Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Sirius Huang and Version 2 by Michał Gackowski.
The widespread role of titanium (IV) oxide (TiO2) in many industries makes this substance of broad scientific interest. TiO2 can act as both a photoprotector and photocatalyst, and the potential for its role in both applications increases when present in nanometer-sized crystals. Its sunlight-scattering properties are used extensively in sunscreens.
- titanium (IV) oxide
- photoprotection
- phototoxicity
- dermal formulations
1. General Information
Crystalline TiO2 may exist in three phases, namely rutile, anatase, and brookite (Figure 1) [28][1].
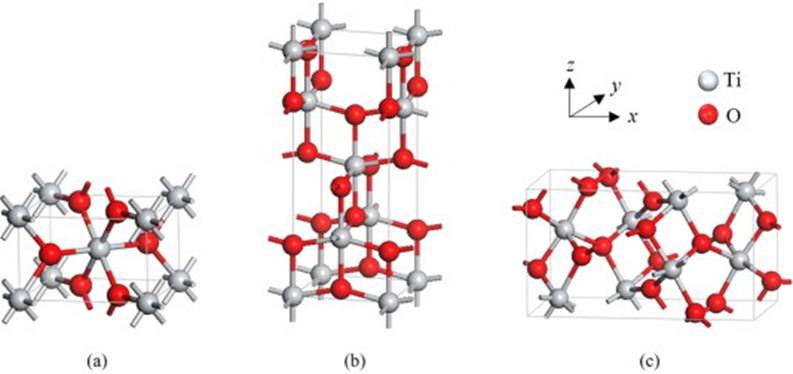
Figure 1.
Unit cell structure of TiO
2
polymorphs rutile (
a
), anatase (
b
), and brookite (
Variations between these polymorphs involve octahedral units arranged in a three-dimensional space. Importantly, each of the crystal structures exhibits different properties—they can differ in shape, structure, refractive index, and photocatalytic activity [30][3]. Furthermore, only rutile is stable, while anatase and brookite are metastable and convert to rutile upon heating. The differences between the polymorphs are particularly apparent when comparing their photocatalytic activity. The higher energy gap of anatase and brookite and their superior electron mobility make these polymorphs more reactive than rutile. Moreover, there is a significant difference in the refractive index of rutile and anatase (2.7 and 2.5, respectively). While there are many arguments in favor of the safety of TiO2, potential risk factors also need to be considered. On the one hand, excellent photoprotective properties are reported, and on the other hand, the risk of inducing photochemical reactions, as shown in the following sections.
2. Titanium Dioxide Nanoparticles (NPs) Photoprotection vs. Phototoxicity
TiO2 nanoparticles (TiO2-NPs) have attracted a considerable attention due to their unique properties, which make them desirable for a wide range of applications. Among industrial applications, they have found a role in cosmetic and pharmaceutical technology. Properties that make it an excellent candidate for the use as a sunscreen include its high photoprotective capacity (strong light scattering) associated with a large photoactive area, insolubility in aqueous media, and transparency. It has been proven that TiO2-NPs can act as a shield for both the skin and drugs against UVA and UVB radiations. Moreover, the argument for using TiO2-NPs as a sunscreen to date is that they are less photoreactive than other chemical ingredients (especially organic sunscreens), and thus less likely irritating to people with sensitive skin. The EU Scientific Committee on Consumer Safety (SCCS) has approved crystalline TiO2-NPs as safe for use in cosmetic products intended for use on healthy, undamaged, or sunburnt skin [31][4]. As a UV filter, TiO2 can be incorporated into cosmetic formulations at a maximum concentration of 25%. However, it has to be considered that the high surface-to-volume ratio makes it legitimate to raise the issue of the increased bio-reactivity of nanomaterials (NMs) and their potential toxicity. Moreover, recent studies and reports on TiO2-induced phototoxicity have brought the topic increasingly into the headlines. Therefore, the question of safety must be asked to consider whether chronic exposure to this material is dangerous or not. Although the phototoxic effect of TiO2-NPs has already been demonstrated for all polymorphs, it is important to note that different phases can induce a reaction of different severities. However, the main problem with the use of TiO2 in products intended for use on the skin surface is its potential to generate ROS [32,33,34,35,36,37,38][5][6][7][8][9][10][11].
3. Illuminated TiO2-NPs Generate Reactive Oxygen Species (ROS)
Reactive oxygen species are a group of chemically reactive molecules/radicals derived from molecular oxygen. Examples of ROS include superoxide (O2●−), hydroxyl radical (HO●) hydrogen peroxide (H2O2), and singlet oxygen (1O2), each of which causes a variety of biological damage and has been implicated in a multitude of diseases, including cancer and ageing [39,40][12][13]. As already proven, ROS are deleterious to biological structures (e.g., membranes, lipids, proteins, and nucleic acids) and may be generated by TiO2-NPs under certain conditions, e.g., when illuminated with UV light and in an aqueous environment [41][14].
TiO2 is a wide bandgap semiconductor (3.2 eV for anatase) [42][15] and can only be photo-activated under UV illumination [43][16]. Only light with a wavelength lower than 387 nm allows for the excitement of an electron from the valence band (VB) to the conduction band (CB). As a result, holes in the VB (h+VB) and electrons in the CB (e−CB) are generated and subsequently migrate to the NPs surface to react with water and adsorbed O2 molecules, respectively [44][17]. These reactions are responsible for the production of ROS in the aquatic and aerobic environment. The reactions mentioned are presented as chemical Equations (1)–(6) [44,45][17][18].
H
2
O + h
+
→
●
OH + H
+
O2 + 2e− + 2H+ → H2O2
O2●− + O2●− +2H+ → H2O2 + O2
H2O2 + e− → ●OH + OH−
H2O2 + O2●− → ●OH + OH− + O2
H
2
O
2
+ hv →
●
OH +
●
OH
The correlation between TiO2-NPs and hydroxyl radical concentrations, UVA light intensity, and concentration of dissolved organic carbon (DOC) with toxicity was assessed by the team of Coral et al. Their study demonstrated that the radical production rate was positively correlated with an increase in TiO2-NPs concentration and UVA intensity, and negatively correlated with increased DOC concentrations. Moreover, the authors showed that the concentration of TiO2-NPs is a poor predictor of toxicity, when considered alone, and that the concentration of generated hydroxyl radical is a better predictor in assessing the overall risk [45][18].
An alternative of TiO2-NPs phototoxicity was also described. Taking into account the energy transfer of O2●− radicals to the holes in the VB, singlet oxygen (1O2) may also be generated according to Equation (7) [46][19].
h
+
+ O
2●−
→
1
O
2
1O2 can oxidize fatty acids or damage nucleotides leading to several oxidative products [46][19]. The presence of 1O2 photo-produced by TiO2-NPs was confirmed by Fenoglio et al. By modification of the NPs surface, the ROS-generating potential can be decreased. For example, by the introduction to the surface carbonaceous or carbonate/carboxylate-like products, that generation of ROS can be decreased, although the presence of singlet oxygen was confirmed [44][17].
4. Factors Influencing the Phototoxic Potential of TiO2-NPs
4.1. Polymorphic Form
As it was previously mentioned, the polymorphic form of TiO2-NPs influences both the material stability as well as the potential of ROS generation. Usually, the rutile phase is less photoreactive than anatase or brookite. This phenomenon is related to a different lifetime of holes (that is longer for rutile) and the electron-trap depth that is shallow for anatase (<0.1 eV), moderate for brookite (~0.4 eV), and high for rutile (~0.9 eV). The deeper the electron trap is located, the less photoreactivity is observed due to lower electron mobility [47][20]. Another difference between polymorphs is the interaction of these materials with oxygen. Molecular oxygen shows a high affinity to oxygen vacancies. In anatase, the interaction of molecular oxygen with the surface is weaker than for rutile. It explains the difference in the concentration of absorbed oxygen in both forms of TiO2-NPs. As shown previously, adsorption of O2 relates to an electron transfer from the surface defects, forming superoxide radical anions, which are well known as ROS with a weaker oxidation ability than hydroxyl radicals [48][21]. The different photoreactivities of anatase and rutile were confirmed in cytotoxicity studies [49][22].
Tests on immortalized human keratinocytes (HaCaT), both with and without UV light were conducted. In the dark, TiO2-NPs (rutile or anatase) did not affect the mitochondrial activity or cell membrane. However, the exposition to rutile NPs may inhibit cell growth and induce OH− generation, which seems to be dependent on the surface properties of nanomaterial, and was confirmed for the hydrophobic surface of rutile NPs. In this study, the cytotoxicity under UVA irradiation was tested and a strong polymorphic-dependent effect was seen. The rutile NPs did not show any cellular effect while the anatase showed strong phototoxicity and induced a marked decrease in mitochondrial activity, cell membrane damage, and the induction of oxidative stress [50][23]. Another research that settled the higher cytotoxic potential of anatase was conducted by Hering et al. By using both the monolayer cell culture and UV light, the authors confirmed the influence of the polymorphic of TiO2-NPs and the material concentration on the cell viability. The results showed that anatase led to a lower effective dose (ED50) for all UV doses, the lowest being when irradiated with UVB light. However, rutile showed higher cell viability compared to anatase at the highest intensity of UVB light [51][24]. The polymorphic-dependent toxicity is mainly connected with the potential to generate ROS, which can damage the cell membrane. This conclusion was also reached by Yin et al. Their work showed that the anatase TiO2-NPs are more photoreactive than rutile, being more phototoxic to HaCaT cells [52][25]. However, some studies confirmed that the mixture of rutile/anatase may be more toxic than smaller pure anatase particles [53,54][26][27].
The cytotoxic effect of various polymorphs is also connected with the cellular uptake of NPs. Although the absorption of particles into the cells depends mostly on their diameter, the hydrophilic and hydrophobic surface properties should also be taken under consideration.
4.2. Material Size, Surface, and Morphology
In physics—scale matters. The size of the material determines its properties, especially when nanomaterials are considered. The reason that properties of nanomaterials are quite different from bulk concerns both the surface effects and quantum size effects.
The atoms in the surface of the material have fewer neighbors than atoms in bulk, and thus generate a lower coordination and dangling bonds. This makes those atoms less stable compared to bulk atoms. The smaller the particle, the greater the number of surface atoms; therefore, the surface-to-volume ratio scales with the inverse of size. Moreover, in semiconductors (e.g., TiO2), the electronic wave functions of conductive electrons are delocalized over the entire particle. Electrons are described as “particles in the box” and the density of state and the energy of particles depend crucially on the size of the box, which leads to a smooth-size dependence. Absorption wavelengths, ionization potentials, and electron affinities are tuned between the atomic values and the work function of the bulk material by varying the size of the NPs. These properties are connected to the availability of electrons for redox reactions. Therefore, the photoreactivity depends on the NPs size [55][28].
Considering the influence of the size on the cytotoxic effect, tests using the Sinorhizobium meliloti bacteria exposed to aqueous dispersions of micrometer-sized TiO2 (44 μm) and TiO2-NPs (21 nm) under various concentrations were conducted. A hundred percent mortality of the bacteria was reached using TiO2-NPs at a concentration of 100 mg/L (under UVA irradiation) and 900 mg/L (under dark conditions). However, the exposure to micrometer-sized TiO2 showed that there is no effect on the bacteria in a concentration of less than 300 mg/L (under dark or UVA light irradiation). One hundred percent mortality was only achieved with a nominal concentration of 600 mg/L or more (UVA light irradiated) [56][29]. This result clearly shows that there is a direct correlation between the effect of material size and the toxic effect.
Sanders et al., tested the uptake and accumulation of TiO2-NPs in ARPE-19 cells and showed that after a short-term or long-term exposure, the NPs were observed inside the cells and remained there for an extended period. Moreover, the amount of NPs inside the cells was dose-dependent. However, no clear effects on viability or morphology were observed. The cells that were treated with any of the TiO2-NPs and remained in the dark were 100% viable up to the concentration of 30 μg/mL, except for the sample treated with 31 nm-sized anatase/rutile, which showed a dose-related decrease in viability beginning at 10 μg/mL. Moreover, the tests conducted under visible light illumination showed no difference in viability compared to the dark conditions, which is not surprising as TiO2 cannot be photo-activated under these irradiation conditions. The differences between samples were observed when cells were exposed to UVA radiation. The outcomes showed clearly that the LC50 decreased with the particles size [57][30]. However, the outcomes of Wyrwoll et al., suggested that these results are only valid on a limited number of particle sizes. The authors showed that NPs of 25 nm were more toxic than those of 10 nm. Although the smaller NPs are able to generate a higher amount of ●OH radicals, the sum of all measured ROS (free and surface attached ●OH and O2●− radicals) was the highest for 25 nm-sized NPs [58][31]. These phenomena may be explained by the different mechanisms that remain behind the photoactivity. For the particles smaller than 25 nm, the mechanism depends more on optical and electronic properties, including light absorption, scattering efficiencies, and charge-carrier dynamics. In contrast, for particles larger than 25 nm, the photoactivity depends more on the surface area available for redox reactions [59][32]. Moreover, Wyrwoll et al., confirm that the ionic strength (IS) of the medium highly influences the material toxicity as a high IS promotes material agglomeration and thus sedimentation; therefore, lowering its phototoxicity [58][31].
Another important factor influencing the toxicity of TiO2-NPs is their specific surface area (SSA). As already mentioned, the smaller the particles, the higher the surface/volume ratio. The large surface area of the material allows it to come into close contact with the cells, which is essential for maximizing the damaging effects of ROS, whose lifetime is extremely short. However, particles with the same size may have a different SSA, and therefore produce various photo- and cytotoxicity effects. This effect was tested on RAW264 cells in the absence and presence of light. It was shown that a larger SSA induced higher cyto- and phototoxicity (UV activated) in cells. The increased interactions between the NPs and biomolecules and the greater number of ROS generated contribute to these effects [60][33].
When considering the physical properties of a material that affect its phototoxic potential, the shape of the particles, which influences their interaction with the cell, should be mentioned. TiO2-NPs are usually produced in spherical shapes. However, TiO2 may also exhibit low-dimensional nanostructures, such as one-dimensional nanotubes, nanorods, nanobelts, and nanowires or two-dimensional nanosheets. By measuring the membrane integrity of bacteria, the evaluation of how different morphologies influence the cytotoxicity of TiO2-NPs was assessed. The outcome showed that the toxic effect strongly depends on the shape with the following order: nanospheres > nanorods > nanosheets ≈ nanorods, and thus indicating that low-dimensional materials are less toxic. Using scanning electron microscopy, it was suggested that the material morphology influences TiO2-NPs phototoxicity by governing the alignment of TiO2-NPs at the bacterial cell surface.
The determination of the cytotoxic and phototoxic effects of TiO2 cannot be described as a simple function of the photocatalytic reactivity or ROS production. Rather, the evaluation of TiO2-NPs phototoxicity must encompass a three-pronged approach, involving the intrinsic photoactivity (influenced by, e.g., polymorphic form, particles size, or specific surface area), aggregation of TiO2-NPs (influenced by, e.g., particles size, ionic strength of the medium, or the materials surface charge), and the TiO2-NPs/cell interactions (influenced by, e.g., material morphology, specific surface area, or ability to penetrate the cell).
5. Cytotoxicity as a Response to Photocatalytic Properties of TiO2-NPs
The photocatalytic properties of TiO2-NPs lead to cytotoxicity, thus inducing apoptosis or, in some cases, necrosis of the cell [62,63][34][35]. Necrosis, as premature cell death, can be caused directly by ROS generated by NPs exposed to UV light, which causes damage to the cell membrane. In contrast, apoptosis is a controlled and programmed cell death. In response to cytotoxicity, cells may undergo apoptosis by activating cell signals that cause the cell to break down and die. The mechanism of apoptosis involves a sequence of biochemical events that results in the activation of proteases, such as caspases, that cleave the cell proteins and DNA, leading to cell death. The cell may be induced to initiate apoptosis by an external signal, e.g., in the form of free-oxygen radicals present in its environment [64][36].
As shown, the type of cell death (i.e., by apoptosis or necrosis) depends on the cell line. Among the mechanisms responsible for cell necrosis, the main one seems to be oxidative stress. By reducing the mitochondrial membrane potential and damaging those structures, the cell becomes devoid of its “engine”. An essential role in the regulation of the mitochondrial membrane potential is played by the mitochondrial permeability transition pore (mPTP)—a non-specific channel between the inner and outer membrane of mitochondria [65][37]. The disorder in the form of abnormal opening of the mPTP in response to TiO2-NPs under UVA irradiation was proven for the HeLa cell line (immortalized epithelial cells), confirming that necrosis plays a key role in cell death. Moreover, the researchers suggest that by regulating the ROS-mPTP pathway, it is possible to influence the cell response to TiO2-NPs [66][38].
However, caspase-dependent apoptosis seems to play a major role in the death of HaCaT cells caused by ROS generated by UV-illuminated TiO2-NPs. Exposure of HaCaT cells to those NPs resulted in accumulation in lysosomes via endocytosis or autophagy. Under UVA light, TiO2-NPs damage the lysosomal membrane and subsequently stimulate ROS production. Therefore, it was suggested that lysosomal membrane permeabilization (LMP)-dependent oxidative stress plays a critical role in the UVA phototoxicity of TiO2-NPs in HaCaT cells [67][39]. Nonetheless, not only ROS have been proven to induce NPs toxicity. Ren et al., point out that reactive nitrogen species (RNS) also induce apoptotic signaling and pathologies in intracellular redox homeostasis. The authors observed the effects of TiO2-NPs on cell glycans—structures that play a regulatory role in various physiological processes, such as sialylation. Sialylation is the glycosylation modification playing a key role in cell adhesion, signaling, and recognition, as well as ageing and senescence [68][40]. Using the HaCaT cells, they have proven the changes in sialic acid concentration in the response of ROS generated by UV-illuminated TiO2-NPs, the changes that overall have been reported to cause cancer [69][41]. Among the biochemical mechanisms that can be influenced by TiO2-NPs, Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway may be disrupted by these NPs. JAK/STAT pathway is known to regulate the development and reproduction in many organisms. In the example of Caenorhabditis elegans, the alteration of this pathway was observed, as a response to oxidative stress. Moreover, down-regulation in glutathione activity also appeared. As the JAK/STAT pathway is cross-talked with transforming growth factor beta (TGF-β; the protein that plays an important role in regulating cell growth and extracellular matrix synthesis), the pathway gene expression of this protein was also tested. As with JAK/STAT, significant changes in this gene expression under the influence of TiO2-NPs (under UV) were observed, indicating a significant reproductive toxicity [70][42].
Although the cytotoxicity was proven in cell tests, there is a significant discrepancy between 3D and 2D cultures. In 2D cell culture, NPs are suspended in the cell media. Due to their physicochemical properties, a sedimentation of NPs was observed, as the result of agglomeration, thus increasing the amount of TiO2 at the bottom of culture wells. This increases the cellular uptake resulting in an overestimated toxic effect [71][43]. Moreover, the 2D models do not consider the natural barrier of the skin. Due to the above, many studies have proven that the toxic effects on 2D cell cultures do not reflect in 3D models [50,51][23][24].
A review that brought together 50 papers focusing on in vitro and in vivo toxicity testing of TiO2 suggests that the genotoxic effect of TiO2 cannot be directly confirmed or ignored. The authors point out that more in vitro than in vivo studies were performed, leading to more positive results of genotoxicity in vitro models than in vivo models. Moreover, they conclude that more well-designed in vivo studies must be performed to assess TiO2 genotoxicity [72][44].
Furthermore, a very recent review including 192 articles with 34 robust datasets does not support a direct DNA damaging mechanism for TiO2 in nano- or micro-form [49][22].
6. Might Modifications of TiO2-NPs Surface Decrease Phototoxicity?
The widespread potential use of TiO2 in medicine and cosmetics has led researchers to try to reduce phototoxicity by modifying the particles surface. Most of the modifications involve the introduction of an inert shell on the surface of the NPs. Among the materials used for coating, the most common are silica (SiO2), alumina (Al2O3), zirconium dioxide (ZrO2), or siloxanes [36][9]. However, the main challenge in synthesizing these types of particles with decreased phototoxicity is to suppress the photoactivity with preserved UV-shielding ability. Moreover, due to the potential application of these particles, the shell must be built of inert materials, the role of which is to inhibit the formation of ROS or to scavenge ROS. However, to date, there is no evidence that this method leads to a complete deactivation of the catalytic activity of TiO2-NPs [36,73][9][45].
One of the most promising materials for shelling TiO2-NPs is SiO2, mainly due to its wide bandgap of ca. 9 eV. To date, several trials have been reported in which the properties of TiO2-NPs have been successfully modified [74,75,76,77][46][47][48][49]. Notable is the decrease in photocatalytic activity by 85% relative to the TiO2 particles while preserving 76% of the original UV-scattering ability [75][47]. El-Toni et al., decreased the photocatalytic activity of TiO2-NPs and the UV-shielding ability was not significantly reduced after coating [78][50].
Nevertheless, the nanoparticle coating procedure has led to a change in the mechanism of the potential generation of toxic effects by the newly formed material. Based on the model of fish gill cells, Martin et al., point out that the coating influences NPs ability to be taken up by cells. They examined three different materials: TiO2/SiO2 (core/shell; hydrophilic), TiO2/Al(OH)3/PDMS (core/shell/shell; hydrophobic), and TiO2/Al2O3/stearic acid (core/shell/shell; hydrophobic). The results showed that particles with hydrophilic surfaces were present inside the cells, whereas those with hydrophobic surfaces were not [79][51]. Moreover, considering that NPs can be incorporated into the cells raises questions about their safety [80][52]. Furthermore, Tang et al., showed that NPs coated with silica or alumina exhibited high phototoxic potency that might originate from the interaction of harmful metal ions released from the oxide coating (e.g., Al3+) [81][53]. However, this effect was not confirmed considering the 3D skin model, as the particles could not penetrate the skin barrier.
Reducing the toxicity of TiO2-NPs by coating with a chemically inert material carries the risk of other toxicity mechanisms of the resulting core/shell NPs. At the same time, the lack of comprehensive in vitro and in vivo studies of the newly engineered materials suggests that further research is needed to confirm the safety of coated TiO2-NPs for medicinal applications.
References
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704.
- Samat, M.H.; Ali, A.M.M.; Taib, M.F.M.; Hassan, O.H.; Yahya, M.Z.A. Hubbard U Calculations on Optical Properties of 3d Transition Metal Oxide TiO2. Results Phys. 2016, 6, 891–896.
- Hiroi, Z. Inorganic Structural Chemistry of Titanium Dioxide Polymorphs. Inorg. Chem. 2022, 61, 8393–8401.
- Revision of the Opinion on Titanium Dioxide (Nano Form). Available online: https://health.ec.europa.eu/publications/revision-opinion-titanium-dioxide-nano-form_en (accessed on 27 March 2023).
- Gupta, S.; Tripathi, M. A Review on the Synthesis of TiO2 Nanoparticles by Solution Route. Open Chem. 2012, 10, 279–294.
- Baldassari, S.; Komarneni, S.; Mariani, E.; Villa, C. Microwave-Hydrothermal Process for the Synthesis of Rutile. Mater. Res. Bull. 2005, 40, 2014–2020.
- Hu, Y.; Tsai, H.-L.; Huang, C.-L. Phase Transformation of Precipitated TiO2 Nanoparticles. Mater. Sci. Eng. A 2003, 344, 209–214.
- Dubey, R.S. Temperature-Dependent Phase Transformation of TiO2 Nanoparticles Synthesized by Sol-Gel Method. Mater. Lett. 2018, 215, 312–317.
- Racovita, A.D. Titanium Dioxide: Structure, Impact, and Toxicity. Int. J. Environ. Res. Public Health 2022, 19, 5681.
- Egerton, T. UV-Absorption—The Primary Process in Photocatalysis and Some Practical Consequences. Molecules 2014, 19, 18192–18214.
- Egerton, T.A.; Tooley, I.R. UV Absorption and Scattering Properties of Inorganic-Based Sunscreens: Absorption and Scattering by TiO2. Int. J. Cosmet. Sci. 2012, 34, 117–122.
- Meo, S.D.; Venditti, P.; Victor, V.M.; Napolitano, G. Harmful and Beneficial Role of ROS 2020. Oxid. Med. Cell. Longev. 2022, 2022, 9873652.
- Martínez de Toda, I.; Vida, C.; Sanz San Miguel, L.; De la Fuente, M. Function, Oxidative, and Inflammatory Stress Parameters in Immune Cells as Predictive Markers of Lifespan throughout Aging. Oxid. Med. Cell. Longev. 2019, 2019, e4574276.
- Gojznikar, J.; Zdravković, B.; Vidak, M.; Leskošek, B.; Ferk, P. TiO2 Nanoparticles and Their Effects on Eukaryotic Cells: A Double-Edged Sword. Int. J. Mol. Sci. 2022, 23, 12353.
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14, 6533–6538.
- Bhuvaneshwari, M.; Sagar, B.; Doshi, S.; Chandrasekaran, N.; Mukherjee, A. Comparative Study on Toxicity of ZnO and TiO2 Nanoparticles on Artemia Salina: Effect of Pre-UV-A and Visible Light Irradiation. Environ. Sci. Pollut. Res. 2017, 24, 5633–5646.
- Fenoglio, I.; Ponti, J.; Alloa, E.; Ghiazza, M.; Corazzari, I.; Capomaccio, R.; Rembges, D.; Oliaro-Bosso, S.; Rossi, F. Singlet Oxygen Plays a Key Role in the Toxicity and DNA Damage Caused by Nanometric TiO2 in Human Keratinocytes. Nanoscale 2013, 5, 6567–6576.
- Coral, J.A.; Kitchens, C.L.; Brumaghim, J.L.; Klaine, S.J. Correlating Quantitative Measurements of Radical Production by Photocatalytic TiO2 with Daphnia Magna Toxicity. Environ. Toxicol. Chem. 2021, 40, 1322–1334.
- Cadet, J.; Douki, T.; Ravanat, J.-L. Oxidatively Generated Damage to the Guanine Moiety of DNA: Mechanistic Aspects and Formation in Cells. Acc. Chem. Res. 2008, 41, 1075–1083.
- Vequizo, J.J.M.; Matsunaga, H.; Ishiku, T.; Kamimura, S.; Ohno, T.; Yamakata, A. Trapping-Induced Enhancement of Photocatalytic Activity on Brookite TiO2 Powders: Comparison with Anatase and Rutile TiO2 Powders. ACS Catal. 2017, 7, 2644–2651.
- Buchalska, M.; Kobielusz, M.; Matuszek, A.; Pacia, M.; Wojtyła, S.; Macyk, W. On Oxygen Activation at Rutile- and Anatase-TiO2. ACS Catal. 2015, 5, 7424–7431.
- Kirkland, D.; Aardema, M.J.; Battersby, R.V.; Beevers, C.; Burnett, K.; Burzlaff, A.; Czich, A.; Donner, E.M.; Fowler, P.; Johnston, H.J.; et al. A Weight of Evidence Review of the Genotoxicity of Titanium Dioxide (TiO2). Regul. Toxicol. Pharmacol. 2022, 136, 105263.
- Horie, M.; Sugino, S.; Kato, H.; Tabei, Y.; Nakamura, A.; Yoshida, Y. Does Photocatalytic Activity of TiO2 Nanoparticles Correspond to Photo-Cytotoxicity? Cellular Uptake of TiO2 Nanoparticles Is Important in Their Photo-Cytotoxicity. Toxicol. Mech. Methods 2016, 26, 284–294.
- Hering, H.; Zoschke, C.; König, F.; Kühn, M.; Luch, A.; Schreiver, I. Phototoxic versus Photoprotective Effects of Tattoo Pigments in Reconstructed Human Skin Models: In vitro Phototoxicity Testing of Tattoo Pigments: 3D versus 2D. Toxicology 2021, 460, 152872.
- Yin, J.-J.; Liu, J.; Ehrenshaft, M.; Roberts, J.E.; Fu, P.P.; Mason, R.P.; Zhao, B. Phototoxicity of Nano Titanium Dioxides in HaCaT Keratinocytes—Generation of Reactive Oxygen Species and Cell Damage. Toxicol. Appl. Pharmacol. 2012, 263, 81–88.
- Coleman, H.M.; Marquis, C.P.; Scott, J.A.; Chin, S.-S.; Amal, R. Bactericidal Effects of Titanium Dioxide-Based Photocatalysts. Chem. Eng. J. 2005, 113, 55–63.
- Uchino, T.; Tokunaga, H.; Ando, M.; Utsumi, H. Quantitative Determination of OH Radical Generation and Its Cytotoxicity Induced by TiO2-UVA Treatment. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2002, 16, 629–635.
- Roduner, E. Size Matters: Why Nanomaterials Are Different. Chem. Soc. Rev. 2006, 35, 583–592.
- Wang, J.; Jia, Y.; Whalen, J.K.; McShane, H.; Driscoll, B.T.; Sunahara, G.I. Evidence That Nano-TiO2 Induces Acute Cytotoxicity to the Agronomically Beneficial Nitrogen-Fixing Bacteria Sinorhizobium meliloti. Can. J. Microbiol. 2021, 68, 67–72.
- Sanders, K.; Degn, L.L.; Mundy, W.R.; Zucker, R.M.; Dreher, K.; Zhao, B.; Roberts, J.E.; Boyes, W.K. In vitro Phototoxicity and Hazard Identification of Nano-Scale Titanium Dioxide. Toxicol. Appl. Pharmacol. 2012, 258, 226–236.
- Wyrwoll, A.J.; Lautenschläger, P.; Bach, A.; Hellack, B.; Dybowska, A.; Kuhlbusch, T.A.J.; Hollert, H.; Schäffer, A.; Maes, H.M. Size Matters—The Phototoxicity of TiO2 Nanomaterials. Environ. Pollut. 2016, 208, 859–867.
- Almquist, C.B.; Biswas, P. Role of Synthesis Method and Particle Size of Nanostructured TiO2 on Its Photoactivity. J. Catal. 2002, 212, 145–156.
- Specific Surface Area of Titanium Dioxide (TiO2) Particles Influences Cyto- and Photo-Toxicity—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/23295712/ (accessed on 11 February 2023).
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J.; et al. Acute Toxicity and Biodistribution of Different Sized Titanium Dioxide Particles in Mice after Oral Administration. Toxicol. Lett. 2007, 168, 176–185.
- Abbasi-Oshaghi, E.; Mirzaei, F.; Pourjafar, M. NLRP3 Inflammasome, Oxidative Stress, and Apoptosis Induced in the Intestine and Liver of Rats Treated with Titanium Dioxide Nanoparticles: In vivo and in vitro Study. Int. J. Nanomed. 2019, 14, 1919–1936.
- Xue, C.; Luo, W.; Yang, X.L. A Mechanism for Nano-Titanium Dioxide-Induced Cytotoxicity in HaCaT Cells under UVA Irradiation. Biosci. Biotechnol. Biochem. 2015, 79, 1384–1390.
- Karch, J.; Molkentin, J.D. Identifying the Components of the Elusive Mitochondrial Permeability Transition Pore. Proc. Natl. Acad. Sci. USA 2014, 111, 10396–10397.
- Geng, R.; Ren, Y.; Rao, R.; Tan, X.; Zhou, H.; Yang, X.; Liu, W.; Lu, Q. Titanium Dioxide Nanoparticles Induced HeLa Cell Necrosis under UVA Radiation through the ROS-MPTP Pathway. Nanomaterials 2020, 10, 2029.
- Kim, I.Y.; Lee, T.G.; Reipa, V.; Heo, M.B. Titanium Dioxide Induces Apoptosis under UVA Irradiation via the Generation of Lysosomal Membrane Permeabilization-Dependent Reactive Oxygen Species in HaCat Cells. Nanomaterials 2021, 11, 1943.
- Pinho, S.S.; Reis, C.A. Glycosylation in Cancer: Mechanisms and Clinical Implications. Nat. Rev. Cancer 2015, 15, 540–555.
- Ren, Y.; Liu, X.; Geng, R.; Lu, Q.; Rao, R.; Tan, X.; Yang, X.; Liu, W. Increased Level of A2,6-Sialylated Glycans on HaCaT Cells Induced by Titanium Dioxide Nanoparticles under UV Radiation. Nanomaterials 2018, 8, 253.
- Kim, H.; Jeong, J.; Chatterjee, N.; Roca, C.P.; Yoon, D.; Kim, S.; Kim, Y.; Choi, J. JAK/STAT and TGF-ß Activation as Potential Adverse Outcome Pathway of TiO2NPs Phototoxicity in Caenorhabditis Elegans. Sci. Rep. 2017, 7, 17833.
- Hering, H.; Zoschke, C.; Kühn, M.; Gadicherla, A.K.; Weindl, G.; Luch, A.; Schreiver, I. TatS: A Novel in vitro Tattooed Human Skin Model for Improved Pigment Toxicology Research. Arch. Toxicol. 2020, 94, 2423–2434.
- Wani, M.R.; Shadab, G. Titanium Dioxide Nanoparticle Genotoxicity: A Review of Recent in vivo and in vitro Studies. Toxicol. Ind. Health 2020, 36, 514–530.
- Smijs, T.G.; Pavel, S. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: Focus on Their Safety and Effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112.
- Choi, J.-W.; Kim, J.-Y.; Lee, W.; Lee, W.H.; Jang, H.-D.; Lee, S.B. UV Protection Characteristics and Fabrication of Modified TiO2 Nanoparticles. J. Ind. Eng. Chem. 2004, 10, 428–434.
- Furusawa, T.; Honda, K.; Ukaji, E.; Sato, M.; Suzuki, N. The Microwave Effect on the Properties of Silica-Coated TiO2 Fine Particles Prepared Using Sol–Gel Method. Mater. Res. Bull. 2008, 43, 946–957.
- Park, O.K.; Kang, Y.S. Preparation and Characterization of Silica-Coated TiO2 Nanoparticle. Colloids Surf. Physicochem. Eng. Asp. 2005, 257–258, 261–265.
- Siddiquey, I.A.; Furusawa, T.; Sato, M.; Honda, K.; Suzuki, N. Control of the Photocatalytic Activity of TiO2 Nanoparticles by Silica Coating with Polydiethoxysiloxane. Dye. Pigment. 2008, 76, 754–759.
- El-Toni, A.M.; Yin, S.; Sato, T.; Ghannam, T.; Al-Hoshan, M.; Al-Salhi, M. Investigation of Photocatalytic Activity and UV-Shielding Properties for Silica Coated Titania Nanoparticles by Solvothermal Coating. J. Alloy. Compd. 2010, 508, L1–L4.
- Martin, N.; Wassmur, B.; Slomberg, D.; Labille, J.; Lammel, T. Influence of TiO2 Nanocomposite UV Filter Surface Chemistry and Their Interactions with Organic UV Filters on Uptake and Toxicity toward Cultured Fish Gill Cells. Ecotoxicol. Environ. Saf. 2022, 243, 113984.
- Camaioni, A.; Massimiani, M.; Lacconi, V.; Magrini, A.; Salustri, A.; Sotiriou, G.A.; Singh, D.; Bitounis, D.; Bocca, B.; Pino, A.; et al. Silica Encapsulation of ZnO Nanoparticles Reduces Their Toxicity for Cumulus Cell-Oocyte-Complex Expansion. Part. Fibre Toxicol. 2021, 18, 33.
- Tang, Y.; Cai, R.; Cao, D.; Kong, X.; Lu, Y. Photocatalytic Production of Hydroxyl Radicals by Commercial TiO2 Nanoparticles and Phototoxic Hazard Identification. Toxicology 2018, 406–407, 1–8.
More
