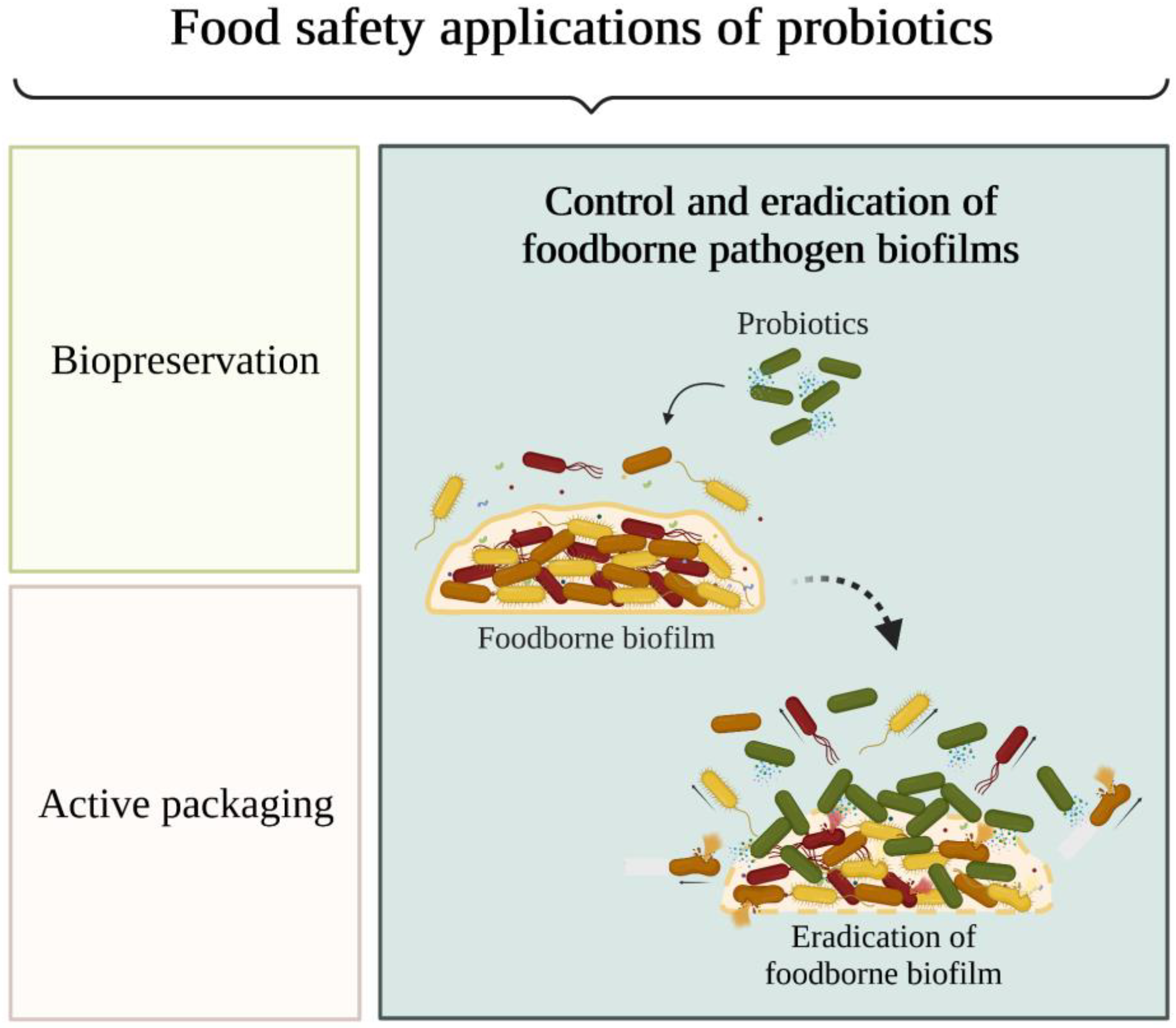
Microorganisms tend to adhere to food contact surfaces and form biofilms, which serve as reservoirs for bacteria that can contaminate food. As part of a biofilm, bacteria are protected from the stressful conditions found during food processing and become tolerant to antimicrobials, including traditional chemical sanitisers and disinfectants. Several studies in the food industry have shown that probiotics can prevent attachment and the consequent biofilm formation by spoilage and pathogenic microorganisms. It shows that the use of probiotics is a promising approach to disrupt biofilms formed by a large spectrum of foodborne microorganisms, with Lactiplantibacillus and Lacticaseibacillus being the most tested genera, both in the form of probiotic cells and as sources of cell-free supernatant.
- biofilm
- food industry
- probiotic
- anti-biofilm activity
1. Introduction
2. The Nature and Extent of Foodborne Diseases
3. The Role of Biofilms in Food Contamination
4. Biofilm Prevention and Control Strategies in the Food Industry
Probiotics can inhibit the growth of microorganisms and biofilm formation through displacement, exclusion, or competition [42]. Displacement consists of adding probiotics and/or their metabolites to disrupt already formed biofilms; exclusion consists of coating food contact surfaces with probiotic biofilms and/or their metabolites to prevent the adhesion of pathogenic microorganisms; competition involves the direct interaction of probiotics and/or their metabolites with foodborne microorganisms [43].
Regarding the displacement strategy, L. monocytogenes and S. aureus are the most studied biofilm-forming pathogens, while Lactiplantibacillus and Lacticaseibacillus are the most tested probiotic genera. Probiotic cells and cell-free supernatants are the most used agents to displace biofilms from abiotic surfaces. Among the antimicrobial substances tested, whole cells showed the most promising results in biofilm displacement, followed by bacteriocins.This entry is adapted from the peer-reviewed paper 10.3390/antibiotics12040754
References
- Tazehabadi, M.H.; Algburi, A.; Popov, I.V.; Ermakov, A.M.; Chistyakov, V.A.; Prazdnova, E.V.; Weeks, R.; Chikindas, M.L. Probiotic Bacilli Inhibit Salmonella Biofilm Formation Without Killing Planktonic Cells. Front. Microbiol. 2021, 12, 615328.
- Gutiérrez, S.; Martínez-Blanco, H.; Rodríguez-Aparicio, L.B.; Ferrero, M.A. Effect of fermented broth from lactic acid bacteria on pathogenic bacteria proliferation. J. Dairy Sci. 2016, 99, 2654–2665.
- Sornsenee, P.; Chatatikun, M.; Mitsuwan, W.; Kongpol, K.; Kooltheat, N.; Sohbenalee, S.; Pruksaphanrat, S.; Mudpan, A.; Romyasamit, C. Lyophilized cell-free supernatants of Lactobacillus isolates exhibited antibiofilm, antioxidant, and reduces nitric oxide activity in lipopolysaccharide-stimulated RAW 264.7 cells. PeerJ 2021, 9, e12586.
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174.
- Divyashree, S.; Anjali, P.G.; Somashekaraiah, R.; Sreenivasa, M.Y. Probiotic properties of Lactobacillus casei—MYSRD 108 and Lactobacillus plantarum—MYSRD 71 with potential antimicrobial activity against Salmonella paratyphi. Biotechnol. Rep. 2021, 32, e00672.
- Qualified Presumption of Safety (QPS): EFSA Journal. Available online: https://efsa.onlinelibrary.wiley.com/doi/toc/10.1002/(ISSN)1831-4732.QPS (accessed on 20 January 2023).
- EUR-Lex—Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32006R1924 (accessed on 20 January 2023).
- Global Overview for Probiotics: Trends, Markets, and Harmonization|RAPS. Available online: https://www.raps.org/news-and-articles/news-articles/2022/9/global-overview-for-probiotics-trends-markets-and?GA_network=x&GA_device=c&GA_campaign=18448087812&GA_adgroup=&GA_target=&GA_placement=&GA_creative=&GA_extension=&GA_keyword=&GA_loc_physical_ms=1011759&GA_landingpage=https\%3a\%2fwww.raps.org\%2fnews-and-articles\%2fnews-articles\%2f2022\%2f9\%2fglobal-overview-for-probiotics-trends-markets-and&gclid=Cj0KCQiAiJSeBhCCARIsAHnAzT-P6bWY8j7jWWPwV1asExzYsInprUJ6LfBJ3im7LfLWkMb7cPA_OQAaAm_UEALw_wcB (accessed on 20 January 2023).
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. 2020, 19, 3390–3415.
- Asaithambi, N.; Singh, S.K.; Singha, P. Current status of non-thermal processing of probiotic foods: A review. J. Food Eng. 2021, 303, 110567.
- The Burden of Foodborne Diseases in the WHO European Region. Available online: https://www.euro.who.int/__data/assets/pdf_file/0005/402989/50607-WHO-Food-Safety-publicationV4_Web.pdf (accessed on 27 October 2022).
- Hossain, M.I.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Toushik, S.H.; Ashrafudoulla, M.; Jahid, I.K.; Lee, J.; Ha, S.-D. Listeria monocytogenes biofilm inhibition on food contact surfaces by application of postbiotics from Lactobacillus curvatus B.67 and Lactobacillus plantarum M.2. Food Res. Int. 2021, 148, 110595.
- Qiao, Z.; Chen, J.; Zhou, Q.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lü, X. Purification, characterization, and mode of action of a novel bacteriocin BM173 from Lactobacillus crustorum MN047 and its effect on biofilm formation of Escherichia coli and Staphylococcus aureus. J. Dairy Sci. 2021, 104, 1474–1483.
- Jaffee, S.; Henson, S.; Unnevehr, L.; Grace, D.; Cassou, E. The Safe Food Imperative: Accelerating Progress in Low- and Middle-Income Countries; The World Bank: Washington, DC, USA, 2019.
- USDA ERS—Cost Estimates of Foodborne Illnesses. Available online: https://www.ers.usda.gov/data-products/cost-estimates-of-foodborne-illnesses.aspx (accessed on 27 October 2022).
- The European Union One Health 2020 Zoonoses Report. Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2021.6971 (accessed on 20 January 2023).
- Tan, X.; Han, Y.; Xiao, H.; Zhou, Z. Pediococcus acidilactici Inhibit Biofilm Formation of Food-Borne Pathogens on Abiotic Surfaces. Trans. Tianjin Univ. 2017, 23, 70–77.
- Sahoo, M.; Panigrahi, C.; Aradwad, P. Management strategies emphasizing advanced food processing approaches to mitigate food borne zoonotic pathogens in food system. Food Front. 2022, 3, 641–665.
- EUR-Lex—Council Regulation (EEC) No 315/93 of 8 February 1993 Laying down Community Procedures for Contaminants in Food. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A31993R0315 (accessed on 20 January 2023).
- Hadawey, A.; Savvas, A.T.; Chaer, I.; Sundararajan, R. Unwrapped food product display shelf life assessment. Energy Procedia 2017, 123, 62–69.
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New Weapons to Fight Old Enemies: Novel Strategies for the (Bio)control of Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 1641.
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014.
- Jara, J.; Pérez-Ramos, A.; del Soar, G.; Rodríguez, J.M.; Fernández, L.; Orgaz, B. Role of Lactobacillus biofilms in Listeria monocytogenes adhesion to glass surfaces. Int. J. Food Microbiol. 2020, 334, 108804.
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898.
- Dhivya, R.; Rajakrishnapriya, V.C.; Sruthi, K.; Chidanand, D.V.; Sunil, C.K.; Rawson, A. Biofilm combating in the food industry: Overview, non-thermal approaches, and mechanisms. J. Food Process. Preserv. 2022, 46, e16282.
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Bonaventura, G.D.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2016, 43, 313–351.
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as Complex Differentiated Communities. Annu. Rev. Microbiol. 2002, 56, 187–209.
- Petrova, O.E.; Sauer, K. Sticky Situations: Key Components That Control Bacterial Surface Attachment. J. Bacteriol. 2012, 194, 2413–2425.
- Toushik, S.H.; Kim, K.-S.; Ashrafudoulla, M.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Kim, Y.; Ha, S.D. Korean kimchi-derived lactic acid bacteria inhibit foodborne pathogenic biofilm growth on seafood and food processing surface materials. Food Control 2021, 129, 108276.
- Kim, N.-N.; Kim, W.J.; Kang, S.-S. Anti-biofilm effect of crude bacteriocin derived from Lactobacillus brevis DF01 on Escherichia coli and Salmonella Typhimurium. Food Control 2019, 98, 274–280.
- Cisneros, L.; Cattelan, N.; Villalba, M.I.; Rodriguez, C.; Serra, D.O.; Yantorno, O.; Fadda, S. Lactic acid bacteria biofilms and their ability to mitigate Escherichia coli O157:H7 surface colonization. Lett. Appl. Microbiol. 2021, 73, 247–256.
- Zhu, T.; Yang, C.; Bao, X.; Chen, F.; Guo, X. Strategies for controlling biofilm formation in food industry. Grain Oil Sci. Technol. 2022, 5, 179–186.
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575.
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmlle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620.
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies. Food Res. Int. 2019, 119, 530–540.
- Wang, N.; Yuan, L.; Sadiq, F.A.; He, G. Inhibitory effect of Lactobacillus plantarum metabolites against biofilm formation by Bacillus licheniformis isolated from milk powder products. Food Control 2019, 106, 106721.
- Kıran, F.; Akoğlu, A.; Çakır, İ. Control of Listeria monocytogenes biofilm on industrial surfaces by cell-free extracts of Lactobacillus plantarum. J. Food Process. Preserv. 2021, 45, e15042.
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; Abd El-Hack, E.M. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156.
- Dobson, A.; Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocin Production: A Probiotic Trait? Appl. Environ. Microbiol. 2012, 78, 1–6.
- Soltani, S.; Biron, E.; Said, L.B.; Subirade, M.; Fliss, I. Bacteriocin-Based Synergetic Consortia: A Promising Strategy to Enhance Antimicrobial Activity and Broaden the Spectrum of Inhibition. Microbiol. Spectr. 2022, 10, e00406–e00421.
- Salman, M.K.; Abuqwider, J.; Mauriello, G. Anti-Quorum Sensing Activity of Probiotics: The Mechanism and Role in Food and Gut Health. Microorganisms 2023, 11, 793.
- Carvalho, F.M.; Teixeira-Santos, R.; Mergulhão, F.J.M.; Gomes, L.C. Targeting biofilms in medical devices using probiotic cells: A systematic review. AIMS Mater. Sci. 2021, 8, 501–523.
- Carvalho, F.M.; Teixeira-Santos, R.; Mergulhão, F.J.M.; Gomes, L.C. The Use of Probiotics to Fight Biofilms in Medical Devices: A Systematic Review and Meta-Analysis. Microorganisms 2021, 9, 27.
