Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Wendy Huang and Version 1 by Victor Enrique Vera-Santander.
Probiotics have been extensively studied within the medical, pharmaceutical, and food fields, as it has been revealed that these microorganisms can provide health benefits from their consumption. Bacterial probiotics comprise species derived from lactic acid bacteria (LAB) (genus Lactobacillus, Leuconostoc, and Streptococcus), the genus Bifidobacterium, and strains of Bacillus and Escherichia coli, among others. The growth of bacterial probiotics is involved in fermentation, which metabolizes the nutrients of the medium and produces a wide variety of metabolic compounds. Probiotics are defined as microorganisms that, when consumed in adequate doses or concentrations, can benefit the consumer’s health.
- probiotic bacteria
- metabolites
- health effects
1. Introduction
The growth of bacterial probiotics is involved in fermentation, which metabolizes the nutrients of the medium and produces a wide variety of metabolic compounds. Metabolites from bacterial probiotics have been studied as they may be the main cause of positive effects on consumer health [1]. Most bacterial probiotics belong to the LAB group, which primarily synthesizes lactic acid from carbohydrate fermentation [2]. Bacterial probiotics excrete secondary metabolites such as organic acids, short-chain fatty acids, enzymes, peptides, teichoic acids, peptidoglycans, exopolysaccharides (EPSs), vitamins, plasmalogens, neurotransmitters, biosurfactants, amino acids, and flavonoid-derived compounds such as desaminotyrosine, equol daidzein, noratirriol, terpenoids, and phenolic compounds, among others [3,4][3][4]. Figure 1 shows a diagram of the health effects that have been studied concerning bacterial probiotic metabolites. Various components have been investigated, including short-chain fatty acids (SCFAs), bacteriocins, enzymes, teichoic acids, EPSs, vitamins, plasmalogens, and biosurfactants.
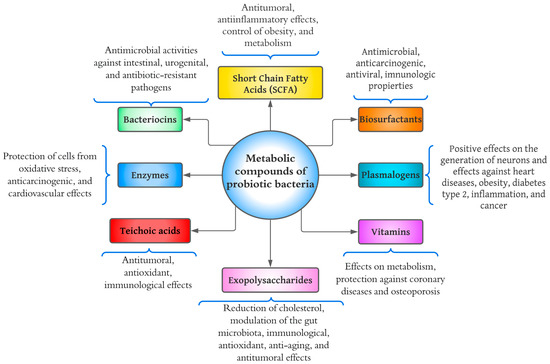
Figure 1.
Diagram of health benefits of metabolic compounds of probiotic bacteria.
2. Short-Chain Fatty Acids
SCFAs are the main metabolites produced by the fermentation of intestinal bacteria; these compounds are produced from the fermentation of non-digestible carbohydrates (prebiotics) by the intestinal microbiota [5]. The SCFAs primarily produced are acetate, propionate, and butyrate. The metabolic pathways of acetate are very diverse and depend on the type of bacteria, while the synthesis of propionate and butyrate depends on a specific substrate [6]. Gill et al. [7] determined that homo-fermentative bacteria synthesize SCFAs from carbohydrate fermentation to produce pyruvate via glycolytic, while heterofermentative bacteria, such as bifidobacterial, do so through the phosphoketolase pathway.
The bacterial probiotics that have been studied are mainly of the genus Lactobacillus and Bifidobacterium. For example, one study investigated the production of SCFA from Bif. longum SP 07/3, Bif. bifidum MF 20/5, L. gasseri PA, 16/8, and L. rhamnosus GG [8]. In the results, the highest production of acetate and propionate was with Bif. bifidum, with a concentration of approximately 125 mM and 130 μM, respectively, while L. rhamnosus GG presented the lowest production of SCFA, with 55 mM of acetate and 100 μM of propionate. SCFAs activate G-specific protein-coupled receptors (GPRs), where GPR41 (known as free fatty acid receptor 3) and GPR43 (free fatty acid receptor 2) have been related to a wide health effect of antitumor and anti-inflammatory benefits on the colon, protection against the development of immune disorders, obesity control, glucose homeostasis control, appetite regulation, and cardiovascular effects [3,7,9][3][7][9]. Gill et al. [7] suggested the consumption of SCFA through the diet as a postbiotic effect, since it has been reported that vinegar and kombucha are the foods with the highest levels of SCFA, with 1015.82 mg of acetate per tablespoon (15 mL) and 1222.95 mg of acetate in a portion of 330 mL, respectively.
In comparison, some cheeses, such as Swiss cheese and blue vein cheese, are high in propionate (109.14 mg) and butyrate (136.54 mg) in a serving of 25 g, respectively [10]. A minimum dose of consumption of SCFAs has not been determined for functional health effects. However, an intervention study tested a beverage with apple cider vinegar containing 25.8 mM of acetate, 0.05 mM of propionate, and 0.04 mM of butyrate. The beverage helped reduce blood pressure, showing potential cardiovascular effects [10]. However, the doses of these compounds and how they can be supplied through food remain to be investigated more thoroughly.
3. Plasmalogens
According to Řezanka et al. [11], plasmalogens are lipid compounds with important functions in organisms such as bacteria, protozoa, invertebrates, and mammals. The largest classes of plasmalogens are glycerophosphoryl ethanolamine and glycerophosphorylcholine with a vinyl ether in the position of glycerol sn-1 instead of a fatty acid. At the position of glycerol sn-2, plasmalogens are enriched with a polyunsaturated fatty acid [12]. Regarding bacterial probiotics, only the production of these compounds with Bif. animalis subsp. lactis has been investigated. This research was conducted by Oberg et al. [13], in which plasmalogens were recognized as endogenous antioxidants induced by hydrogen peroxide (H2O2). The effects caused by these compounds are positively involved in neuro-regeneration and the alleviation of heart disease; it has positive effects against type 2 diabetes, obesity, inflammation, and cancer [12].
Regarding neuro-regeneration, plasmalogens are the main components of neuronal tissue, contributing between 50% and 80% of the total glycerophosphoryl ethanolamines of the gray and white matter of the brain [14]. As proof of this claim, Hossain et al. [15] observed that a daily intake of plasmalogens could improve cognition in Alzheimer’s patients. However, research into these metabolites is still very early, and more research is needed concerning their production in bacterial probiotics and their potential health benefits.
4. Enzymes
Enzymes excreted by microorganisms have a variety of biochemical, physiological, and regulatory functions. As shown in Figure 1, the health benefits of proteases are the protection of cells from oxidative stress and effects against heart disease and cancer [5]. Microorganisms of the genus Bacillus are probably the most important bacterial source of proteases, as they are capable of producing high amounts of neutral and alkaline proteolytic enzymes with remarkable properties, such as high stability at extreme temperatures, pH, organic solvents, detergents, and oxidizing compounds [16]. The proteases (subtilisin and glutamyl endopeptidase) of B. pumilus have been shown to degrade polymeric extracellular components and significantly eradicate the biofilm generated by Serratia marcescens, known as an opportunistic pathogen responsible for several hospital-acquired infections [17]. Although enzymes have been widely studied in science, more research is still needed regarding their effects in the human body.
5. Bacteriocins
Bacteriocins are polypeptides and proteins that form pores in bacterial membranes and inhibit cell wall synthesis [3]. Currently, there are five classes of bacteriocins, as follows:
-
Class I: Small proteolytic and heat-resistant peptides substantially modified by transcriptionally specific enzymes. Examples: lantibiotics (nisin), sactipeptide, and loop peptides [18].
-
Class II: Divided into four subtypes that are (1) pediocin-like, (2) two peptides, (3) circular, and (4) linear, not pediocin-like. They comprise small peptides resistant to temperature and pH [18].
-
Class III: Large thermolabile peptides (>30 kDa) with complex activity and structure. This group includes helveticin, acidophylline, and lactacins (A and B) [19].
-
Class V: Peptides with circular structures without post-translational modifications, including enterokine AS-48 and gasericin A [19].
Nisin is one of the most studied and used bacteriocins in food science and industry. This bacteriocin is produced by Lactococcus. lactis subsp. lactis and is approved by the FDA as a Generally Recognized as Safe Additive (“GRAS”) [20]. The antimicrobial spectrum of nisin is broad and can inhibit the growth of Staphylococcus aureus, Cutibacterium acnes, Mycobacterium smegmatis, and strains of Bacillus, Clostridium, Enterococcus, Mycobacterium, and Streptococcus [21]. Furthermore, bacteriocins from Enterococcus faecalis and L. casei have been shown to be effective against urogenital, intestinal, and antibiotic-resistant pathogens [22,23][22][23].
6. Exopolysaccharides (EPSs)
EPSs are biopolymers produced by microorganisms during their growth. They vary according to the degree of branching from linear to highly branched molecules [24]. EPSs are divided into homopolysaccharides and heteropolysaccharides. Homopolysaccharides are composed of monosaccharides identical in their structure; for example, starch and cellulose. In comparison, heteropolysaccharides consist of different monosaccharides such as xanthan gum, pectin, and galactomannans, among others [25]. The probiotics that have been studied reagrding EPSs belong to the genus Lactobacillus. In a study by Xu et al. [26], they presented that EPSs produced by L. buchneri TCP016 (doses of 200–800 mg) were able to reduce liver damage by regulating the intestinal microbiota, since EPSs reduced the growth of Helicobacteraceae, Lachnospiraceae, and Enterobacteriaceae, but increased the proliferation of Lactobacillus, Rikenellaceae, Bacteroidaceae, and Prevotellaceae. In another study, Wang et al. [27] studied the EPS from L. fermentum S1 by applying doses of 1–4 mg/mL; these EPS showed potent antioxidant activities and prevented the formation of biofilms of E. coli and S. aureus. In addition, EPS from L. casei SB27 showed an increase in the concentration of these compounds (600 μg/mL), and antitumor effects in vitro were increased [28]. EPSs have demonstrated various health effects such as antioxidants, cholesterol-lowering effects, immunity, anti-aging, modulation of the intestinal microbiota, and antitumor [26,27,28,29][26][27][28][29].
7. Teichoic Acids
According to Van der Es et al. [30], teichoic acids are composed of anionic glycopolymers (ribitol) with repeated units of polyols attached to phodiester. There are two types of teichoic acids: Lipoteichoic Acids (LTAs), which are anchored to the membrane by a glycolipid, and Wall Teichoic Acids (WTAs), which are covalently bound to peptidoglycan [31]. Teichoic acids have important roles in bacteria, such as determining cell shape, regulating cell division, and providing vital metabolic aspects for cell physiology, and may confer pathogenesis and antibiotic resistance of Gram-positive bacteria [3]. The most studied teichoic acids in science are LTAs, due to their immune, antitumor, and antioxidant functional properties [32]. For example, several studies have shown that LTAs from L. delbrueckii, L. sakei, L. rhamnosus GG, and L. plantarum K8 have potential anti-inflammatory effects [33,34,35][33][34][35]. Wang et al. [36] studied the LTAs of L. paracasei D3–5 using a dose of 200 mg /kg per day. Their results showed that the compounds benefited the physical, cognitive, and anti-inflammatory functions in mice.
8. Vitamins
Vitamins are essential in the diet in small amounts to facilitate various body biological processes. LeBlanc et al. [37] noted that these compounds are important in regulating biochemical reactions in the cell, since some participate as precursors of intracellular coenzymes. Currently, thirteen vitamins are known as essential to human health, and these are classified as fat-soluble (vitamins A, D, E, and K) and water-soluble (vitamin C and vitamins of the B complex) [37]. The human body cannot synthesize most vitamins; therefore, consuming them externally through food is an excellent alternative. Bacterial probiotics can synthesize a large amount of vitamins, such as vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B9 (folate), vitamin B12 (cobalamin), and vitamin K [5,3][3][5]. As can be seen, the B complex vitamins are the most produced by bacterial probiotics; production is important, since the B vitamins act in synergy in the body’s homeostasis by participating in metabolic processes, such as energy generation and the synthesis of red blood cells [37]. Deptula et al. [38] noted that vitamin B12 produced through microbial fermentation is preferable to chemical synthesis. Nataraj et al. [3] argued that using microorganisms in vitamin production is more economically feasible than fortifying with chemically synthesized pseudo-vitamins. Bacterial probiotics such as L. brevis, L. fermentum, L. reuteri, and L. salivarius have complete genes (ribA, ribB, ribG, and ribH) for riboflavin synthesis [39]. Bardosono et al. [40] conducted a clinical trial consisting of Bif. animalis subsp. lactis HNO19 supplementation in pregnant women. Their results showed that probiotic supplementation increased the concentration of vitamin B6 in the blood and vitamin B12 in the second and third trimesters, respectively. Therefore, consuming probiotics or foods with probiotics is vital to staying healthy, as vitamins can also be synthesized in the digestive tract.
9. Biosurfactants
Biosurfactants represent a wide diversity of polymers synthesized during the early and late logarithmic stationary phases of a microorganism’s growth cycle [41]. Biosurfactants consist of glycolipids, lipopeptides, phospholipids, neutral lipids, protein-polysaccharide complexes, and free fatty acids [3]. The species of Lactobacillus are the most studied concerning biosurfactants. The biosurfactants (methylpentadecanoic acid and eicosanoic acid) of L. jensenii P6A and L. gasseri P6 have shown antimicrobial properties against several pathogens, such as E. coli, Candida albicans, Staphylococcus saprophyticus, Enterobacter aerogenes, and Klebsiella pneumonia [42]. Merghni et al. [43] showed that the biosurfactants of L. casei ATCC 393 have antioxidant and antimicrobial properties against S. aureus (present in the oral cavity), suggesting that biosurfactants could have a significant role in the prevention of oral diseases. Fracchia et al. [44] noted that biosurfactants exhibit a wide variety of properties such as emulsion stabilization, anti-adhesion capacity, anti-biofilm, anticancer, antiviral, immunological, and antimicrobial.
References
- Aguilar-Toalá, J. E.; Garcia-Varela, R.; Garcia, H. S.; Mata-Haro, V.; González-Córdova, A. F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. https://doi.org/10.1016/j.tifs.2018.03.009.
- Stiles, M. E. Biopreservation by Lactic Acid Bacteria. Antonie Van Leeuwenhoek 1996, 70 (2–4), 331–345. https://doi.org/10.1007/BF00395940.
- Nataraj, B. H.; Ali, S. A.; Behare, P. V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Cell Factories 2020, 19 (1), 168. https://doi.org/10.1186/s12934-020-01426-w.
- Erginkaya, Z.; Konuray-Altun, G. Potential Biotherapeutic Properties of Lactic Acid Bacteria in Foods. Food Biosci. 2022, 46, 101544. https://doi.org/10.1016/j.fbio.2022.101544.
- Hernández-Granados, M. J.; Franco-Robles, E. Postbiotics in Human Health: Possible New Functional Ingredients? Food Res. Int. 2020, 137, 109660. https://doi.org/10.1016/j.foodres.2020.109660.
- Morrison, D. J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7 (3), 189–200. https://doi.org/10.1080/19490976.2015.1134082.
- Gill, P. A.; van Zelm, M. C.; Muir, J. G.; Gibson, P. R. Review Article: Short Chain Fatty Acids as Potential Therapeutic Agents in Human Gastrointestinal and Inflammatory Disorders. Pharmacol. Ther. 2018, 48 (1), 15–34. https://doi.org/10.1111/apt.14689.
- LeBlanc, J. G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L. G.; Courau, S.; Langella, P. Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria. Cell Factories 2017, 16 (1), 79. https://doi.org/10.1186/s12934-017-0691-z.
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; Parmentier, M.; Detheux, M. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. Biol. Chem. 2003, 278 (28), 25481–25489. https://doi.org/10.1074/jbc.M301403200.
- Gill, P. A.; Bogatyrev, A.; Zelm, M. C.; Gibson, P. R.; Muir, J. G. Delivery of Acetate to the Peripheral Blood after Consumption of Foods High in Short‐Chain Fatty Acids. Nutr. Food Res. 2021, 65 (4), 2000953. https://doi.org/10.1002/mnfr.202000953.
- Řezanka, T.; Křesinová, Z.; Kolouchová, I.; Sigler, K. Lipidomic Analysis of Bacterial Plasmalogens. Folia Microbiol. (Praha) 2012, 57 (5), 463–472. https://doi.org/10.1007/s12223-012-0178-6.
- Wallner, S.; Schmitz, G. Plasmalogens the Neglected Regulatory and Scavenging Lipid Species. Phys. Lipids 2011, 164 (6), 573–589. https://doi.org/10.1016/j.chemphyslip.2011.06.008.
- Oberg, T. S.; Ward, R. E.; Steele, J. L.; Broadbent, J. R. Identification of Plasmalogens in the Cytoplasmic Membrane of Bifidobacterium Animalis Subsp. Lactis. Environ. Microbiol. 2012, 78 (3), 880–884. https://doi.org/10.1128/AEM.06968-11.
- Han, X.; Holtzman, D. M.; McKeel, D. W. Plasmalogen Deficiency in Early Alzheimer’s Disease Subjects and in Animal Models: Molecular Characterization Using Electrospray Ionization Mass Spectrometry: Plasmalogen Deficiency in Alzheimer’s Disease. Neurochem. 2001, 77 (4), 1168–1180. https://doi.org/10.1046/j.1471-4159.2001.00332.x.
- Hossain, Md. S.; Tajima, A.; Kotoura, S.; Katafuchi, T. Oral Ingestion of Plasmalogens Can Attenuate the LPS-Induced Memory Loss and Microglial Activation. Biophys. Res. Commun. 2018, 496 (4), 1033–1039. https://doi.org/10.1016/j.bbrc.2018.01.078.
- Contesini, F. J.; Melo, R. R. de; Sato, H. H. An Overview of Bacillus Proteases: From Production to Application. Rev. Biotechnol. 2018, 38 (3), 321–334. https://doi.org/10.1080/07388551.2017.1354354.
- Mitrofanova, O.; Mardanova, A.; Evtugyn, V.; Bogomolnaya, L.; Sharipova, M. Effects of Bacillus Serine Proteases on the Bacterial Biofilms. BioMed Res. Int. 2017, 2017, 1–10. https://doi.org/10.1155/2017/8525912.
- Hols, P.; Ledesma-García, L.; Gabant, P.; Mignolet, J. Mobilization of Microbiota Commensals and Their Bacteriocins for Therapeutics. Trends Microbiol. 2019, 27 (8), 690–702. https://doi.org/10.1016/j.tim.2019.03.007.
- Preciado, G. M.; EscalanteMinakata, P.; Castro, J. A. O.; Junquera, V. I.; Chávez, J. A. M.; González, C. N. A.; Herrera, R. R. Bacteriocinas: características y aplicación en alimentos. 2013, 8.
- Makhal, S.; Kanawjia, S. K.; Giri, A. Effect of MicroGARD on Keeping Quality of Direct Acidified Cottage Cheese. Food Sci. Technol. 2015, 52 (2), 936–943. https://doi.org/10.1007/s13197-013-1055-2.
- Shin, J. M.; Gwak, J. W.; Kamarajan, P.; Fenno, J. C.; Rickard, A. H.; Kapila, Y. L. Biomedical Applications of Nisin. Appl. Microbiol. 2016, 120 (6), 1449–1465. https://doi.org/10.1111/jam.13033.
- Abanoz, H. S.; Kunduhoglu, B. Antimicrobial Activity of a Bacteriocin Produced by Enterococcus Faecalis KT11 against Some Pathogens and Antibiotic-Resistant Bacteria. Korean J. Food Sci. Anim. Resour. 2018, 38 (5), 1064–1079. https://doi.org/10.5851/kosfa.2018.e40.
- Hasan, F. B.; Reza, M.; Masud, H. A. A.; Uddin, M. K.; Uddin, M. S. Preliminary Characterization and Inhibitory Activity of Bacteriocin like Substances from Lactobacillus Casei against Multi-Drug Resistant Bacteria. Bangladesh J. Microbiol. 2019, 36 (1), 1–6. https://doi.org/10.3329/bjm.v36i1.44259.
- Welman, A. D.; Maddox, I. S. Exopolysaccharides from Lactic Acid Bacteria: Perspectives and Challenges. Trends Biotechnol. 2003, 21 (6), 269–274. https://doi.org/10.1016/S0167-7799(03)00107-0.
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of Lactic Acid Bacteria: Structure, Bioactivity and Associations: A Review. Polym. 2019, 207, 317–332. https://doi.org/10.1016/j.carbpol.2018.11.093.
- Xu, R.; Aruhan; Xiu, L.; Sheng, S.; Liang, Y.; Zhang, H.; Liu, Y.; Tong, H.; Du, R.; Wang, X. Exopolysaccharides from Lactobacillus Buchneri TCP016 Attenuate LPS- and d -GalN-Induced Liver Injury by Modulating the Gut Microbiota. Agric. Food Chem. 2019, 67 (42), 11627–11637. https://doi.org/10.1021/acs.jafc.9b04323.
- Wang, K.; Niu, M.; Song, D.; Song, X.; Zhao, J.; Wu, Y.; Lu, B.; Niu, G. Preparation, Partial Characterization and Biological Activity of Exopolysaccharides Produced from Lactobacillus Fermentum S1. Biosci. Bioeng. 2020, 129 (2), 206–214. https://doi.org/10.1016/j.jbiosc.2019.07.009.
- Di, W.; Zhang, L.; Wang, S.; Yi, H.; Han, X.; Fan, R.; Zhang, Y. Physicochemical Characterization and Antitumour Activity of Exopolysaccharides Produced by Lactobacillus Casei SB27 from Yak Milk. Polym. 2017, 171, 307–315. https://doi.org/10.1016/j.carbpol.2017.03.018.
- Guo, Y.; Pan, D.; Li, H.; Sun, Y.; Zeng, X.; Yan, B. Antioxidant and Immunomodulatory Activity of Selenium Exopolysaccharide Produced by Lactococcus Lactis Subsp. Lactis. Food Chem. 2013, 138 (1), 84–89. https://doi.org/10.1016/j.foodchem.2012.10.029.
- Van der Es, D.; Hogendorf, W. F. J.; Overkleeft, H. S.; van der Marel, G. A.; Codée, J. D. C. Teichoic Acids: Synthesis and Applications. Soc. Rev. 2017, 46 (5), 1464–1482. https://doi.org/10.1039/C6CS00270F.
- Brown, S.; Santa Maria, J. P.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Rev. Microbiol. 2013, 67 (1), 313–336. https://doi.org/10.1146/annurev-micro-092412-155620.
- Lebeer, S.; Claes, I.; Tytgat, H. L. P.; Verhoeven, T. L. A.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; de Vos, W. M.; De Keersmaecker, S. C. J.; Vanderleyden, J. Functional Analysis of Lactobacillus Rhamnosus GG Pili in Relation to Adhesion and Immunomodulatory Interactions with Intestinal Epithelial Cells. Environ. Microbiol. 2012, 78 (1), 185–193. https://doi.org/10.1128/AEM.06192-11.
- Kim, K. W.; Kang, S.-S.; Woo, S.-J.; Park, O.-J.; Ahn, K. B.; Song, K.-D.; Lee, H.-K.; Yun, C.-H.; Han, S. H. Lipoteichoic Acid of Probiotic Lactobacillus Plantarum Attenuates Poly I:C-Induced IL-8 Production in Porcine Intestinal Epithelial Cells. Microbiol. 2017, 8, 1827. https://doi.org/10.3389/fmicb.2017.01827.
- Claes, I. J.; Segers, M. E.; Verhoeven, T. L.; Dusselier, M.; Sels, B. F.; De Keersmaecker, S. C.; Vanderleyden, J.; Lebeer, S. Lipoteichoic Acid Is an Important Microbe-Associated Molecular Pattern of Lactobacillus Rhamnosus GG. Cell Factories 2012, 11 (1), 161. https://doi.org/10.1186/1475-2859-11-161.
- Ahn, J. E.; Kim, H.; Chung, D. K. Lipoteichoic Acid Isolated from Lactobacillus Plantarum Maintains Inflammatory Homeostasis through Regulation of Th1- and Th2- Induced Cytokines. Microbiol. Biotechnol. 2019, 29 (1), 151–159. https://doi.org/10.4014/jmb.1809.09001.
- Wang, S.; Ahmadi, S.; Nagpal, R.; Jain, S.; Mishra, S. P.; Kavanagh, K.; Zhu, X.; Wang, Z.; McClain, D. A.; Kritchevsky, S. B.; Kitzman, D. W.; Yadav, H. Lipoteichoic Acid from the Cell Wall of a Heat Killed Lactobacillus Paracasei D3-5 Ameliorates Aging-Related Leaky Gut, Inflammation and Improves Physical and Cognitive Functions: From C. Elegans to Mice. GeroScience 2020, 42 (1), 333–352. https://doi.org/10.1007/s11357-019-00137-4.
- LeBlanc, J. G.; Laiño, J. E.; del Valle, M. J.; de Giori, G. S.; Sesma, F.; Taranto, M. P. B-Group Vitamins Production by Probiotic Lactic Acid Bacteria. In Biotechnology of Lactic Acid Bacteria; Mozzi, F., Raya, R. R., Vignolo, G. M., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2015; pp 279–296. https://doi.org/10.1002/9781118868386.ch17.
- Deptula, P.; Chamlagain, B.; Edelmann, M.; Sangsuwan, P.; Nyman, T. A.; Savijoki, K.; Piironen, V.; Varmanen, P. Food-Like Growth Conditions Support Production of Active Vitamin B12 by Propionibacterium Freudenreichii 2067 without DMBI, the Lower Ligand Base, or Cobalt Supplementation. Microbiol. 2017, 8. https://doi.org/10.3389/fmicb.2017.00368.
- Thakur, K.; Lule, V.; Kumar, N.; Mandal, S.; Anand, S.; Kumari, V.; Tomar, S. Riboflavin Producing Probiotic Lactobacilli as a Biotechnological Strategy to Obtain Riboflavin-Enriched Fermented Foods. Pure Appl. Microbiol. 2016, 10, 161–166.
- Bardosono, S.; Wibowo, N.; Sutanto, L. B.; Irwinda, R.; Cannan, R.; Rowan, A.; Dekker, J. Plasma Folate, Vitamin B6 and B12 in Their Relationship to the Presence of Probiotic Strain Bifidobacterium Animalis Subsp. Lactis HNO19 (DR10TM) Among Indonesian Pregnant Women in Their Third Semester. World Nutr. J. 2019, 2 (2), 56. https://doi.org/10.25220/WNJ.V02.i2.0009.
- Satpute, S. K.; Kulkarni, G. R.; Banpurkar, A. G.; Banat, I. M.; Mone, N. S.; Patil, R. H.; Cameotra, S. S. Biosurfactant/s from Lactobacilli Species: Properties, Challenges and Potential Biomedical Applications: Biosurfactant/s from Lactobacilli Species. Basic Microbiol. 2016, 56 (11), 1140–1158. https://doi.org/10.1002/jobm.201600143.
- Morais, I. M. C.; Cordeiro, A. L.; Teixeira, G. S.; Domingues, V. S.; Nardi, R. M. D.; Monteiro, A. S.; Alves, R. J.; Siqueira, E. P.; Santos, V. L. Biological and Physicochemical Properties of Biosurfactants Produced by Lactobacillus Jensenii P6A and Lactobacillus Gasseri P65. Cell Factories 2017, 16 (1), 155. https://doi.org/10.1186/s12934-017-0769-7.
- Merghni, A.; Dallel, I.; Noumi, E.; Kadmi, Y.; Hentati, H.; Tobji, S.; Ben Amor, A.; Mastouri, M. Antioxidant and Antiproliferative Potential of Biosurfactants Isolated from Lactobacillus Casei and Their Anti-Biofilm Effect in Oral Staphylococcus Aureus Strains. Pathog. 2017, 104, 84–89. https://doi.org/10.1016/j.micpath.2017.01.017.
- Fracchia, L.; J. Banat, J.; Cavallo, M.; Ceresa, C.; M. Banat, I.; 1 Department of Pharmaceutical Sciences, Università del Piemonte Orientale “A. Avogadro”, Largo Donegani 2, 28100, Novara, Italy; Potential Therapeutic Applications of Microbial Surface-Active Compounds. AIMS Bioeng. 2015, 2 (3), 144–162. https://doi.org/10.3934/bioeng.2015.3.144
References
- Aguilar-Toalá, J. E.; Garcia-Varela, R.; Garcia, H. S.; Mata-Haro, V.; González-Córdova, A. F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. https://doi.org/10.1016/j.tifs.2018.03.009.
- Stiles, M. E. Biopreservation by Lactic Acid Bacteria. Antonie Van Leeuwenhoek 1996, 70 (2–4), 331–345. https://doi.org/10.1007/BF00395940.
- Nataraj, B. H.; Ali, S. A.; Behare, P. V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Cell Factories 2020, 19 (1), 168. https://doi.org/10.1186/s12934-020-01426-w.
- Erginkaya, Z.; Konuray-Altun, G. Potential Biotherapeutic Properties of Lactic Acid Bacteria in Foods. Food Biosci. 2022, 46, 101544. https://doi.org/10.1016/j.fbio.2022.101544.
- Hernández-Granados, M. J.; Franco-Robles, E. Postbiotics in Human Health: Possible New Functional Ingredients? Food Res. Int. 2020, 137, 109660. https://doi.org/10.1016/j.foodres.2020.109660.
- Morrison, D. J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7 (3), 189–200. https://doi.org/10.1080/19490976.2015.1134082.
- Gill, P. A.; van Zelm, M. C.; Muir, J. G.; Gibson, P. R. Review Article: Short Chain Fatty Acids as Potential Therapeutic Agents in Human Gastrointestinal and Inflammatory Disorders. Pharmacol. Ther. 2018, 48 (1), 15–34. https://doi.org/10.1111/apt.14689.
- LeBlanc, J. G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L. G.; Courau, S.; Langella, P. Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria. Cell Factories 2017, 16 (1), 79. https://doi.org/10.1186/s12934-017-0691-z.
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; Parmentier, M.; Detheux, M. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. Biol. Chem. 2003, 278 (28), 25481–25489. https://doi.org/10.1074/jbc.M301403200.
- Gill, P. A.; Bogatyrev, A.; Zelm, M. C.; Gibson, P. R.; Muir, J. G. Delivery of Acetate to the Peripheral Blood after Consumption of Foods High in Short‐Chain Fatty Acids. Nutr. Food Res. 2021, 65 (4), 2000953. https://doi.org/10.1002/mnfr.202000953.
- Řezanka, T.; Křesinová, Z.; Kolouchová, I.; Sigler, K. Lipidomic Analysis of Bacterial Plasmalogens. Folia Microbiol. (Praha) 2012, 57 (5), 463–472. https://doi.org/10.1007/s12223-012-0178-6.
- Wallner, S.; Schmitz, G. Plasmalogens the Neglected Regulatory and Scavenging Lipid Species. Phys. Lipids 2011, 164 (6), 573–589. https://doi.org/10.1016/j.chemphyslip.2011.06.008.
- Oberg, T. S.; Ward, R. E.; Steele, J. L.; Broadbent, J. R. Identification of Plasmalogens in the Cytoplasmic Membrane of Bifidobacterium Animalis Subsp. Lactis. Environ. Microbiol. 2012, 78 (3), 880–884. https://doi.org/10.1128/AEM.06968-11.
- Han, X.; Holtzman, D. M.; McKeel, D. W. Plasmalogen Deficiency in Early Alzheimer’s Disease Subjects and in Animal Models: Molecular Characterization Using Electrospray Ionization Mass Spectrometry: Plasmalogen Deficiency in Alzheimer’s Disease. Neurochem. 2001, 77 (4), 1168–1180. https://doi.org/10.1046/j.1471-4159.2001.00332.x.
- Hossain, Md. S.; Tajima, A.; Kotoura, S.; Katafuchi, T. Oral Ingestion of Plasmalogens Can Attenuate the LPS-Induced Memory Loss and Microglial Activation. Biophys. Res. Commun. 2018, 496 (4), 1033–1039. https://doi.org/10.1016/j.bbrc.2018.01.078.
- Contesini, F. J.; Melo, R. R. de; Sato, H. H. An Overview of Bacillus Proteases: From Production to Application. Rev. Biotechnol. 2018, 38 (3), 321–334. https://doi.org/10.1080/07388551.2017.1354354.
- Mitrofanova, O.; Mardanova, A.; Evtugyn, V.; Bogomolnaya, L.; Sharipova, M. Effects of Bacillus Serine Proteases on the Bacterial Biofilms. BioMed Res. Int. 2017, 2017, 1–10. https://doi.org/10.1155/2017/8525912.
- Hols, P.; Ledesma-García, L.; Gabant, P.; Mignolet, J. Mobilization of Microbiota Commensals and Their Bacteriocins for Therapeutics. Trends Microbiol. 2019, 27 (8), 690–702. https://doi.org/10.1016/j.tim.2019.03.007.
- Preciado, G. M.; EscalanteMinakata, P.; Castro, J. A. O.; Junquera, V. I.; Chávez, J. A. M.; González, C. N. A.; Herrera, R. R. Bacteriocinas: características y aplicación en alimentos. 2013, 8.
- Makhal, S.; Kanawjia, S. K.; Giri, A. Effect of MicroGARD on Keeping Quality of Direct Acidified Cottage Cheese. Food Sci. Technol. 2015, 52 (2), 936–943. https://doi.org/10.1007/s13197-013-1055-2.
- Shin, J. M.; Gwak, J. W.; Kamarajan, P.; Fenno, J. C.; Rickard, A. H.; Kapila, Y. L. Biomedical Applications of Nisin. Appl. Microbiol. 2016, 120 (6), 1449–1465. https://doi.org/10.1111/jam.13033.
- Abanoz, H. S.; Kunduhoglu, B. Antimicrobial Activity of a Bacteriocin Produced by Enterococcus Faecalis KT11 against Some Pathogens and Antibiotic-Resistant Bacteria. Korean J. Food Sci. Anim. Resour. 2018, 38 (5), 1064–1079. https://doi.org/10.5851/kosfa.2018.e40.
- Hasan, F. B.; Reza, M.; Masud, H. A. A.; Uddin, M. K.; Uddin, M. S. Preliminary Characterization and Inhibitory Activity of Bacteriocin like Substances from Lactobacillus Casei against Multi-Drug Resistant Bacteria. Bangladesh J. Microbiol. 2019, 36 (1), 1–6. https://doi.org/10.3329/bjm.v36i1.44259.
- Welman, A. D.; Maddox, I. S. Exopolysaccharides from Lactic Acid Bacteria: Perspectives and Challenges. Trends Biotechnol. 2003, 21 (6), 269–274. https://doi.org/10.1016/S0167-7799(03)00107-0.
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of Lactic Acid Bacteria: Structure, Bioactivity and Associations: A Review. Polym. 2019, 207, 317–332. https://doi.org/10.1016/j.carbpol.2018.11.093.
- Xu, R.; Aruhan; Xiu, L.; Sheng, S.; Liang, Y.; Zhang, H.; Liu, Y.; Tong, H.; Du, R.; Wang, X. Exopolysaccharides from Lactobacillus Buchneri TCP016 Attenuate LPS- and d -GalN-Induced Liver Injury by Modulating the Gut Microbiota. Agric. Food Chem. 2019, 67 (42), 11627–11637. https://doi.org/10.1021/acs.jafc.9b04323.
- Wang, K.; Niu, M.; Song, D.; Song, X.; Zhao, J.; Wu, Y.; Lu, B.; Niu, G. Preparation, Partial Characterization and Biological Activity of Exopolysaccharides Produced from Lactobacillus Fermentum S1. Biosci. Bioeng. 2020, 129 (2), 206–214. https://doi.org/10.1016/j.jbiosc.2019.07.009.
- Di, W.; Zhang, L.; Wang, S.; Yi, H.; Han, X.; Fan, R.; Zhang, Y. Physicochemical Characterization and Antitumour Activity of Exopolysaccharides Produced by Lactobacillus Casei SB27 from Yak Milk. Polym. 2017, 171, 307–315. https://doi.org/10.1016/j.carbpol.2017.03.018.
- Guo, Y.; Pan, D.; Li, H.; Sun, Y.; Zeng, X.; Yan, B. Antioxidant and Immunomodulatory Activity of Selenium Exopolysaccharide Produced by Lactococcus Lactis Subsp. Lactis. Food Chem. 2013, 138 (1), 84–89. https://doi.org/10.1016/j.foodchem.2012.10.029.
- Van der Es, D.; Hogendorf, W. F. J.; Overkleeft, H. S.; van der Marel, G. A.; Codée, J. D. C. Teichoic Acids: Synthesis and Applications. Soc. Rev. 2017, 46 (5), 1464–1482. https://doi.org/10.1039/C6CS00270F.
- Brown, S.; Santa Maria, J. P.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Rev. Microbiol. 2013, 67 (1), 313–336. https://doi.org/10.1146/annurev-micro-092412-155620.
- Lebeer, S.; Claes, I.; Tytgat, H. L. P.; Verhoeven, T. L. A.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; de Vos, W. M.; De Keersmaecker, S. C. J.; Vanderleyden, J. Functional Analysis of Lactobacillus Rhamnosus GG Pili in Relation to Adhesion and Immunomodulatory Interactions with Intestinal Epithelial Cells. Environ. Microbiol. 2012, 78 (1), 185–193. https://doi.org/10.1128/AEM.06192-11.
- Kim, K. W.; Kang, S.-S.; Woo, S.-J.; Park, O.-J.; Ahn, K. B.; Song, K.-D.; Lee, H.-K.; Yun, C.-H.; Han, S. H. Lipoteichoic Acid of Probiotic Lactobacillus Plantarum Attenuates Poly I:C-Induced IL-8 Production in Porcine Intestinal Epithelial Cells. Microbiol. 2017, 8, 1827. https://doi.org/10.3389/fmicb.2017.01827.
- Claes, I. J.; Segers, M. E.; Verhoeven, T. L.; Dusselier, M.; Sels, B. F.; De Keersmaecker, S. C.; Vanderleyden, J.; Lebeer, S. Lipoteichoic Acid Is an Important Microbe-Associated Molecular Pattern of Lactobacillus Rhamnosus GG. Cell Factories 2012, 11 (1), 161. https://doi.org/10.1186/1475-2859-11-161.
- Ahn, J. E.; Kim, H.; Chung, D. K. Lipoteichoic Acid Isolated from Lactobacillus Plantarum Maintains Inflammatory Homeostasis through Regulation of Th1- and Th2- Induced Cytokines. Microbiol. Biotechnol. 2019, 29 (1), 151–159. https://doi.org/10.4014/jmb.1809.09001.
- Wang, S.; Ahmadi, S.; Nagpal, R.; Jain, S.; Mishra, S. P.; Kavanagh, K.; Zhu, X.; Wang, Z.; McClain, D. A.; Kritchevsky, S. B.; Kitzman, D. W.; Yadav, H. Lipoteichoic Acid from the Cell Wall of a Heat Killed Lactobacillus Paracasei D3-5 Ameliorates Aging-Related Leaky Gut, Inflammation and Improves Physical and Cognitive Functions: From C. Elegans to Mice. GeroScience 2020, 42 (1), 333–352. https://doi.org/10.1007/s11357-019-00137-4.
- LeBlanc, J. G.; Laiño, J. E.; del Valle, M. J.; de Giori, G. S.; Sesma, F.; Taranto, M. P. B-Group Vitamins Production by Probiotic Lactic Acid Bacteria. In Biotechnology of Lactic Acid Bacteria; Mozzi, F., Raya, R. R., Vignolo, G. M., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2015; pp 279–296. https://doi.org/10.1002/9781118868386.ch17.
- Deptula, P.; Chamlagain, B.; Edelmann, M.; Sangsuwan, P.; Nyman, T. A.; Savijoki, K.; Piironen, V.; Varmanen, P. Food-Like Growth Conditions Support Production of Active Vitamin B12 by Propionibacterium Freudenreichii 2067 without DMBI, the Lower Ligand Base, or Cobalt Supplementation. Microbiol. 2017, 8. https://doi.org/10.3389/fmicb.2017.00368.
- Thakur, K.; Lule, V.; Kumar, N.; Mandal, S.; Anand, S.; Kumari, V.; Tomar, S. Riboflavin Producing Probiotic Lactobacilli as a Biotechnological Strategy to Obtain Riboflavin-Enriched Fermented Foods. Pure Appl. Microbiol. 2016, 10, 161–166.
- Bardosono, S.; Wibowo, N.; Sutanto, L. B.; Irwinda, R.; Cannan, R.; Rowan, A.; Dekker, J. Plasma Folate, Vitamin B6 and B12 in Their Relationship to the Presence of Probiotic Strain Bifidobacterium Animalis Subsp. Lactis HNO19 (DR10TM) Among Indonesian Pregnant Women in Their Third Semester. World Nutr. J. 2019, 2 (2), 56. https://doi.org/10.25220/WNJ.V02.i2.0009.
- Satpute, S. K.; Kulkarni, G. R.; Banpurkar, A. G.; Banat, I. M.; Mone, N. S.; Patil, R. H.; Cameotra, S. S. Biosurfactant/s from Lactobacilli Species: Properties, Challenges and Potential Biomedical Applications: Biosurfactant/s from Lactobacilli Species. Basic Microbiol. 2016, 56 (11), 1140–1158. https://doi.org/10.1002/jobm.201600143.
- Morais, I. M. C.; Cordeiro, A. L.; Teixeira, G. S.; Domingues, V. S.; Nardi, R. M. D.; Monteiro, A. S.; Alves, R. J.; Siqueira, E. P.; Santos, V. L. Biological and Physicochemical Properties of Biosurfactants Produced by Lactobacillus Jensenii P6A and Lactobacillus Gasseri P65. Cell Factories 2017, 16 (1), 155. https://doi.org/10.1186/s12934-017-0769-7.
- Merghni, A.; Dallel, I.; Noumi, E.; Kadmi, Y.; Hentati, H.; Tobji, S.; Ben Amor, A.; Mastouri, M. Antioxidant and Antiproliferative Potential of Biosurfactants Isolated from Lactobacillus Casei and Their Anti-Biofilm Effect in Oral Staphylococcus Aureus Strains. Pathog. 2017, 104, 84–89. https://doi.org/10.1016/j.micpath.2017.01.017.
- Fracchia, L.; J. Banat, J.; Cavallo, M.; Ceresa, C.; M. Banat, I.; 1 Department of Pharmaceutical Sciences, Università del Piemonte Orientale “A. Avogadro”, Largo Donegani 2, 28100, Novara, Italy; Potential Therapeutic Applications of Microbial Surface-Active Compounds. AIMS Bioeng. 2015, 2 (3), 144–162. https://doi.org/10.3934/bioeng.2015.3.144
More
