Cinnamon is an evergreen and tropical plant of the family
Lauraceae [
1], growing particularly in Sri Lanka [
2], whose aqueous extract contains different substances such as the essential oils cinnamic aldehyde and cinnamyl aldehyde. The possibility of using natural substances as a cure is the fundamental of complementary and alternative medicines (CAMs), and cinnamon aqueous extract could have a crucial role in this field because of its anti-oxidant and anti-inflammatory effects [
1]. Its range of activity is wide; in fact, cinnamon is used especially as a flavoring agent and a spice [
3], and Sri Lankan medicine uses its extract from years to treat diabetes [
4,
5], allergies, microbial and parasitic diseases because of its action on different pathogens such as Mycobacterium tuberculosis and Streptococcus pneumoniae [
6]. Cinnamon also has an anti-cancer effect due to the interaction of its molecules in different pathways involved in the proliferation, survival, spread and programmed death of cells [
7]. The aqueous extract can be obtained from crushing cinnamon bark and leaving it to stand for three hours in hot water; it is filtered and frozen, becoming a sort of powder, and is finally mixed with sterilized water at a specific dose. The concentration of its principal compounds was measured, resulting in 2.9 mg/g of extract and 7.9 mg/g of extract of cinnamic aldehyde and cinnamyl aldehyde [
1]. Studies also investigated the possible effects of aqueous cinnamon extract on hepatic and renal functions after administration of five, ten and twenty times the effective dosage of it in healthy mice and demonstrated that, comparing a group of rats treated with aqueous cinnamon extract with another one of untreated animals, after an administration of a dose twenty times higher than the effective one, a minimum augment in alkaline phosphatase and transaminases can be observed in rats which received the substance, but after seventy-two hours a normalization of these values occurs. As far as renal function is concerned, cinnamon extract determines a decrease in serum creatinine level and, in contrast, a rise in urea and uric acid levels compared with untreated rats [
8].
2. Cinnamon and Cancer: Pathways and Mechanisms of Action
Lifestyle, including diet, has a crucial role in the pathogenesis of cancer; in fact, it is well-known that the Mediterranean diet, based on regular hydration with above two liters of water daily; an adequate intake of vegetables, fruits, legumes and unrefined cereals rich in vitamins and antioxidants substances; and a minimum quantity of red meat and other foods, represents a valid protective factor against cancer [
9,
10].
Foods from vegetal world, plants and herbs are the object of different studies with goals to attempt to understand the mechanisms of interaction between vegetal molecules and human diseases. Aqueous cinnamon extract has been tested in breast cancer cells and it demonstrated to be able to up-regulate the function of specific genes such as the peroxisome proliferator-activated receptor (PPARG) [
11]: it encodes for a nuclear receptor which, in breast cancer cells, has an essential role in homeostasis, cellular metabolism and neoplastic progression. In particular, PPARG acts via suppressing the proliferation and migration of cells and stimulating apoptosis. Studies put in evidence that, in patients with breast cancer, high levels of PPARG are related to a major survival rate compared with those with lower expressions of this nuclear receptor [
12]. In prostate cancer cells, aqueous cinnamon extract has been tested as an anti-proliferative molecule and as a proteasome inhibitor. In fact, it is capable of selectively inhibiting the proliferation of cancer cells. Proteasome is a system of proteins which, through ubiquitination, regulates the proliferation and degradation of cells [
13]; it is the target for drugs’ so-called proteasome inhibitors such as Bortezomib, used in the treatment of multiple myeloma and other tumors [
14]. The use of natural substances, and in particular of aqueous cinnamon extract, as proteasome inhibitors in prostate cancer could represent a fascinating field of study and lead to new instruments for curing patients [
15].
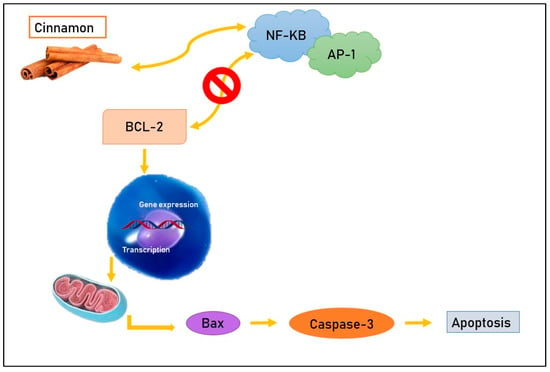
2.1. Inhibition of NF-KB and AP-1
In vivo experiments [
1] suggest that aqueous cinnamon extract acts on specific molecules such as the transcription factors nuclear factor kappa-light-chain-enhancer of activated B cells (NF-KB) and Activator protein 1 (AP-1), which have a crucial role in cell survival and in the regulation of the cell cycle and apoptosis. In fact, NF-KB and AP-1 have as a target the anti-apoptotic protein Bcl-2 [
16] and, normally, cause an increase in this molecule with the consequent inhibition of programmed death of tumor cells [
17]. Cinnamon compounds tie to NF-KB and AP-1, determining a decrease in the correspondent RNA expression and translation and preventing the anti-apoptotic action of Bcl-2, which they commonly stimulate [
1] (
Figure 1). In another in vivo study [
9], mice in which melanoma cells (B16F10) were subcutaneously transplanted were divided into two subsets: in one group, animals received cinnamon extract daily at a dose of 400 μg/g of weight, and in the other group animals received phosphate-buffered saline (PBS). After thirty days of treatment, the tumor size was evaluated based on tumor weight and, interestingly, an important decrease was observed in the subset of mice that received cinnamon (tumor weight 6.2 g) compared with those that received PBS (tumor weight 12.2 g). These results show that cinnamon aqueous extract, acting on the transcription factors NF-KB and AP-1, has an important anti-cancer effect in vivo [
1]. Even if aqueous cinnamon extract has not lead to morphological changes in cells 24 h from exposure, a progressive augment in apoptotic cells has been observed [
18] and it seems that the apoptosis rate gradually increases depending on the time from cinnamon administration; in fact, in order to verify this condition, neoplastic cells of colorectal adenocarcinoma have been treated with this natural compound and showed a similar time-related pattern in increasing apoptosis rate. Furthermore, aqueous cinnamon extract proved to be capable of stimulating the expression of pro-apoptotic genes such as Bim and Bax [
19,
20,
21,
22,
23,
24,
25,
26].
Figure 1. Cinnamon compounds tie to NF-KB and AP-1, preventing their bond with the anti-apoptotic protein Bcl-2 and leading, consequently, to apoptosis.
2.2. Activation of Cytotoxic CD8+ T Cells
Cytotoxic CD8+ T cells have a fundamental function in the immune response against tumors and prevent the so-called “immune escape”; thanks to neoplastic cells, they are able not to be recognized as non-self-agents [
18], but their activity is sometimes suppressed from the tumoral microenvironment or from alterations such as reduced expression of perforins, inefficacy of adhesion, abnormal production of cytokines and defective action of exocytosis granules [
27,
28,
29]. CD8+ T cells, stimulated by ingredients in cinnamon extract, determine an increase in the expression of molecules with cytolytic action such as granzymes B, granzymes C, interferon-C and tumor necrosis factor-alpha (TNF-α) [
18,
30,
31,
32,
33,
34]. In detail, there is evidence in vitro of this effect in cells from breast cancer, colorectal cancer, melanoma and liver cancer [
17]. It has been also demonstrated that there are differences between oral and subcutaneous administration of aqueous cinnamon extract; in fact, an oral treatment of more of 48 h inhibits the growth of tumor cells via apoptosis more strongly than that of a subcutaneous one through an important reduction in pro-angiogenic molecules and pro-inflammatory cytokines, while a subcutaneous administration determines a major activation of cytotoxic CD8+ T cells with an anti-cancer effect. These observations could indicate a possible effect of cinnamon extract as an anti-cancer molecule thanks to its capacity to recruit cytotoxic CD8+ T cells and potentiate their activity against neoplastic cells.
2.3. Inhibition of COX-2 and Inflammatory Cells
Inflammation has a crucial role as a defense mechanism against pathogens and other external agents. It starts after a trigger, for example a virus, activates a cascade of intracellular and intercellular signals, leading to the release of inflammatory molecules such as nitric oxide (NO), prostaglandins (PGs) and TNF-α [
35]. This situation causes a disequilibrium between pro-inflammatory and anti-inflammatory cytokines with a prevalence of the first ones and, as a consequence, an increased incidence of chronic diseases such as tumors has been observed [
36,
37,
38,
39,
40,
41]. It is well-known that macrophages and other cells produce nitric oxide and prostaglandins and that between these two components there is a cross-talk. In fact, both NO and PGs are blocked by nitric oxide synthase-inhibitors (NOS-inhibitors), but this effect can be suppressed through coincubation with L-arginine, which is the precursor of nitric oxide. Furthermore, inhibition of cyclooxygenase-2 (COX-2) in inflammation determines a change in the L-arginine-nitric oxide pathway, while COX-2 inhibition causes a reduction in NOS effect in human platelets [
42]. In vivo experiments [
42] show an anti-inflammatory effect of cinnamon aqueous extract in a mouse model of paw edema similar to indomethacin, a drug acting as a strong inhibitor of COX-2. As observed, edema reaches the maximum volume in its third phase, when there is infiltration of neutrophils and the release of free radicals from them. Cinnamic aldehyde, contained in cinnamon, impeded neutrophils’ infiltration of the edema, determining, consequently, a decrease in inflammation. Furthermore, it is clear that myeloperoxidase (MPO), an enzyme contained in azurophilic granules of neutrophils, is the molecule that causes tissue damage. There is evidence of an effect of cinnamon extract in inhibiting MPO, preventing tissue injury [
43]. As far as the role of chronic inflammation in the pathogenesis of cancer, evidence about the ability of cinnamon extract to inhibit inflammatory molecules could be strong encouragement for scientists to go on in this field of research because of the real effects that have been reached with this substance not only in vitro, but also in in vivo models.
2.4. Inhibition of PI3K/Akt/mTOR, MAPK-P38alfa and DHFR
PI3K/Akt/mTOR signals, promoted by point mutations of PI3K and Akt and by inactivity of the phosphatase and tensin homolog (PTEN), play an important role in the regulation of the proliferation and apoptosis of cancer cells, especially from ovarian, gastric and breast neoplasms [
44,
45,
46]. In detail, an irregular function of PI3K/Akt/mTOR determines an increased expression of fusin, which subsequently stimulates C-X-C chemokine receptor type 4 (CXCR-4)-related STAT3; this cascade of signals allows the maintenance of stemness in cancer cells [
47]. Experiments with oral cancer cells exposed to aqueous cinnamon extract allowed the detection of an inhibitory action of the natural ingredients of this compound on cellular growth due to the fact that it down-regulates different molecules of the PI3K/Akt/mTOR pathway, preventing its activity [
6]. Obviously, more and more evidence is needed before declaring that the programmed death of cells observed is due to an up-regulation of the PI3K/Akt/mTOR pathway from compounds in cinnamon extract, but the experiment cited surely represents a starting point. MAPKP-38α is a kinase activated by pro-inflammatory molecules, oxidative stress and heat shock, all mechanisms involved in tumorigenesis [
48,
49,
50,
51,
52,
53,
54,
55]. Various evidence [
9] exists about the action of cinnamon compounds as a substrate for MAPKP-38α, preventing the bond of the “real” substrate and, consequently, blocking the function of this pathway, including growth and survival stimulation [
6]. Other evidence comes from dihydrofolate reductase (DHFR), a protein that induces the transformation of dihydrofolate in tetrahydrofolate, which is finally the cofactor in the synthesis of different molecules fundamental in cell proliferation such as thymidylate, purines and some amino acids [
15]; for this reason, DHFR has been used as a target for drugs in the treatment of various tumors, such as with methotrexate [
16]. The mechanism of action for aqueous cinnamon extract is the same as that for MAPKP-38α: it ties as a substrate to DHFR, preventing the bond of its “real” substrate and, consequently, blocking its proliferative stimulation of cancer cells. This evidence could indicate a possible effect of cinnamon extract as an anti-cancer molecule due to the fact that it is capable of inhibiting the activity of MAPK-P38alfa and dihydrofolate reductase, molecules which normally have crucial roles in the proliferation and survival of neoplastic cells. In detail, aqueous cinnamon extract interacts with catalytic residues of MAPKP38α and DHFR, such as Val38 and Tyr35, for the first and with Ala9 and Tyr121 for the second, blocking or significantly modifying their function [
6].
2.5. Inhibition of Angiogenesis
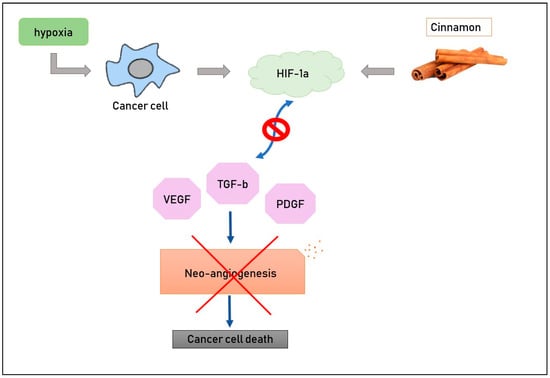
The effect of aqueous cinnamon extract has been studied in a mouse melanoma model [
17] in order to evaluate its possible action in inhibiting angiogenesis. It is well-known that cancer cells need more and more nutrients to grow, and these compounds come from blood, so it is crucial for tumors to have new blood vessels in their macroenvironment. Hypoxia and pro-angiogenic molecules such as vascular endothelial growth factor (VEGF), platelet-derived endothelial growth factor (PDGF) and transforming growth factor beta (TGF-b) constitute the main stimuli for neo-vascularization. Scientists treated mouse melanoma cells with cinnamon extract at various doses (0.3 or 0.5 mg/mL) and then evaluated the levels of pro-angiogenic molecules; RT-PCR and ELISA methods were used to detect the expression of VEGF, PDGF and TGF-b, and it was demonstrated that aqueous cinnamon extract inhibited these factors both in mRNA and in proteins (
Figure 2). Its activity is clinically evident, too, if we pay attention to the dimensions of the spleen and lymph nodes, which are significantly smaller in mice treated with this extract than those in untreated mice [
56]. HIF-1a is a transcription factor whose activity depends on tissue oxygen levels, [
8,
18]. In fact, hypoxia causes an increase in the presence of pro-angiogenic molecules and stimulates neo-vascularization. The administration of cinnamon extract down-regulates levels of HIF1a, conducting a reduction in the formation of new blood vessels. This evidence allows us to state that cinnamon inhibits neo-angiogenesis [
56].
Figure 2. Cinnamon compounds down-regulate levels of HIF-1a, with the results being a decrease in pro-angiogenic factors and, consequently, of new blood vessel formation.