Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Infectious Diseases
The WHO declared coronavirus disease 2019 (COVID-19) a pandemic in March 2020, which was caused by novel coronavirus severe acute respiratory coronavirus 2 (SARS-CoV-2). SARS-CoV-2 made its first entry into the world in November 2019, and the first case was detected in Wuhan, China. Mutations in the SARS-CoV-2 genome distressed life in almost every discipline by the extended production of novel viral variants.
- COVID-19
- SARS-CoV-2
- vaccines
1. Genome Structure and Characteristic of COVID-19
The causative agent of the current global pandemic also known as coronavirus disease (COVID-19) is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was previously known as novel coronavirus (2019-nCoV). As shown in Figure 1, SARS-CoV-2 is an enveloped ribonucleic acid (RNA) virus packaged by nucleocapsid phosphoproteins that is diversely found in humans and wildlife. Coronavirus is known as one of the largest RNA viruses (26–32 kb) [1]. The genome of SARS-CoV consists of 14 open reading frames (ORFs) which encodes 27 structural and non-structural proteins. The primary structural proteins of coronavirus are membrane (M), spike (S) and nucleocapsid (N) proteins [2]. A total of seven species have been identified as moderately pathogenic to humans which includes alphacoronaviruses HCoV-229E and HCoV-NL63 and betacoronaviruses HCoV-OC43, HCoV-HKU1, SARS-CoV, MERS-CoV and SARS-CoV-2 [3]. They are known to infect the neurological, respiratory, enteric, and hepatic systems [1,2,3,4].
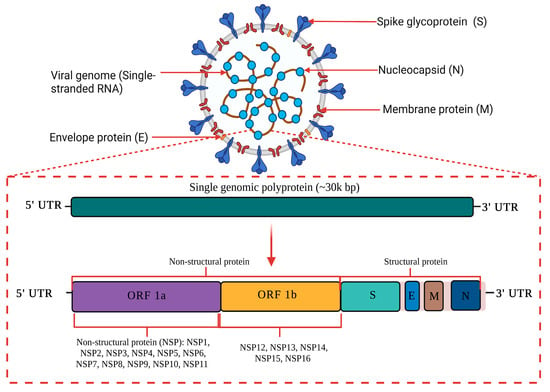
Figure 1. A schematic diagram of SARS-CoV-2 genome structure. SARS-CoV-2 is an enveloped RNA virus with an approximately 30 kilo base pair (kbp) genome size. The primary structural proteins of SARS-CoV-2 are membrane (M), spike (S) and nucleocapsid (N) proteins. The genome of SARS-CoV-2 consists of 14 open reading frames (ORFs) which encode for 27 structural and non-structural proteins. This image was created with Biorender.com.
2. Epidemiology of SARS-CoV-2
The past few decades have seen endemic coronavirus outbreaks in the Middle East (2012) and China (2002). Yet again, we see the emergence of another outbreak due to a new strain called the SARS-CoV-2 virus, which is highly contagious [5]. The most recent outbreak initially presented as pneumonia of unknown etiology in a cluster of patients in Wuhan, China [6]. The repeated emergence of coronavirus points toward the animal-to-human and human-to-human transmission of this virus and the evolution of new coronaviruses in the future.
Since the 12 December 2019, Wuhan, China has been the epicenter of coronavirus activity [7,8]. It was first reported as an acute respiratory illness that nobody had heard of before. Some studies have suggested that bats may be the likely source of the SARS-CoV-2 virus. However, there is no evidence to suggest that the SARS-CoV-2 virus originated in a seafood market. This theory remains unproven. To a lesser extent, bats serve as a natural reservoir for a wide variety of coronaviruses, including viruses that are similar to MERS-Co-V and SARS-Co-V [9,10,11]. SARS-CoV-2 was anticipated to spread mostly through direct contact with wild animals through ingestion or through intermediary host species. On the other hand, neither the route(s) of transmission nor the source(s) of SARS-CoV-2 have been definitively established.
3. Mutant Variant of SARS-CoV-2
After the outbreak of COVID-19 in late 2019, the virus remained genetically stable for approximately 11 months. Subsequently, in late 2020, COVID-19 development has been characterized by the emerging of clusters of mutations of SARS-CoV-2 known as ‘variants of concern’ (VOC), which affect viral features that include virus transmissibility and antigenicity. It emerged most likely in response to the shifting of the human population immunological profile. Furthermore, Harvey and colleagues have extensively discussed SARS-CoV-2 mutant variants, particularly the spike protein (the main antigen) [12]. It was reported that most of the mutation found in genomes from circulating SARS-CoV-2 virions are likely to be neutral or modestly deleterious. This is because ‘neutral’ amino acid alterations, which have no apparent influence on the virus’s efficiency or adaptability, are much more common than the rare ‘high-effect’ mutations that are necessary for adaptation.
For instance, the spike protein amino acid change D614G was shown to be occurring more often and many times in the global SARS-CoV-2 population in April 2020 [13,14]. Additionally, the high dN/dS ratio in the coding sequence suggests a positive selection at codon position 614 [13,14]. This was further supported by subsequent investigations which revealed that D614G has improved infectivity [15,16] and transmission [17]. In November 2021, the SARS-CoV-2 variant known as Omicron (B.1.1.529) was discovered in Botswana, South Africa, and Hong Kong, and it has since been a cause for alarm as VOC. Concerns regarding Omicron’s possible high transmissibility stem from the fact that it has been detected in at least 85 countries, as indicated by SARS-CoV-2 genome data published on GISAID (https://www.gisaid.org), accessed on 18 October 2022. The multitude of mutations in the S protein, some of which are shared with past VOCs and others that are novel, is the most alarming component of Omicron. According to preliminary investigations, the ability of Omicron to elude humoral immune responses is quite high, as seen by the rapid decline in the neutralizing effectiveness of infection- and vaccine-elicited antibodies and sera against Omicron [18,19,20,21,22]. Nevertheless, it is unclear how well Omicron can elude cellular immunological responses, which is the other component of the adaptive immune system mediated by T cells. Table 1 showed the circulating VOCs and Omicron subvariants under monitoring (as of 12 October 2022) published on the WHO (https://www.who.int/activities/tracking-SARS-CoV-2-variants), accessed on 18 October 2022.
Most importantly, a better knowledge of the phenotypic effects of mutations across the SARS-CoV-2 genome and their implications for variant survivability may aid in elucidating the mechanisms of virus transmission and evolutionary success.
4. Clinical Pathology of COVID-19
Clinical manifestations associated with COVID-19 present a broad spectrum which include asymptomatic patients to multiorgan dysfunction. COVID-19 can be categorized based on severity of infection such as mild, moderate, severe and critical. The common symptoms of COVID-19-infected patients could be ordered as major and minor symptoms. Major symptoms include fever (98.6%), fatigue (69.6%), dry cough and body ache, whereby minor symptoms comprise dyspnea, gastrointestinal disorders headaches and skin lesions [23]. Rare cases of vasculitis-type skin eruption in association with COVD-19 have been reported in patients infected with SARS-CoV-2 [24]. Overall, the spectrum of symptoms was found to be varied continuously in respect to different variants. For instance, the Omicron variant of SARS-CoV-2, a highly contagious variant, caused less severe disease with lower replication in lung parenchyma in comparison with the previous variants. In a study conducted by Boscolo-Rizzo and team, a significant reduction has been observed in self-reported chemosensory dysfunction such as taste and smell when comparing patients infected with the Omicron variant and previous variants including Alpha, Beta, and Delta [25]. A multiorgan inflammatory syndrome associated with SARS-CoV-2 has been observed in older children linked with abdominal pain, cardiac dysfunction and shock having resemblance with Kawasaki disease [26]. Patients infected with SARS-CoV-2 may have minimal to no symptoms related to acute respiratory distress syndrome (ARDS) which can result into multiorgan failure. Additionally, the severity of symptoms depends on the age of patient and is probably associated with a weaker immune system. The most common comorbidities related to elderly patients infected with SARS-CoV-2 include hypertension, neurological disorders, diabetes, and cardiovascular diseases [25].
5. Immunopathology of COVID-19
As a member of the cytopathic virus family, during the replication, SARS-CoV-2 damages the host tissues by targeting cells [27]. Angiotensin-Converting Enzyme 2 (ACE2) is regarded as the primary receptor which enables the entry of SARS-CoV-2 into host cells. ACE2 receptors are commonly present on the surface of alveolar epithelial cells (Type II), which are vital for the gaseous exchange in lungs. ACE2 is also expressed on endothelial cells, type I pneumocytes and outside pulmonary tissues such as intestine, blood vessels, heart, urinary bladder, and kidney [28,29]. Patients infected with SARS-CoV-2 have reported a greater level of pro-inflammatory cytokines: mainly Interleukin-6 (IL-6), Interleukin-8 (IL-8), Interleukin-1β (IL-1β), Tumor Necrosis Factor Alpha (TNF-α), Interferon-inducible protein 10 (IP10), Monocyte chemoattractant protein-1 (MCP-1), and regulated upon activation, normal T cell expressed and secreted (RANTES) similar to MERS virus. Monocytes and macrophages are activated by the release of cytokines and chemokines, which resulted in the generation of cytokines to prime T cell adaptive immune response [30]. Most of the time, the activation of macrophages and monocytes will invade the virus and results in reduced inflammation. Whereby, in a few cases, an adverse inflammatory response associated with a storm of cytokines has been observed where patients have previous underlying medical conditions or immunocompromised immune systems. This storm of cytokines is commonly classified by elevated plasma cytokines levels including Interleukin-2 (IL-2), Interleukin-7 (IL-7), Interleukin-10 (IL-10), Granulocyte colony-stimulating factor (GCSF), IP-10, MCP-1, MIP-1α, and TNF-α [29,30,31,32]. Such pulmonary inflammation is uncontrolled and most likely causes death. Moreover, thrombosis and pulmonary embolism have also been linked with the severe infections of SARS-CoV-2. In this case, platelets are hyperactivated, which accumulates platelets at thrombin suboptimal concentrations probably due to the higher production of a clotting factor by the liver caused by inflammatory cytokines [33]. Similarly, it has been reported that the receipt of angiotensin receptor blockers (ARB) increases the risk of SARS-CoV-2 infections particularly in close communities [34]. Whereby, in another retrospective study, the use of ARBs did not present any significant affiliation with the severity or mortality or elevation in the diagnosis of COVID-19 [35]. Nevertheless, the mechanism behind the immune response and associated molecular pathways to SARS-CoV-2 are still vivid and vague. It is very important to clarify these pathways to develop an effective therapeutic agent especially for upcoming new variants of SARS-CoV-2.
This entry is adapted from the peer-reviewed paper 10.3390/diseases11020064
This entry is offline, you can click here to edit this entry!
