1. Introduction
Interactions of oxygen molecules with electrons leaked from enzymatic and non-enzymatic processes produces reactive oxygen species (ROS), such as superoxide (O
2−•) and hydrogen peroxides (H
2O
2) [
1,
2,
3]. Hydroxyl radicals (HO•) are the most reactive type of ROS and are likely produced by the reaction of H
2O
2 and ferrous iron, via the so called Fenton reaction [
4], although there is some debate as to which ROS are the primary products in this reaction [
5,
6]. Singlet molecular oxygen (
1O
2), is a high-energy oxygen species but possesses unique properties that are different from other ROS [
7,
8,
9]. While most ROS are produced by electron transfer reactions,
1O
2 is generated by the transfer of energy to the ground state, triplet molecular oxygen (
3O
2), the most abundant oxygen molecule in atmosphere. The type II photodynamic reaction promoted by the presence of photosensitizing molecules is widely employed to generate
1O
2 in biological systems [
10,
11]. However, the problem with the photodynamic action is that other ROS are also generated as byproducts [
12,
13]. This methodological issue appears to have hindered progress in research on the biological effects of
1O
2, despite its importance.
1O
2 possesses high energy and is considered to be a major cause for skin damage induced by ultraviolet (UV) irradiation [
14,
15]. Meanwhile, due to its strong cytotoxicity,
1O
2 is the molecule that is responsible for killing tumor cells during photodynamic therapy (PDT) [
10,
11].
1O
2 as well as HO• preferentially reacts with conjugated double bonds, and hence polyunsaturated fatty acids (PUFA), which are dominantly present in the form of phospholipids in the cell membrane, are likely targets [
9]. It is known that, upon mitotic stimuli, a small amount of ROS, notably H
2O
2, is produced, and this species modulates phosphorylation-mediated signaling pathways [
16,
17]. While signal modulation by H
2O
2 involves the transient oxidation of cysteine (Cys), reactions with
1O
2 tend to result in irreversible oxidation. In most cases, exposure to
1O
2 impairs cellular function but also occasionally stimulates tumorigenic cell growth [
18,
19]. Concerning cell death, results reported in many studies indicate that the apoptotic pathway is activated by
1O
2 [
20,
21,
22]. However, recent studies suggest that ferroptosis, an iron-dependent necrotic cell death [
23,
24], is also involved in
1O
2-promoted cell death [
25,
26].
2. Properties of 1O2 as a Potent Oxidant
Oxidative stress is induced by either the production of large amounts of ROS or an insufficient amount of antioxidants which include enzymes that eliminate ROS or small antioxidant compounds, such as glutathione (GSH), carotenoids and tocopherols [
2]. Electrons that are leaked from enzymatic and non-enzymatic reactions initiate the generation of ROS, as represented by O
2−•, H
2O
2 and HO•, and hence, the radical electron plays pivotal roles in the development of oxidative stress in many situations [
1,
3]. However,
1O
2 is generated when the oxygen molecule in the ground triplet state
3O
2 is excited by receiving energy without the transfer of an electron. The
1O
2-generating system involves enzymatic reactions, such as myeloperoxidase, lipoxygenase and cyclooxygenase as well as chemical reactions, such as O
2−•-mediated GSH oxidation and the interaction of peroxides with hypochlorite or peroxynitrite [
13,
28,
29,
30]. Photoaging and PDT are the subjects that have been most extensively investigated in terms of
1O
2–mediated reactions that are associated with human physiology and the pathogenesis of related diseases. In spite of the high oxidizing power, reactions of
1O
2 are thought to exert only limited effects compared to those of HO• in biological systems.
HO• is considered to be the most reactive ROS and appears to be responsible for a variety of pathological conditions. However, the half-life of HO• is quite short (10 nsec), so it only reacts with molecules that are in close proximity to the site where it is generated. On the other hand, the half-life of
1O
2 is approximately 4 µsec in aqueous solution, which allows it to diffuse 150–220 nm [
31,
32]. Thus,
1O
2 may react at various locations beyond where it is generated and, therefore, can affect surrounding molecules and organelles more widely compared to HO•. Nevertheless, this distance is insufficient for extracellularly produced
1O
2 to move to the interior of a cell. Therefore,
1O
2 that is generated inside the cell has the ability to damage various cellular components including DNA and organelles.
3. Chemical Probes for Detecting 1O2
Analyses employing a cell biological approach are essential for answering basic questions as to which part of the cell produces
1O
2 in photoaging and during PDT and how cellular responses proceed in such situations. For that purpose, the use of a fluorescent chemical probe is the most convenient approach.
1O
2 sensor green (SOSG) is a prototype that is popularly used in studies for detecting
1O
2, although it has some disadvantages such as lack in membrane permeability [
33]. Other compounds have been designed to overcome the disadvantage of SOSG. For example, Aarhus Sensor Green, which is a tetrafluoro-substituted fluorescein derivative that is covalently linked to a 9,10-diphenyl anthracene moiety [
34] and the classic indocyanine green probe may also be applicable for this objective in certain experiments [
35]. To increase the cellular delivery of SOSG, biocompatible nanosensors, with SOSG encapsulated within their hydrophobic core, have been developed, and these modifications appear to improve its delivery [
36,
37,
38].
The cell membrane permeable far-red fluorescence probe Si-DMA, which is composed of silicon-containing rhodamine and anthracene moieties as a chromophore, has also been developed [
39]. Upon reaction with
1O
2, Si-DMA is converted into an endoperoxide at the anthracene moiety that emits strongly. The use of Si-DMA reportedly enables the visualization of
1O
2 generated in a single mitochondrial tubule during PDT. After the treatment of cells with the endoperoxide, dose-dependent increases in fluorescence of Si-DMA were observed [
40]. Thus, these results suggest that chemical probes may be applicable for studies concerning the cellular effects of
1O
2. This compound is now commercially available. Another compound, a rhodamine 6G-aminomethylanthracene-linked donor–acceptor molecule (RA), was reported to exhibit unique properties [
41]. RA acts as a fluorogenic
1O
2 sensor molecule and also acts as a photosensitizer to generate
1O
2 upon exposure to green light. Other fluorescent reagents, such as one based on an aminocoumarin-methylanthracene-based electron donor–acceptor molecule [
42], are also being developed. Since information on the use of these newly developed probes is currently limited, it is necessary to carefully choose which compounds are suitable for the intended research.
4. Photodynamic Reaction as a 1O2 -Generating System
PDT mainly contributes to
1O
2 generation in biological systems through type II mechanisms that involve energy transfer from triplet excited molecules to triplet oxygen. Photosensitizers may also act according to competitive type I photosensitization mechanism that mostly involves charge transfer between suitable targets and a photosensitizer in its triplet excited state [
43,
44]. In order to protect cells against the deteriorating action of UV light, the effects of
1O
2 on skin tissues have been extensively investigated. In the meantime,
1O
2 is considered to be the main molecule that promotes cytotoxic processes during PDT, and hence, multiple studies are currently underway with the aim of understanding the mechanism responsible for
1O
2-mediated cell death and developing efficient photosensitizers [
8,
10]. UV radiation causes skin photoaging and oxidatively generated damage to dermal cells and is especially troublesome in cases of sunburn which occurs by exposure to excessive UV for long periods of time [
14,
45]. UVB (280–315 nm) comprises approximately 5% of the solar UV and causes the direct photodamage to many molecules including DNA and proteins in skin tissues through its high energy photochemical reactions. Genetic damage caused by the oxidative modification of DNA and other molecules emerges in a short time after exposure to UVB. In the case of UVA (315–400 nm) that accounts for approximately 95% of the solar UV, cellular damage occurs through the activation of chromophores that act as photosensitizers to generate
1O
2 and other ROS, and hence, the oxidative reaction proceeds indirectly via the ROS that are generated. It is rather difficult to determine if changes in cells that have been exposed to UVA are the consequence of the generation of either
1O
2 or other ROS because they are produced simultaneously by the photodynamic reactions and result in essentially the same end products [
46].
In order to elucidate the reactions caused by
1O
2, reliable methods for generating
1O are required [
12]. The most common method for this purpose is irradiation of the photosensitizer with UV or visible light because it is simple and easy to control its production [
27,
47].
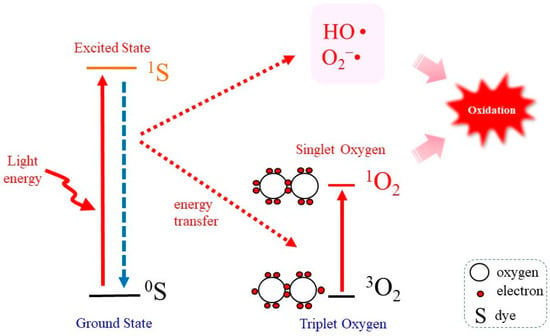
Figure 1 represents the “Jablonski diagram” that depicts conceptual images of the generation of
1O
2 by the irradiation of a photosensitizing molecule (S) with light followed by transferring energy to
3O
2 [
48]. When a photosensitizer is exposed to light, most likely the UVA in natural light, photon energy converts the photosensitizer in the ground state (
0S) to that in the excited state (
1S). On returning to the ground state
0S, a part of the released photoenergy can be transferred to
3O
2, which results in the electron spin state being altered and the generation of
1O
2. Under this situation, photodynamic action generates not only
1O
2 but also other ROS such as O
2−• and HO• [
8].
Figure 1. Photodynamic 1O2 generation. Photoirradiation of a photosensitizing molecule in the ground state (0S) leads the production of the excited form (1S). On returning to the ground state, energy is transferred to 3O2, which becomes excited to 1O2. In the meantime, however, other ROS such as O2−• and HO• may also be produced.
To observe cellular responses to
1O
2, cell-permeable and non-cytotoxic compounds need to be used as the photosensitizer. For example, Rose Bengal and methylene blue meet the conditions and, hence, are popularly used for the purpose of examining biological action of
1O
2 [
47]. Since PDT is a useful therapeutic for eliminating tumors, many attempts have been made to improve the treatment by developing convenient photosensitizers [
11,
27,
49].
5. Endoperoxides as Donor Compounds for Generating Pure 1O2
Endoperoxides that release
1O
2 without other ROS have been developed to evaluate its unique reaction [
13,
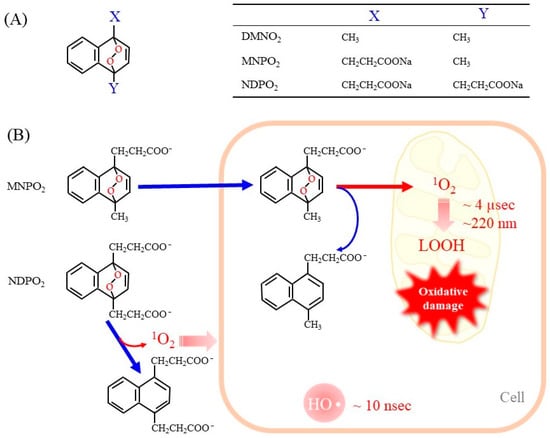
50]. Naphthalene endoperoxide-based
1O
2 donor compounds were first developed, and the structure–function relationships of the compounds have been described in detail in a review article [
13]. Consequently, several naphthalene endoperoxides have been established and utilized for the in vitro evaluation of the effects of
1O
2, as representative structures in
Figure 2A. Upon mild heating at 37 °C, the endoperoxides spontaneously release pure
1O
2, which then directly reacts with surrounding compounds and organelles. Heat-labile endoperoxides are considered clean sources of
1O
2 for highly specific oxidation of cellular biomolecules and have been applied for
1O
2-mediated oxidation of the DNA guanine base in cells [
51,
52].
Figure 2. Representative endoperoxides and the release of 1O2. (A) The general structure of naphthalene endoperoxides is shown on the left. The table on the right shows the chemical groups attached to the naphthalene ring. DMNO2, 1,4-dimethylnaphthalene endoperoxide; MNPO2, 1-methylnaphthalene-4-propanonate endoperoxide; NDPO2, 1,4-naphthalenedipropanoate endoperoxide. (B) Endoperoxides represented by MNPE and NDPE generate 1O2 spontaneously at physiologic temperature 37 °C. MNPE, which is relatively hydrophobic, can enter the cell, but NDPE cannot cross the cell membrane. The short life of 1O2 (~4 µsec) makes it diffuse only 150–220 nm in aqueous solution. As a result, 1O2 released from NDPE is present outside the cell only, while 1O2 from MNPE can act inside the cell. For reference, hydroxy radicals are also shown.
Here discuss the advantages and disadvantages of 1O2 donor compounds in comparison with the photodynamic action. The benefits include the following: (1) Endoperoxides produce pure 1O2. (2) The concentrations of the released 1O2 are easily controlled. (3) Heating at physiological temperature, generally under cell culture conditions, can promote the release of 1O2 from endoperoxides. (4) It may be possible to design endoperoxides that are localized to a specific organelle by appropriate chemical modification of the compounds. Limitations include the following: (1) A high concentration of endoperoxides is required to generate sufficient levels of 1O2. (2) It is essential to consider effects of the raw material after the release of 1O2 because they are sometimes toxic to cells. (3) Endoperoxides may not be evenly distributed inside cells due to their chemical nature. (4) The amount of 1O2 released is initially maximal but then gradually decreases with increasing endoperoxide consumption.
When endoperoxides are applied to a cell culture system, it is necessary to use compounds that are able to pass through the cell membrane. In fact, the side chains of naphthalene endoperoxides determine whether they enter cells or remain outside of cells (
Figure 2B) [
53]. 1-Methylnaphthalene-4-propanonate endoperoxide (MNPO
2) is cell membrane-permeable and generates
1O
2 within cells. However, 1,4-naphthalenedipropanoate endoperoxide (NDPO
2) cannot enter cells. Accordingly, while MNPO
2 induces cell damage, NDPO
2 at the same concentration has no effects, although both compounds trigger the release of cyt c from isolated mitochondria to a similar extent [
53].
While the generation of
1O
2 by a light-irradiated photosensitizer is frequently used, the use of
1O
2 donor compounds has been limited because they are complex molecules that are difficult to synthesize. Some naphthalene endoperoxides are now commercially available. New compounds other than naphthalene-based endoperoxides are also being developed. For the efficient delivery of a
1O
2 donor to cancer cells, a porphyrin-based covalent organic framework that contains a naphthalene endoperoxide has also been developed [
54]. Trials to develop new types of endoperoxides, which are based on 2-pyridone and anthracene, are also underway [
50]. Two
1O
2-producing systems, photodynamic reactions and naphthalene endoperoxides, have provided rather consistent results so far [
13], implying that the contribution of other byproducts may be negligible.
6. Natural or Synthetic 1O2-Scavenging Compounds
The body is protected from oxidatively generated damage by a variety of natural and synthetic compounds that scavenge
1O
2. The quantitative evaluation of the
1O
2-scavenging ability of a compound provides useful information not only for basic research but also for developing functional foods and medicines concerning antioxidation [
55]. Many nutritional compounds, such as tocopherols, carotenoids and flavonoids, possess antioxidant capacity and protect susceptible molecules from
1O
2. The oxygen radical absorption capacity (ORAC) assay is a representative method for the detection of
1O
2-scavenging ability of food ingredients [
56]. Thereafter, a simple method called a singlet oxygen absorption capacity (SOAC) assay has been established for the evaluation of
1O
2-scavenging ability [
56,
57]. These methods are useful in exploring popularly used
1O
2-scavenging compounds, especially in the field of food chemistry.
While carotenoids react with
1O
2 more rapidly than α-tocopherol, in a nearly diffusion-limited manner (~10
10 M
−1s
−1) [
58,
59], lycopene, which is found in fruits and vegetables such as tomato, is one of the strongest natural carotenoids [
60]. By employing the SOAC assay method, carotenoids have been found to quench
1O
2 approximately 30–100 times faster than α-tocopherol [
57]. After transferring excitation energy to carotenoids,
1O
2 returns to the ground state. The excited carotenoids spontaneously release thermal energy and then return to the ground state. Hence, carotenoids are spontaneously recycled and have the advantage of quenching
1O
2 without affecting other molecules. This chemical property of
1O
2 is a major difference from other radicals that are generated by electron transfer reactions that require another radical to quench.
Based on in vitro data on the action of anti-oxidants, the biological benefit of carotenoids has also been examined by some studies on humans. The administration of a representative carotenoid β-carotene to humans failed to alleviate sunburn reactions under the conditions used [
61]. However, a later study reported that carotenoids, β-carotene and lycopene effectively protect erythema formation induced using a solar light simulator [
62,
63]. Lycopene has also attracted attention as a nutrient with anticancer effects [
64]. For another example, lutein is a xanthophyll carotenoid that is found in foods such as dark green leafy vegetables and exhibits strong antioxidant activity via its ability to scavenge ROS including
1O
2 and lipid peroxyl radicals [
65]. Lutein appears to exert anti-inflammatory actions against some diseases, including neurodegenerative disorders, eye diseases, cardiovascular diseases and skin diseases.
Many synthetic antioxidant compounds that scavenge ROS including
1O
2 have been developed as medicines. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) is a compound that eliminates a variety of radical species and was the first approved compound for use as a medicine for the treatment of acute brain infarctions. Edaravone can scavenge
1O
2 that is generated by activated human neutrophils [
66] and by photoactivated Rose Bengal [
67]. The plasma lipid peroxidation caused by
1O
2, however, cannot be suppressed by edaravone and other clinical drugs with antioxidant ability, which include roglitazone, probucol, carvedilol, pentoxifylline and ebselen, although they exhibit suppressive effects on lipid peroxidation caused by free radicals, peroxynitrite, hypochlorite, and lipoxygenase reactions [
68]. Because blood plasma contains high concentrations of proteins and many other compounds that could potentially interfere with the scavenging reaction by these chemicals, such biological compounds may have influenced the results.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28104085