, 502.
- Mandal, S.; Prathipati, P.K.; Belshan, M.; Destache, C.J. A potential long-acting bictegravir loaded nano-drug delivery system for HIV-1 infection: A proof-of-concept study. Antivir. Res. 2019, 167, 83–88.
- Yang, H.; Li, J.; Patel, S.K.; Palmer, K.E.; Devlin, B.; Rohan, L.C. Design of poly(Lactic-co-glycolic acid) (plga) nanoparticles for vaginal co-delivery of griffthsin and dapivirine and their synergistic effect for HIV prophylaxis. Pharmaceutics 2019, 11, 184.
- Vacas-Córdoba, E.; Maly, M.; de la mata, F.J.; Gómez, R.; Pion, M.; Muñoz-Fernández, M.Á. Antiviral mechanism of polyanionic carbosilane dendrimers against HIV-I. Int. J. Nanomed. 2016, 11, 1281–1294.
- Guerrero-Beltran, C.; Rodriguez-Izquierdo, I.; Serramia, M.J.; Araya-Duran, I.; Márquez-Miranda, V.; Gomez, R.; De la Mata, F.J.; Leal, M.; González-Nilo, F.; Muñoz-Fernandez, M.A. Anionic Carbosilane Dendrimers Destabilize the GP120-CD4 Complex Blocking HIV-1 Entry and Cell to Cell Fusion. Bioconjug. Chem. 2018, 29, 1584–1594.
- Peña-González, C.E.; Garcia-Broncano, P.; Ottaviani, M.F.; Cangiotti, M.; Fattori, A.; Hierro-Olvia, M.; González-Martín, M.L.; Perez-Serrano, J.; Gomez, R.; Muñoz-Fernandez, M.A.; et al. Dendronized Anionic Gold Nanoparticles: Synthesis, Characterization, and Antiviral Activity. Chem. A Eur. J. 2016, 22, 2987–2999.
- Peña-González, C.E.; Pedziwiatr-Werbicka, E.; Scharbin, D.; Guerrero-Beltran, C.; Abashkin, V.; Loznikova, S.; Jimenez, J.L.; Muñoz-Fernandez, M.A.; Bryszewska, M.; Gomez, R.; et al. Gold nanoparticles stabilized by cationic carbosilane dendrons: Synthesis and biological properties. Dalt. Trans. 2017, 46, 8736–8745.
- Guerrero-Beltrán, C.; Ceña-Diez, R.; Sepulveda-Crespo, D.; De la Mata, J.; Gomez, R.; Leal, M.; Muñoz-Fernandez, M.A.; Jimenez, J.L. Carbosilane Dendrons with Fatty Acids At the Core As a New Potential Microbicide Against Hsv-2/Hiv-1 Co-Infection. Nanoscale 2017, 9, 17263–17273.
- Tyo, K.M.; Lasnik, A.B.; Zhang, L.; Mahmoud, M.; Jenson, A.B.; Fuqua, J.L.; Palmer, K.E.; Steinbach-Rankins, J.M. Sustained-release Griffithsin nanoparticle-fiber composites against HIV-1 and HSV-2 infections. J. Control. Release 2020, 321, 84–99.
- Nunes, R.; Araujo, F.; Barreiros, L.; Bártolo, I.; Segundo, M.A.; Taveira, N.; Sarmento, B.; Das Neves, J. Noncovalent PEG Coating of Nanoparticle Drug Carriers Improves the Local Pharmacokinetics of Rectal Anti-HIV Microbicides. ACS Appl. Mater. Interfaces 2018, 10, 34942–34953.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21228647
