Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
The diagnosis of infective endocarditis (IE) during pregnancy is accompanied by a poor prognosis for both mother and fetus in the absence of prompt management by multidisciplinary teams.
- infective endocarditis
- pregnancy
- cardiovascular maternal risk
1. Infective Endocarditis during Pregnancy—An Introduction to a Complex Issue
Cardiovascular disease is responsible for complications in 1–4% of pregnancies and 25% of maternal deaths [1]. Infective endocarditis (IE) is a rare condition during pregnancy, with an extremely rare incidence rate of less than 0.01% [2]. From 1893 when the first case of IE was reported by William Bart Osler to the present day, advances in diagnostic methods and the development of new therapeutic molecules have allowed for prompt diagnosis as well as the possibility of quickly administering a targeted antibiotic regimen to reduce the risk of morbidity and mortality for both mother and fetus. The use of multiple imaging assessment methods affords the possibility of surgical intervention during the acute infectious process as well as the development of therapeutic guidelines with clear indications for the use of antibiotics in pregnancy which have laid the foundation for the clear management of this pathology, but with multiple challenges remaining for both mother and fetus [3,4].
IE is an infection of the endocardium and predominantly affects the anatomical structures on the left side of the heart [5]. The emergence of bacterial agents resistant to standard antibiotic therapy, the increasing identification of “modern” risk factors such as intracardiac devices and intravenous drug administration and the increasing prevalence of nosocomial infections are current challenges with multiple implications for pregnant women with IE, both medically, socially and economically [6,7].
The prognosis of patients with IE depends on the age at which pregnancy is achieved, as it is known that young age is associated with a high risk of obstetric complications. Epidemiological data in the literature highlights the high mortality rate of these pathologies, both for the mother (22%) and the fetus (15%) in the absence of prompt management from multidisciplinary teams [8].
Cardiovascular risk assessment is important to perform at every pregnancy, especially in patients with a history of heart disease prior to pregnancy. The guidelines of the European Society of Cardiology [9] for the assessment of cardiovascular disease recommend the application of the World Health Organization (WHO) classification of maternal cardiovascular risk assessment, with prognostic and therapeutic implications alike. A large proportion of clinical cases reported in the literature present as complications or cause of death the occurrence of heart failure or an acute embolic cardiovascular event [10].
2. From Pathophysiology to Multidisciplinary Assessment
2.1. Hemodynamic and Immunologic Adaptations during Pregnancy
The main physiological changes occur in the first weeks of pregnancy when some of the pregnant patients will exhibit clinical manifestations suggestive for various cardiovascular pathologies which were subclinical until that moment [11]. During pregnancy, a number of hemodynamic changes occur at the cardiovascular level predominantly affecting the hemodynamic status [12,13].
Heart rate increases on average by up to 25%, steady throughout pregnancy until the third trimester [14,15,16]. Starting from the 25th week of pregnancy, there is an increase in plasma volume on the one hand and on the other hand a 30% reduction in red blood cells which leads to dilution anemia [17,18].
The cardiac output increases as the pregnancy progresses, reaching in the third trimester 45% higher than baseline. Some patients may experience increases in cardiac output both at onset through increased stroke volume and in progression through vena cava compression [19].
Changes in blood pressure values should be carefully monitored during pregnancy to assess the hemodynamic impact on both mother and fetus [20,21]. Vascular resistance occurring in the first trimester of pregnancy will lead to a decrease in blood pressure values, especially for the systolic component where values can be up to 15 mmHg lower [11,22,23]. In a patient with right heart IE, the increased blood volume and hemodynamic changes described above create an increased pressure environment that can easily lead to pulmonary embolism. Systemic embolisms can occur in a similar way in pregnant patients with left heart IE [24].
During pregnancy a number of immunological adaptations occur to adapt to the fetus which increase the risk of infections [25,26]. During pregnancy, there are oscillations in immune status, with data in the literature showing a global suppression [27]. Different stages of pregnancy are associated with a distinct maternal immunological profile. Thus, if in the first trimester studies have shown the existence of a significant inflammatory substrate, the second trimester is characterized by a reduced inflammatory status creating a predisposition for the occurrence of infections [28]. If we corroborate these data with the presence of the risk factors mentioned above, we get a picture of a complex pathology, which requires a rapid and efficient multidisciplinary approach.
2.2. Demographics and Risk Factors
-
Demographic Data
The risk of developing IE for women with congenital heart disease is lower than that of men due to better dental and hand hygiene as well as lower rates of smoking and intravenous drug use [29,30,31]. Increased life expectancy and the rise in healthcare-associated infections have led to an increased incidence of IE among women, while in men studies have reported a decrease in incidents with age [32]. Gender also influences the mortality rate, with women having a higher risk than men [33]. An increased susceptibility to infections secondary to the relative immunosuppression associated with pregnancy exacerbates the negative impact of IE on both mother and fetus, both during pregnancy and in the first weeks postpartum [34].
Kebed et al. [35] conducted a systematic review based on an analysis of 72 clinical trials including 90 patients with peripartum IE. 98% of patients had native valve infections, with the most common cardiac structure involved being the mitral valve. In the antibiotic era, IE is no longer a disease of underdeveloped countries. Similar epidemiological phenomena have been reported in high income countries due to rising living standards and prophylactic antibiotic administration [36,37].
-
Risk Factors
The risk factors responsible for the development of IE in pregnant women are similar to those of the general population, the three main etiologies being intravenous drug administration, congenital heart disease and rheumatic heart disease [38,39]. 0.5% of patients with congenital heart disease develop IE during pregnancy [9].
In the past, the most common causes of IE in young people were rheumatic heart disease or congenital heart disease. Advances in technology have led to changes in the panel of predisposing factors over time, with age, frailty or the presence of comorbidities increasingly being blamed instead of prosthetic valves, hemodialysis, intravenous catheters or immunosuppression [40,41] (Figure 1).
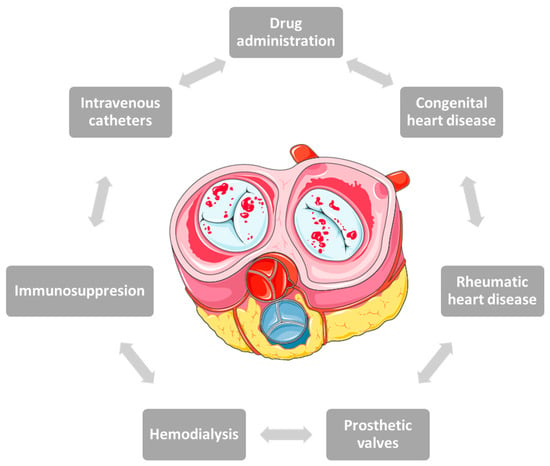
Figure 1. Risk factors involved in the onset of infective endocarditis in pregnancy.
Over 75% of patients who develop IE secondary to intravenous drug administration have endocardial damage, most commonly secondary to septic embolization at injection sites [42]. Tricuspid valve involvement is objectified in most cases, whereas pulmonary valve lesions are much rarer [43,44]. Isolated pulmonary valve IE is a rare entity, with few such cases reported in the literature, especially in pregnant women. Risk factors include pulmonary valve abnormalities, intravenous drugs or the presence of right heart catheters [45].
There are few cases in the literature of IE involving devices used to close atrial septal defects in pregnant patients one year after the procedure. In a case series published by Amedro et al. [46], none of the 22 patients with atrial septal defects closed with minimally invasive surgery developed IE at more than one year. This clinical finding has a pathophysiological basis in the observation that bacterial insemination occurs before neo-endothelialization of implanted devices, most commonly in the first 3 months [47].
Pregnant women may associate a partially immunocompromised status that increases their risk of developing IE in the context of the presence of comorbidities with prognostic implications on the course of pregnancy or fetal viability. This justifies the continuation of antibiotic therapy beyond the 6-month period indicated by current clinical guidelines [48,49]. The susceptibility of pregnant women to certain infectious diseases or the modulation of their severity is closely related to the placental immune response and its tropism for certain pathogens [49,50]. These rare cases present diagnostic and treatment challenges, due to the lack of accurate therapeutic recommendations. Most pregnant women have been excluded from clinical trials that have investigated the indications for percutaneous closure of atrial septal defects or patent foramen ovale [51,52,53].
Bicuspid aortic valve is the most common congenital disease, affecting 1–2% of the general population. The occurrence of IE in these patients involves the presence of a susceptible endothelium which allows for the aggregation of platelets and fibrin at this level, with the consequent formation of thrombi which represent a favorable site for the proliferation of microorganisms [54].
In the literature, there are extremely few clinical cases reported of patients with mitral valve IE requiring simultaneous valve replacement and assisted cesarean delivery. One of the main concerns in these situations is the safety of the fetus which may be jeopardized by the need for a cardiopulmonary bypass [55]. The highest risks have been reported in fetuses with a gestational age of 26 weeks and a reported survival rate of less than 28% [56].
-
Etiological Agents
Streptococci and staphylococci are the main etiological determinants of IE [57]. Staphylococci are the most common pathogens, with Staphylococcus aureus being an increasingly isolated pathogen that has a negative prognostic role [58,59,60]. IE can coexist with other infections, usually caused by the same pathogen. The etiological agents of left heart IE are predominantly streptococci, with staphylococci more commonly associated with right heart involvement [61]. These cases are extremely rare in pregnancy, with very few such clinical scenarios reported in the literature. One such example is a Streptococcus oralis infection that caused IE and meningitis simultaneously [62]. Streptococcus oralis causes meningitis in patients undergoing spinal anesthesia or certain dental procedures, and is rarely associated with neonatal meningitis or maternal sepsis [63].
Bacillus cereus is a rare pathogen responsible for the occurrence of IE in the general population, with less than 20 such clinical cases reported to date. It occurs most commonly in drug users or those with implanted cardiac devices. Shah et al. [64] isolated Bacillus cereus in the tricuspid valve of a 25-week pregnant patient, this being the first case of Bacillus cereus infection in a pregnant woman. Khafaga et al. [58] reported for the first time Staphylococcus lugdunensis as an etiologic agent of IE in a pregnant woman. Staphylococcus lugdunensis is a coagulase-negative, skin commensal staphylococcus that colonizes the perineum [65,66].
Most cases of pregnant IE reported in the literature have a bacterial infection as a microbiological substrate, with rare cases of fungi underlying this potentially fatal complication for both mother and fetus [67,68]. IE with fungal pathogens such as Zygomycetes are frequently nosocomial, secondary to prolonged antibiotic therapy or intravenous catheterization [69,70]. Such a clinical situation was encountered in a pregnant woman previously diagnosed with positive serology for hepatitis B [67].
2.3. Diagnosis and Management
The diagnostic and therapeutic management of these cases must be prompt, taking into account the maternal impact of the associated morbidity and mortality as well as the high risk of fetal death [29,35,71]. The management of IE in pregnant women must be carried out in multidisciplinary teams that individualize the therapeutic plan and identify the optimal time for surgery (in selected situations) and pregnancy termination [72]. Literature data show a reduction of in-hospital (p < 0.001) and one-year mortality for pregnant patients treated with multidisciplinary teams [73,74,75]. The development of such models of good practice for the benefit of patients is an ongoing concern of clinicians and researchers in the field alike, with the well-being of the mother and the fetus at the center [75,76].
-
Clinical Picture
The clinical picture of pregnant patients with IE may be the classic one with fever, vascular murmurs, petechiae and clinical signs associated with anemia and embolization [2], or may be partially masked by other symptoms associated with pregnancy. Special attention should be paid to fever in pregnancy, as it is a symptom frequently associated with various causes such as chorioamnionitis, pneumonia, various viral infections and pyelonephritis [77].
-
Diagnostic Methods
The diagnostic algorithm of pregnant patients with IE is similar to that of the pathology in the general population. Identification of the infectious agent is achieved by bacterial cultures which remain the standard test for pregnancy IE. In patients with negative cultures, direct serological testing is performed. The guidelines of the European Society of Cardiology recommend the collection of three blood cultures from different venipuncture sites, taken at an interval of at least one hour between the first and the last [31,78]. Alternatively, molecular testing (e.g., PCR testing for Tropheryma whipplei) or histopathological testing using resected valves can be used. The modified Duke criteria help establish the diagnosis of IE based on clinical, echocardiographic or microbiological findings [79,80].
Cell-free deoxyribonucleic acid (DNA) next-generation sequencing is a useful diagnostic tool, superior to the methods presented above because of the longer time interval in which it can identify the pathogen compared to standard blood cultures [81,82,83]. Circulating cell-free DNA was first discovered in 1948 by Mandel et al. [84,85]. Blood contains small amounts of it, predominantly from bacteria, and is a veritable reservoir of genetic material from all the body’s cells [86,87]. There are different types of cell-free DNA, the most common being circulating tumor DNA, cell-free mitochondrial DNA, cell-free fetal DNA and donor-derived cell-free DNA [88]. Recent studies have appreciated cell-free DNA as a potential clinical biomarker associated with endothelial dysfunction [89].
Cell-free DNA sequencing provides a rapid, non-invasive diagnosis, representing the only diagnostic resource in some cases of IE in which the infectious agent could not be identified by conventional microbiological identification methods [90]. In addition to diagnosis, this method can also be used for monitoring infectious pathologies or for early identification of recurrences, but further studies are needed on the decay of it in blood after treatment [91]. Decay kinetics after treatment have not been extensively reviewed in the literature to date, with few reports in the literature. Solanky et al. [91] presented the case of a 53-year-old patient diagnosed with IE involving Bartonella quintana at the level of the biological aortic prosthesis and periodically monitored decay kinetics after parenteral antibiotic therapy and valve resection. The group of investigators observed that after 4 weeks of parenteral antibiotic therapy, the cell-free DNA sequencing signal decreased by approximately 80%. Following excision of the aortic bioprosthesis, the decrease in cell-free DNA sequencing occurred in two phases, a rapid one in the first 24 h and a slow one occurring up to 48 h after surgery, which justifies its use as a method of monitoring the response to antibiotic therapy.
Cell-free DNA sequencing and other state-of-the-art molecular methods are showing their usefulness in the etiological diagnosis of IE, especially in those with negative bacterial cultures [92,93]. A representative example is metagenomic next-generation sequencing, which according to clinical studies published to date has a higher sensitivity than classical diagnostic methods [94,95]. Duan et al. [96] analyzed a group of 109 patients, both with and without different infectious pathologies, and demonstrated that although the sensitivity of the method is superior, no statistically significant differences were reported in specificity compared to cultures (p = 0.41). Comparing the infectious and non-infectious groups of patients, the investigators demonstrated that the duration of hospitalization and the 28-day death rate in the first group were statistically significant and superior. Advanced statistical techniques identified age as a determinant parameter in obtaining positive metagenomic next-generation sequencing analysis results.
Microbial cell-free DNA has a sensitivity of 89.3% and a specificity of 74.3% compared to blood cultures, with each additional day that positive results are reported associated with a 2.89-fold increased risk of metastatic infection (p = 0.0011) [97]. A recently published systematic review included a total of 13 clinical trials using this genetic diagnostic method in IE. Until August 2022, metagenomic next-generation sequencing has been used to identify gram positive cocci (8.9% of cases), coagulase-negative staphylococci (17.6% of cases), streptococci (37.5% of cases) and Enterococcus faecalis (6.6% of cases) [92].This method not only enjoys advantages such as reduced processing time, the provision of real-time information and ease of obtaining a blood sample, but also a number of disadvantages such as the high cost of the molecular extraction technique and the lack of guidelines to give this method the status of an alternative technique to conventional diagnostic methods due to the lack of large clinical studies on large groups of patients confirming the data existing so far from limited reports (Figure 2) [98].
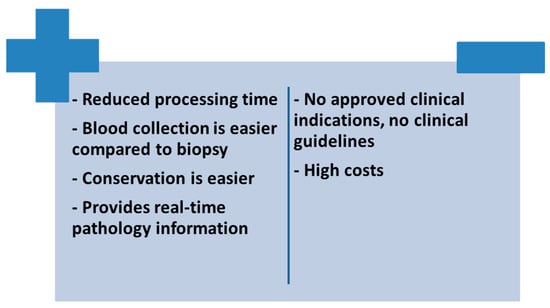
Figure 2. Advantages and disadvantages of using cell-free DNA sequencing in patients with IE.
Cell-Free DNA has also been identified in pregnant patients in the maternal circulation. In 1997, a group of investigators identified Y-specific DNA fragments in the serum and plasma of pregnant patients, representing approximately 3.4–6.2% of the plasma [99]. This discovery led over time to the use of this genetic material for prenatal aneuploidy screening. Particularly for pregnant women, the significant correlations are between the vegetation length and the serum matrix metalloproteinase-9 level and the occurrence of embolic events. Based on this observation, Thruny et al. [100] demonstrated that 64% of patients with new embolic events had vegetations greater than 10 mm in size and a serum matrix metalloproteinase-9 titer greater than 167 ng/mL [101].
-
Multimodal Imaging Assessment
The imaging assessment of a pregnant woman with IE should focus on reducing the risk of fetal irradiation. The most commonly used methods of assessment and diagnosis are transthoracic echocardiography and nuclear magnetic resonance [77,102,103]. Echocardiographic identification of vegetations requires transesophageal echocardiography as soon as possible in patients at high risk of complications [78] (Figure 3). The CAPREG II study evaluated pregnant patients with various cardiovascular pathologies to identify predictors associated with a high risk of maternal complications such as mechanical prosthesis, high-risk associated aortic disease, pulmonary hypertension and chronic coronary syndrome [104]. Echocardiography also identifies a number of negative prognostic factors such as the presence of systolic dysfunction, the presence of a subaortic gradient of more than 30 mmHg or a pulmonary artery systolic pressure value of more than 50 mmHg in the absence of obstruction in the right ventricular outflow tract [105]. In addition to transthoracic echocardiography, transesophageal echocardiography is an additional imaging investigation required for patients with IE and mechanical valve prosthesis [106].
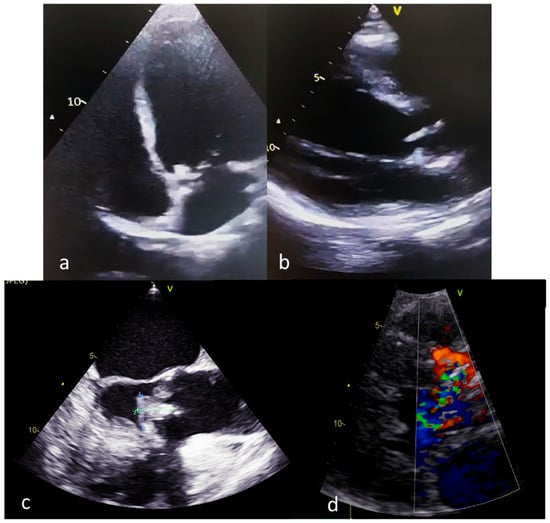
Figure 3. Echocardiographic findings in pregnant patients with IE (a,b): transthoracic echocardiography, aortic valve vegetations; (c): transesophageal echocardiography; (d): transthoracic echocardiography, aortic regurgitation secondary to aortic valve vegetation).
This entry is adapted from the peer-reviewed paper 10.3390/medicina59050939
This entry is offline, you can click here to edit this entry!
