Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Rita Xu and Version 2 by Rita Xu.
Occlusions in the blood vessels caused by blood clots, referred to as thrombosis, and the subsequent outcomes are leading causes of morbidity and mortality worldwide. In vitro and in vivo models of thrombosis have advanced ourthe understanding of the complex pathways involved in its development and allowed the evaluation of different therapeutic approaches for its management.
- animal thrombosis models
- endothelial dysfunction
- hypercoagulation
1. Introduction
Thrombosis refers to pathological clot formation within the blood vasculature that may limit or block the blood flow. This may lead to severe conditions such as stroke, pulmonary embolism (PE), myocardial infarction (MI), organ and tissue ischemia, or other conditions depending on the site where the clot has formed. This disorder is among the leading causes of morbidity and mortality worldwide, as it was estimated to account for one in four deaths in 2010 [1]. Stroke and MI are among the most threatening thrombotic incidents [2]. Decreased quality of life, poor prognosis, and shorter life expectancy are associated with several thrombotic-related disorders, like chronic thromboembolic pulmonary hypertension (CTEPH), which occurs due to obstruction in major arteries that supply the lungs and is considered a long-term complication of PE [3,4][3][4].
Several factors that affect normal coagulation and homeostasis contribute to thrombosis, which may be acquired, inherited, or a combination of both. Homeostasis imbalance due to endothelial defects, changes in the blood flow, or alternation in fibrinolytic and coagulation components leading to a hypercoagulable state all contribute to thrombosis development [5].
2. Models for Thrombosis
2.1. In Vitro Models
Reproducing a vascular disorder outside the biological system is a complicated procedure, which usually requires microfluidic devices to mimic the biological system. Traditional in vitro models mostly evaluate a single aspect or component of the vascular disorder, like the platelet aggregation assay, which mainly evaluates the influence of agonists or antagonists on the platelets’ functions [15][6]. Among the earliest in vitro thrombosis models was the capillary thrombometer, described by Morawitz and Jürgens in 1930, which consisted of a horizontal glass capillary connected to two glass columns and was used to assess in vitro thrombus formation rate through the movement of blood back and forth in the glass capillary until the thrombus was formed [16][7]. On the other hand, the more recent thrombosis in vitro models, like thrombus on a chip, include more components, with better-controlled conditions to accurately mimic the biological system, including the vascular structure, blood cellular components, and signaling molecules.2.1.1. Macrofluidic- and Microfluidic-Based Models
The emergence of microfluidics technology has allowed the development of in vitro models of the vascular system with precise manipulation of the blood flow and cellular components, also providing a major advantage of using smaller blood volume as compared to earlier methods. Before this technology, macrofluidic systems were used, including a cone-and-plate device and two-disc rheometer, as shown in Figure 1, which rely on the rotation of the cone or disc for shear stress induction, and have been used to evaluate the influence of varying shear stress on endothelial cells [17][8]. The cone-and-plate device was the first to report that endothelial cells’ function and morphology are modulated by altered shear stresses [18][9]; however, they are not as common nowadays as the microfluidic systems.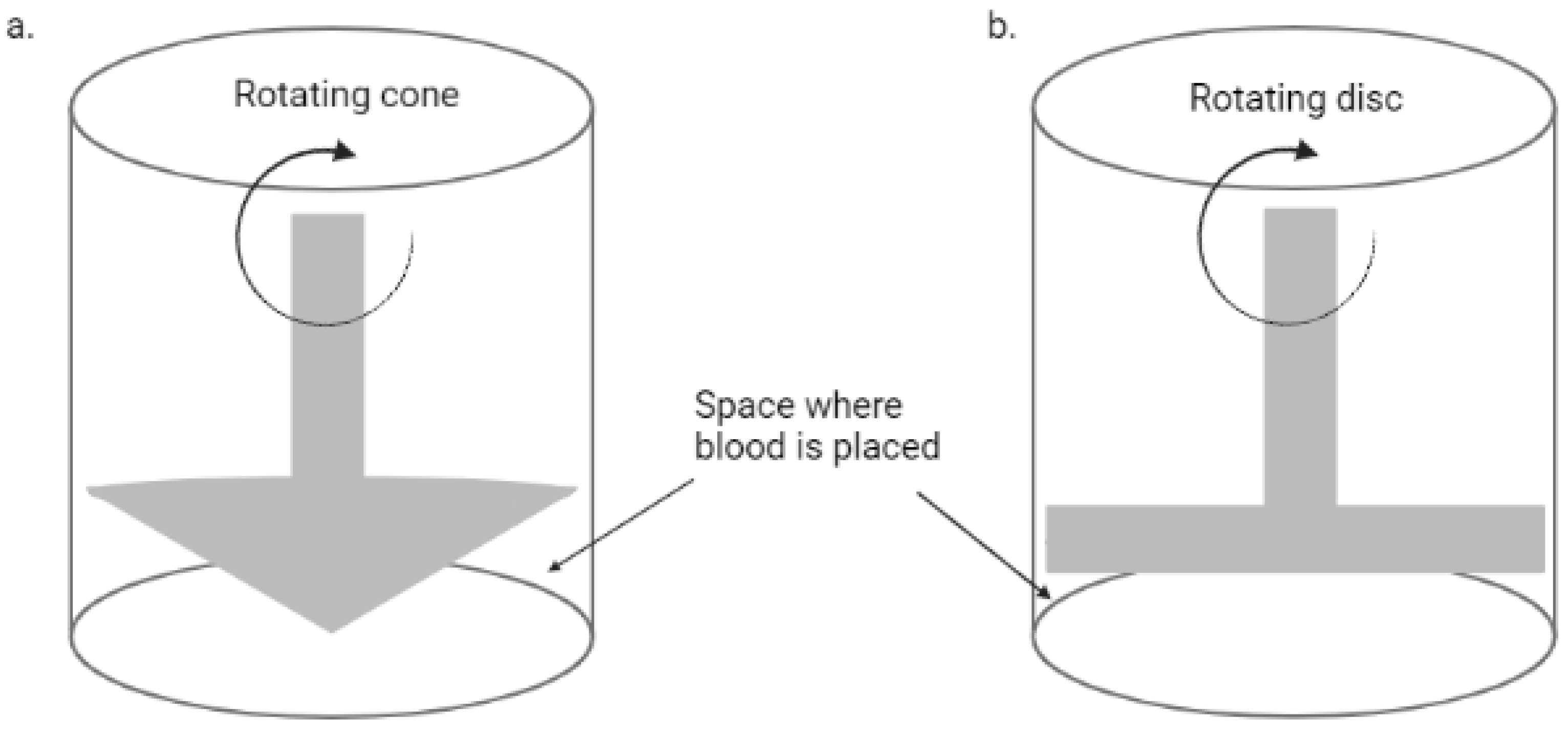
Figure 1. A simplified diagram of a rotational rheometer. (a) Cone and plate rheometer; (b) two-disc rheometer.
Flow Chambers
This is among the earliest methods used to model thrombosis in vitro, which generally represents a channel which blood passes through. This device has gone through many modifications throughout time and is commonly used for quantitative assays to measure in vitro thrombus formation [22][13]. Different flow chamber-based devices have been developed, ranging from simple to more complicated systems. These devices vary in size and flow surface coatings, which include endothelial cells, collagen, or other synthetic peptides, and are applied mainly to assess platelets’ function and hemostasis [22,23][13][14]. Several devices are classified under this technology, including viscometers, parallel-plate, annular, and tubular flow chambers, some of which have a constant flow of fluid while others have a controlled flow rate [24][15]. Also, custom-made devices are developed by a number of laboratories relying on this method [25][16]. A common approach in designing a flow chamber-based thrombosis model is the perfusion of blood sample over a surface coated with a platelet-activating or thrombogenic agent, as shown in Figure 2, in which collagen is the most commonly used. However, other agents like fibrin, fibrinogen, von Willebrand factor (vWF), and others are also employed [26,27][17][18]. Generally, the method involves coating a glass coverslip with the thrombogenic agent, followed by blood perfusion over the coverslip under controlled shear rate, and in some cases the process is monitored in real time [28,29][19][20]. Some studies reported the use of cell-free homogenate of atherosclerotic plaque as a thrombogenic agent and were reported to generate in vitro thrombosis through direct activation of platelets; thus, this method can be used as a model for arterial thrombosis and to evaluate novel anti-thrombogenic agents [29,30][20][21].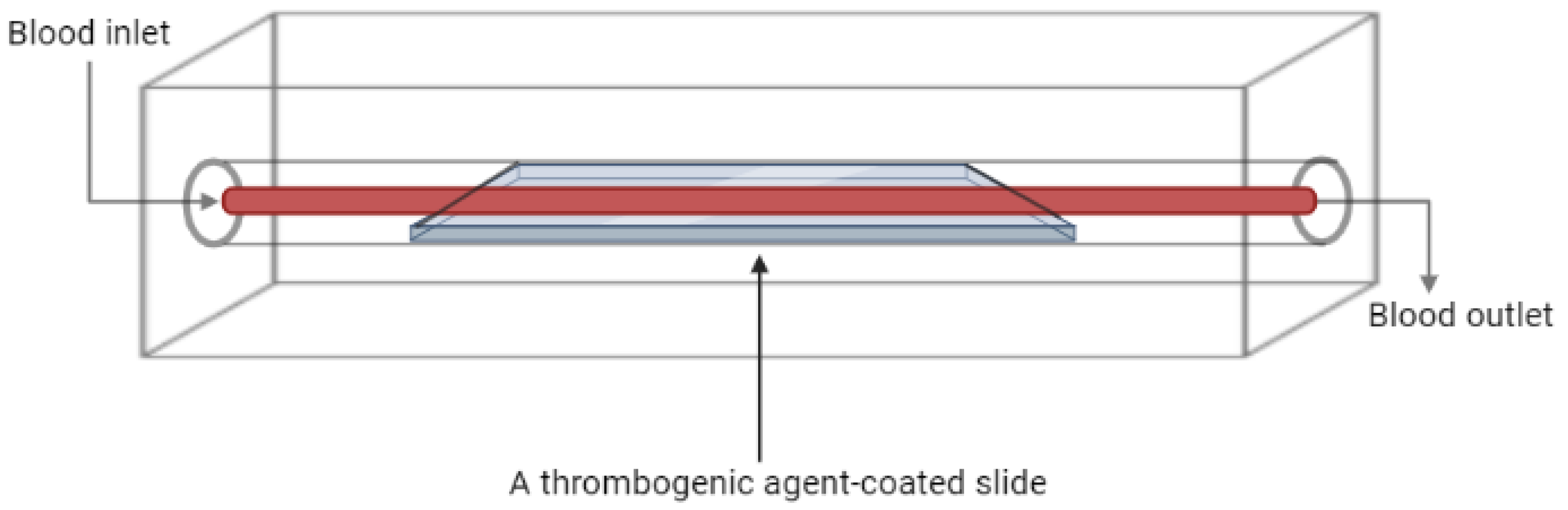
Figure 2. Schematic diagram of a flow chamber-based thrombosis model.
Thrombosis on a Chip
These models also employ the same concept as flow chambers but can be considered unique in terms of the ‘chip’ feature. These chips can be designed using soft lithography or bioprinting technology where microchannels are created using different materials and can be functionalized with endothelial cells [31][22]. Figure 3 shows a thrombosis-on-a-chip model that employs a novel method to develop occlusive thrombosis and can be used to evaluate the inhibition of thrombus formation by different compounds. In this device, a bifurcation system is designed where blood enters through a single inlet, passes through two different branches, and leaves from two outlets, which is believed to induce the formation of occlusive thrombus [32][23]. In one channel, a patch of collagen and tissue factor (TF) is placed to mimic plaque rupture. Two additional inlets linked to each arm of the device were also designed to incorporate ethylenediaminetetraacetic acid (EDTA) downstream of the collagen and TF patch, which was used to assess the efficacy of an antiplatelet drug [33][24].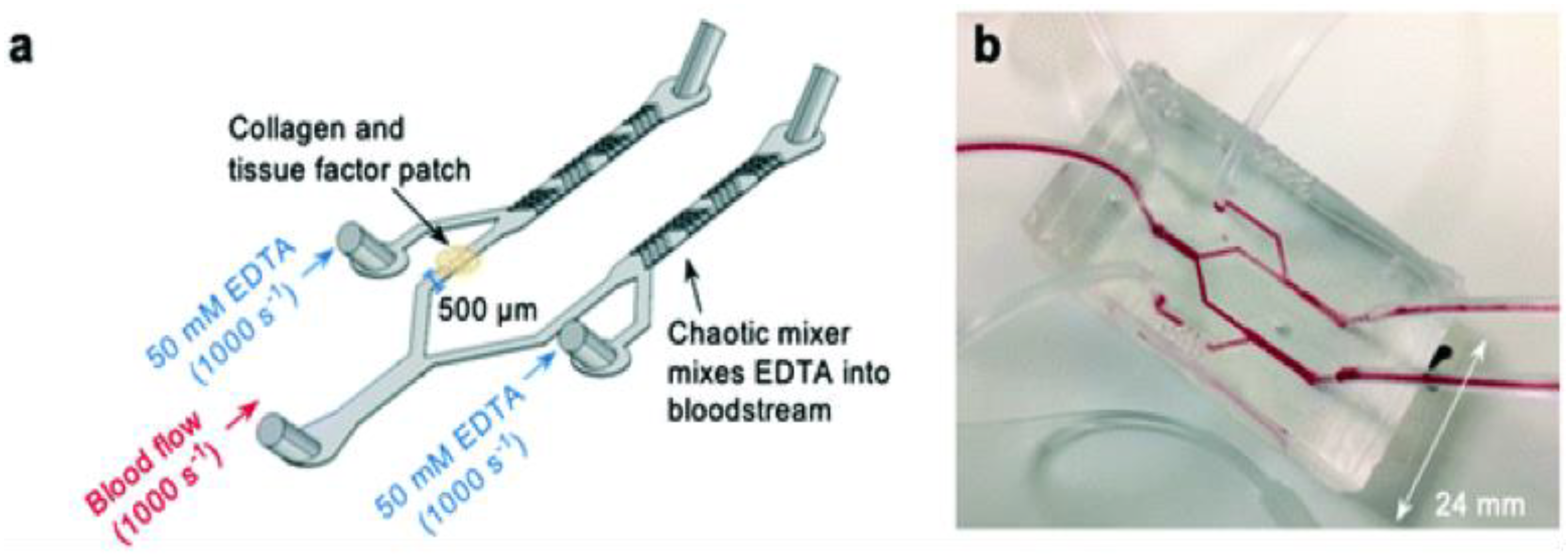
Figure 3. Novel thrombosis-on-a-chip device to measure occlusion time. (a) A schematic illustration of the ‘EDTA-quenched’ device; (b) a photograph of the device.
Other Microfluidic-Based Models
Considering the pathophysiology of venous thrombosis and the time it usually takes to develop, in vitro venous thrombosis models are not as common as arterial thrombosis. However, some studies reported the development of in vitro models of venous thrombosis. In one study, a microfluidic device with geometries similar to human venous valves was designed, employing primary and secondary vortex characterized by the point of vortical flow and the point of low shear rate, respectively, that are believed to mimic human conditions and support thrombus formation [37,38][28][29]. The study reported in vitro venous thrombosis development through a three-step process, including initial fibrin formation at the site of the secondary vortex, followed by platelet delivery at the site between the primary vortex and the formed fibrin with the aid of red blood cells, and then platelet adherence to the fibrin, resulting in thrombus growth and escape to the bulk flow [39][30]. This device is among the few in vitro thrombosis models that recapitulate venous thrombosis. Some in vitro models were created to recapitulate shear stress induced by blood-contacting medical devices. This is an important issue that has been characterized and addressed by Chen et al., 2015, using a novel blood-shearing device designed to mimic the pathological flow conditions of high shear stress and short exposure time caused by medical devices like catheters, stents, heart valves, and many others, helping in understanding the tendency and mechanism of developing thrombosis in patients implanted with these devices [40,41,42][31][32][33].2.2. In Vivo Models
In vivo thrombosis models have been developed using various techniques that relied on the Virchow’s triad, which describes the three main elements involved in thrombus formation, which are damage to the vascular wall, disturbance in blood flow, and the presence of a hypercoagulable state [55][34]. Different methods can be applied to initiate any of these conditions, like promoting coagulation through the use of certain chemicals, directly injuring the vasculature to damage the endothelium, or artificially inducing stenosis to alter blood flow, among different other methods [56,57][35][36].2.2.1. Murine Models
Rats and mice are among the most common animal models that are used to recapitulate different human disorders; being mammals means they share some common physiological processes with humans. They also share similarities with human genetics, as almost every disease-associated gene in humans has a counterpart in rats and mice [36][27]. They also provide numerous opportunities for genetic manipulation, and their small size allows ease of handling for maintenance and performing of different tests. As previously mentioned, in vivo thrombus development involves the creation of endothelial injury, promoting a local hypercoagulable state, or artificial induction of vascular stasis or stenosis, and all these parameters have been employed in murine thrombosis models either alone or in combination with each other using different techniques, as described below.Induction of Endothelial Injury
This can be done with the use of laser- or photochemical-induced injuries, where a beam of laser is focused on a blood vessel that is mostly but not always exposed through surgery. In photochemical-induced injury, the model is first treated with a chemical, like the commonly used Rose Bengal, followed by subsequent light illumination to further promote endothelial damages [58][37]. In these cases, the developed thrombus can be monitored over time using intravital microscopy and fluorophores that specifically target the thrombus [59][38]. On the other hand, direct mechanical injuries can also be induced by scrapping the endothelial wall using forceps to pinch a surgically exposed blood vessel [60][39], or through excision and ligation [61][40] or any other method that physically damages the endothelium. Chemicals like ferric chloride (FeCl3) are commonly used and have been proven to induce occlusive thrombus in a dose-dependent manner, where they can be applied to surgically expose vessels to promote endothelial injuries via free radical generation and induction of oxidative stress [62,63][41][42].Promoting Hypercoagulation
Factors that promote hypercoagulation are normally used in combination with other methods, like stasis, stenosis, or endothelial injury, to accelerate thrombus development. Serum, tissue factor, and high-fat diet (HFD) are among the common agents that are used to promote coagulation. Hyperlipidemia is known to induce a hypercoagulable state by promoting several coagulation factors and fibrin deposition on the vascular wall, and the state is shown to be reversed when the high fat is withdrawn from the diet [57][36]. When combined with arteriovenous (AV) shunt models or ferric chloride-treated models, diabetic fatty rats were shown to develop thrombosis at a faster rate [64][43]. Serum and tissue factor are also commonly used as hypercoagulable agents in animal models, where the administration of serum is usually accompanied with vascular ligation to facilitate thrombus formation. One study reported that heterologous serum obtained from human blood samples had a stronger thrombogenic potential in rats than homologous serum [65][44]. Alternating the serum components can help elucidate the mechanism of thrombus development and the factors involved in thrombogenicity [66][45]. On the other hand, tissue factor, which is normally a glycoprotein that is expressed at the site of vascular injury, is used to induce thrombosis by direct infusion into the blood vessel [65,67,68][44][46][47]. Also, any antifibrinolytic agents that inhibit fibrin degradation can be employed to promote hypercoagulation, like tranexamic acid, which acts by binding to the surface of plasminogen or plasmin, preventing it from binding to and degrading fibrin, and was used to induce acute hypercoagulation in rat models by a single intragastric administration [69][48]. Transgenic murine models with hypercoagulable states are also commonly employed in thrombosis studies. In this regard, one study reported that transgenic mice with altered thrombomodulin (TM) gene, that plays a role in anticoagulation, referred to as the TMPro/Pro model, has an increased vascular fibrin deposition and a higher tendency to develop thrombosis when endothelial injury or stasis is induced by FeCl3 treatment or arterial ligation, respectively [70][49]. A review on the available transgenic mouse models of venous thrombosis has been reported by Audrey et al., 2007 [71][50].Induction of Stasis or Stenosis
This method is mostly employed in larger vessels and refers to complete or partial blockage of blood flow, which is most commonly accomplished by narrowing the vessels by tying them with a ligature or compressing with a forceps; the method was first introduced in 1976 [72][51], and is referred to as Folt’s model. One study applied vascular ligation in mice of different ages and found that older mice have a vascular environment that promotes thrombus development [73][52]. Figure 4 shows a simplified diagram of stasis and stenosis obtained through tightening of the blood vessel.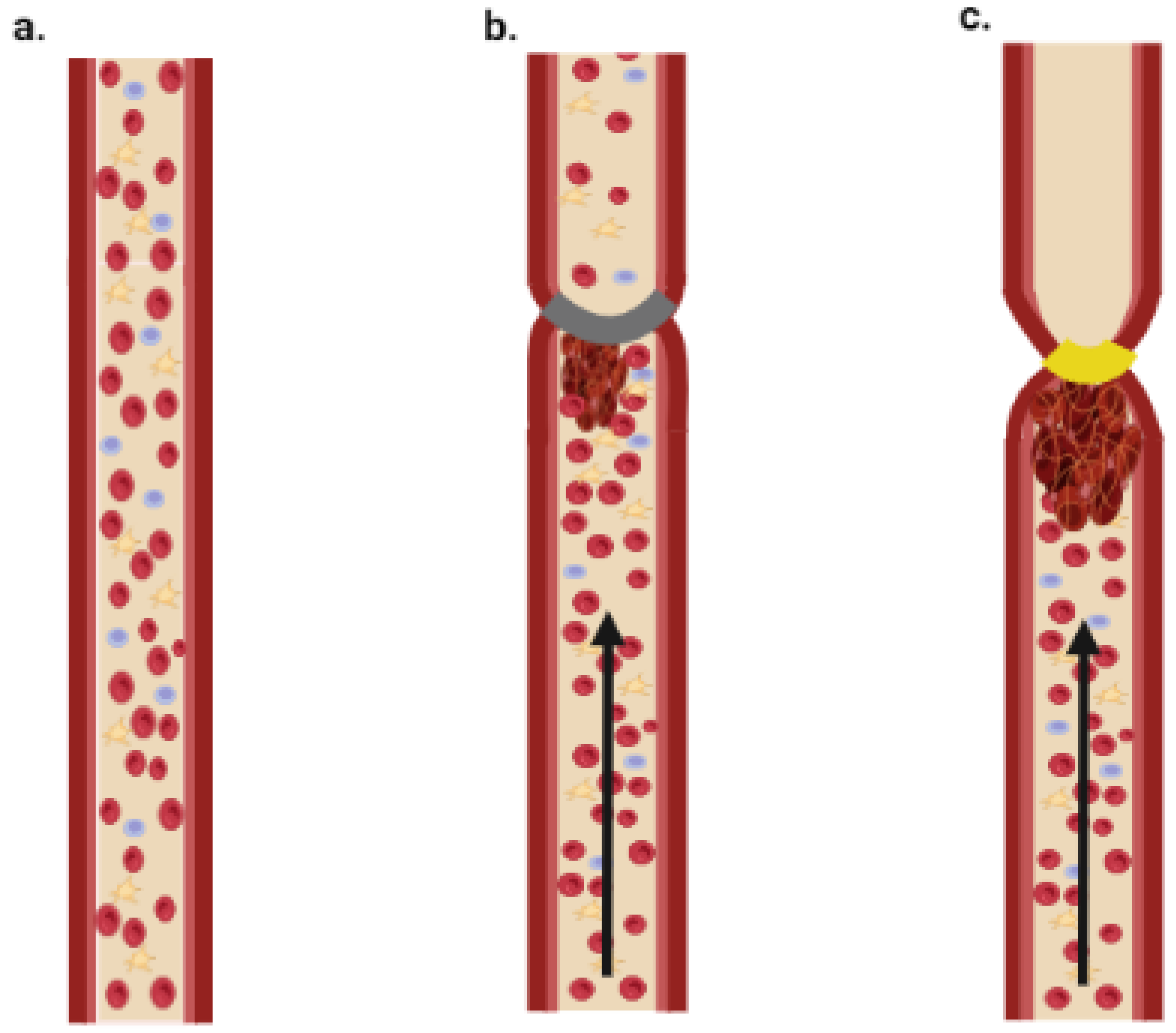
Figure 4. Schematic illustration of stasis and stenosis models. (a) Normal blood flow; (b) stenosis model—thrombus is formed due to partial block of blood flow; (c) stasis model—thrombus is formed due to complete block of blood flow.
2.2.2. Porcine Models
In addition to sharing more genetic similarities with humans than mice do [74][53], pigs also provide the advantage of large size, which means that thrombosis models resemble humans more closely than smaller species and vascular interventions can be employed more easily. Also, porcine hemodynamics, coagulation cascade, and the vascular system overall are closer to humans [75,76,77][54][55][56]. Considering their large size, porcine thrombosis models mostly involve surgical interventions and, thus, are considered more complicated and practically demanding in comparison to other smaller animal models.Endothelial Dysfunction
In pigs, different methods can alter endothelial integrity and functions, thus promoting coagulopathies and thrombosis. One study reported that balloon angioplasty in carotid arteries promotes erosion of the endothelial cells at the inflation site, with some sites showing tears in the underlying layers and excessive platelet deposition, eventually leading to thrombus development with complete occlusion in some cases. Such models can be used to evaluate therapeutic options to prevent endothelial injury and vascular occlusion following angioplasty, which is considered a common consequence following this therapeutic procedure [78][57]. Other approaches involve the use of balloon angioplasty wrapped with metallic coil to facilitate endothelial injury upon balloon inflation [79][58]. Also, stents—whether bare metals or drug eluting stents—can be employed where they are implanted in the vessel and used as models to study restenosis and stent thrombosis that can occur following these surgical procedures, which limits their application [80][59]. Electrical stimulation can also induce endothelial injury and complete vascular occlusion without the need of vascular constriction. This phenomenon is believed to occur due to platelet deposition at the site of injury, thus making this model useful to compare between different antiplatelet drugs [81][60]. Other studies reported that pigs fed with a high-cholesterol diet for at least nine weeks display attenuated endothelial functions with non-responsiveness to serotonin or bradykinin in terms of vasorelaxation, which is a prominent sign of endothelial dysfunction [82,83][61][62].Induction of Stasis or Stenosis
Balloon catheters or stent-based balloon catheters, which are normally used to dilate atherosclerotic blood vessels, as shown in Figure 5, are also used to limit blood flow or completely block it by controlling the degree of inflation. This method is employed in large animals like porcine models to study thrombosis development [84][63].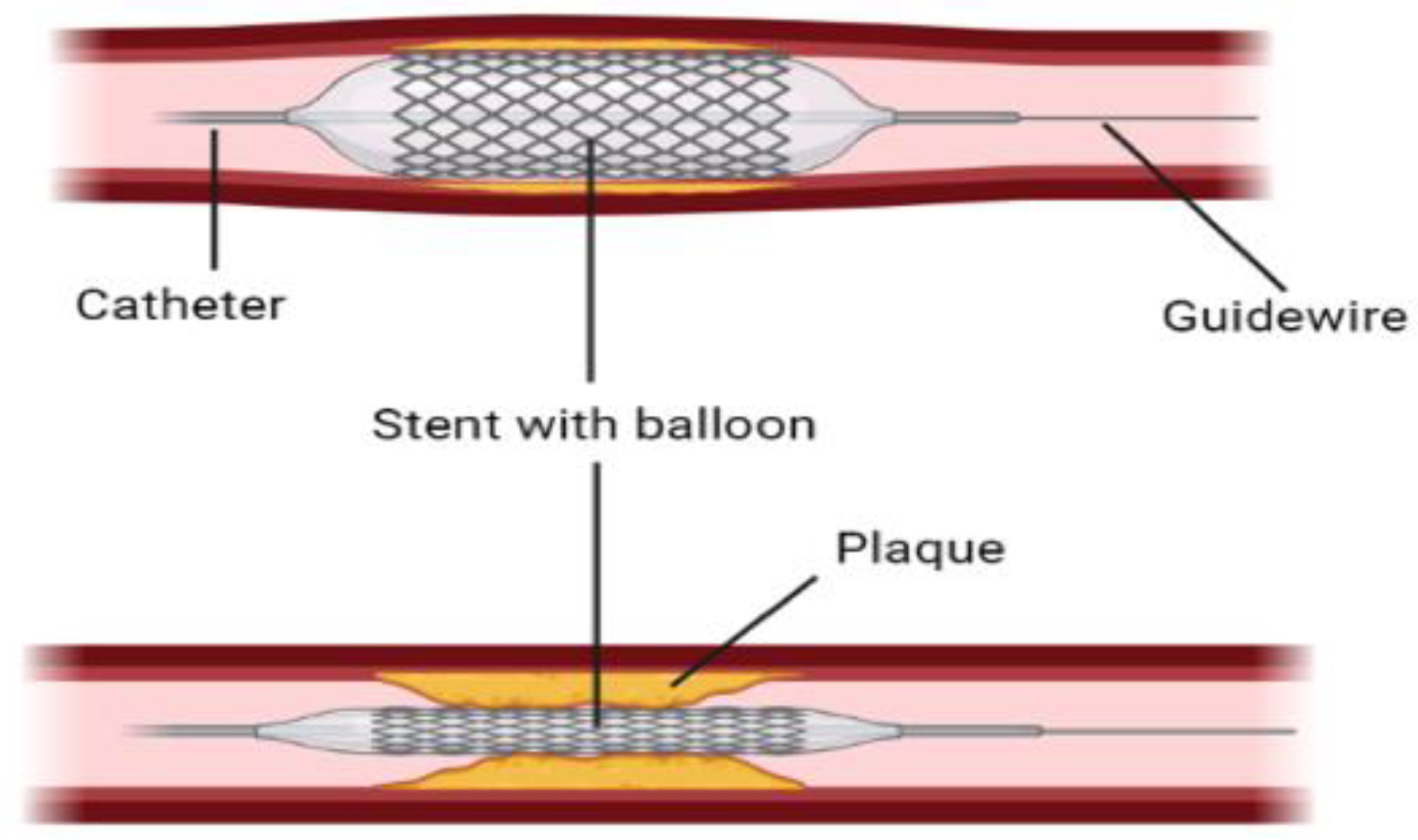
Figure 5.
A graphical demonstration of stent with balloon angioplasty to open narrowed arteries.
References
- Wendelboe, A.M.; Raskob, G.E. Global burden of thrombosis: Epidemiologic aspects. Circ. Res. 2016, 118, 1340–1347.
- Mackman, N. Triggers, targets and treatments for thrombosis. Nature 2008, 451, 914–918.
- Zipes, D.P. Braunwald’s heart disease: A textbook of cardiovascular medicine. BMH Med. J. 2018, 5, 63.
- Klok, F.; Van der Hulle, T.; Exter, P.D.; Lankeit, M.; Huisman, M.; Konstantinides, S. The post-PE syndrome: A new concept for chronic complications of pulmonary embolism. Blood Rev. 2014, 28, 221–226.
- Rasche, H. Haemostasis and thrombosis: An overview. Eur. Heart J. Suppl. 2001, 3, Q3–Q7.
- Tsoupras, A.; Zabetakis, I.; Lordan, R. Platelet aggregometry assay for evaluating the effects of platelet agonists and antiplatelet compounds on platelet function in vitro. MethodsX 2019, 6, 63–70.
- Morawitz, P.; Jürgens, R. Gibt es eine Thrombasthenie. Münch. Med. Wschr. 1930, 77, 2001.
- Hosseini, V.; Mallone, A.; Nasrollahi, F.; Ostrovidov, S.; Nasiri, R.; Mahmoodi, M.; Haghniaz, R.; Baidya, A.; Salek, M.M.; Darabi, M.A. Healthy and diseased in vitro models of vascular systems. Lab A Chip 2021, 21, 641–659.
- Dewey, C., Jr.; Bussolari, S.R.; Gimbrone, M.A., Jr.; Davies, P.F. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 1981, 103, 177–185.
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278.
- Lam, W.A. Thrombosis-on-a-Chip: A new way to model a complex process. Blood 2017, 130, SCI-10.
- Sebastian, B.; Dittrich, P.S. Microfluidics to mimic blood flow in health and disease. Annu. Rev. Fluid Mech. 2018, 50, 483–504.
- Roest, M.; Reininger, A.; Zwaginga, J.; King, M.; Heemskerk, J. Biorheology Subcommittee of the SSC of the ISTH. In Flow Chamber-Based Assays to Measure Thrombus Formation In Vitro: Requirements for Standardization; Wiley Online Library: New York, NY, USA, 2011; 9, pp. 2322–2324.
- Zwaginga, J.; Nash, G.; King, M.; Heemskerk, J.; Frojmovic, M.; Hoylaerts, M.; Sakariassen, K. Biorheology Subcommittee of the SSC of the ISTH. Flow-based assays for global assessment of hemostasis. Part 1: Biorheologic considerations 1. J. Thromb. Haemost. 2006, 4, 2486–2487.
- Slack, S.M.; Turitto, V.T. Flow chambers and their standardization for use in studies of thrombosis. Thromb. Haemost. 1994, 72, 777–781.
- Van Kruchten, R.; Cosemans, J.M.; Heemskerk, J.W. Measurement of whole blood thrombus formation using parallel-plate flow chambers–a practical guide. Platelets 2012, 23, 229–242.
- Jen, C.; Lin, J. Direct observation of platelet adhesion to fibrinogen-and fibrin-coated surfaces. Am. J. Physiol. Heart Circ. Physiol. 1991, 261, H1457–H1463.
- Savage, B.; Saldívar, E.; Ruggeri, Z.M. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 1996, 84, 289–297.
- Kuijpers, M.J.; Schulte, V.; Bergmeier, W.; Lindhout, T.; Brakebusch, C.; Offermanns, S.; Fässler, R.; Heemskerk, J.W.; Nieswandt, B. Complementary roles of platelet glycoprotein VI and integrin α2β1 in collagen-induced thrombus formation in flowing whole blood ex vivo. FASEB J. 2003, 17, 685–687.
- Siljander, P.R.-M.; Munnix, I.C.; Smethurst, P.A.; Deckmyn, H.; Lindhout, T.; Ouwehand, W.H.; Farndale, R.W.; Heemskerk, J.W. Platelet receptor interplay regulates collagen-induced thrombus formation in flowing human blood. Blood 2004, 103, 1333–1341.
- Cosemans, J.M.; Kuijpers, M.J.; Lecut, C.; Loubele, S.T.; Heeneman, S.; Jandrot-Perrus, M.; Heemskerk, J.W. Contribution of platelet glycoprotein VI to the thrombogenic effect of collagens in fibrous atherosclerotic lesions. Atherosclerosis 2005, 181, 19–27.
- Zhang, Y.S.; Oklu, R.; Albadawi, H. Bioengineered in vitro models of thrombosis: Methods and techniques. Cardiovasc. Diagn. Ther. 2017, 7 (Suppl. 3), S329.
- Tsai, M.; Kita, A.; Leach, J.; Rounsevell, R.; Huang, J.N.; Moake, J.; Ware, R.E.; Fletcher, D.A.; Lam, W.A. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J. Clin. Investig. 2011, 122, 408–418.
- Berry, J.; Peaudecerf, F.J.; Masters, N.A.; Neeves, K.B.; Goldstein, R.E.; Harper, M.T. An “occlusive thrombosis-on-a-chip” microfluidic device for investigating the effect of anti-thrombotic drugs. Lab A Chip 2021, 21, 4104–4117.
- Jain, A.; Graveline, A.; Waterhouse, A.; Vernet, A.; Flaumenhaft, R.; Ingber, D.E. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat. Commun. 2016, 7, 1–10.
- Yeom, E.; Park, J.H.; Kang, Y.J.; Lee, S.J. Microfluidics for simultaneous quantification of platelet adhesion and blood viscosity. Sci. Rep. 2016, 6, 1–11.
- Herbig, B.A.; Yu, X.; Diamond, S.L. Using microfluidic devices to study thrombosis in pathological blood flows. Biomicrofluidics 2018, 12, 42201.
- Lurie, F.; Kistner, R.L.; Eklof, B.; Kessler, D. Mechanism of venous valve closure and role of the valve in circulation: A new concept. J. Vasc. Surg. 2003, 38, 955–961.
- Karino, T.; Goldsmith, H.L.; Motomiya, M.; Mabuchi, S.; Sohara, Y. Flow Patterns in Vessels of Simple and Complex Geometries a. Ann. N. Y. Acad. Sci. 1987, 516, 422–441.
- Lehmann, M.; Schoeman, R.M.; Krohl, P.J.; Wallbank, A.M.; Samaniuk, J.R.; Jandrot-Perrus, M.; Neeves, K.B. Platelets drive thrombus propagation in a hematocrit and glycoprotein VI–dependent manner in an in vitro venous thrombosis model. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1052–1062.
- Chen, Z.; Mondal, N.K.; Ding, J.; Gao, J.; Griffith, B.P.; Wu, Z.J. Shear-induced platelet receptor shedding by non-physiological high shear stress with short exposure time: Glycoprotein Ibα and glycoprotein VI. Thromb. Res. 2015, 135, 692–698.
- Chen, Z.; Mondal, N.K.; Ding, J.; Koenig, S.C.; Slaughter, M.S.; Griffith, B.P.; Wu, Z.J. Activation and shedding of platelet glycoprotein IIb/IIIa under non-physiological shear stress. Mol. Cell. Biochem. 2015, 409, 93–101.
- Chen, Z.; Mondal, N.K.; Ding, J.; Koenig, S.C.; Slaughter, M.S.; Wu, Z.J. Paradoxical Effect of Nonphysiological Shear Stress on Platelets and v on W illebrand Factor. Artif. Organs. 2016, 40, 659–668.
- Kushner, A.; West, D.; Pillarisetty, L.S. Virchow triad. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Albadawi, H.; Witting, A.A.; Pershad, Y.; Wallace, A.; Fleck, A.R.; Hoang, P.; Khademhosseini, A.; Oklu, R. Animal models of venous thrombosis. Cardiovasc. Diagn. Ther. 2017, 7 (Suppl. 3), S197.
- De Curtis, A.; D’Adamo, M.C.; Amore, C.; Polishchuck, R.; Di Castelnuovo, A.; Donati, M.B.; Iacoviello, L. Experimental Arterial Thrombosis in Genetically or Diet Induced Hyperlipidemia in Rats. Thromb. Haemost. 2001, 86, 1440–1448.
- Rosen, E.D.; Raymond, S.; Zollman, A.; Noria, F.; Sandoval-Cooper, M.; Shulman, A.; Merz, J.L.; Castellino, F.J. Laser-induced noninvasive vascular injury models in mice generate platelet-and coagulation-dependent thrombi. Am. J. Pathol. 2001, 158, 1613–1622.
- Kamocka, M.; Mu, J.; Liu, X.; Chen, N.; Zollman, A.; Sturonas-Brown, B.; Dunn, K.; Xu, Z.; Chen, D.Z.; Alber, M.S. Two-photon intravital imaging of thrombus development. J. Biomed. Opt. 2010, 15, 16020.
- Pierangeli, S.S.; Barker, J.H.; Stikovac, D.; Ackerman, D.; Anderson, G.; Barquinero, J.; Acland, R.; Harris, E.N. Effect of human IgG antiphospholipid antibodies on an in vivo thrombosis model in mice. Thromb. Haemost. 1994, 71, 670–674.
- Sawyer, P.N.; Pate, J.W.; Weldon, C.S. Relations of abnormal and injury electric potential differences to intravascular thrombosis. Am. J. Physiol. Leg. Content 1953, 175, 108–112.
- Kurz, K.; Main, B.; Sandusky, G. Rat model of arterial thrombosis induced by ferric chloride. Thromb. Res. 1990, 60, 269–280.
- Wang, X.; Xu, L. An optimized murine model of ferric chloride-induced arterial thrombosis for thrombosis research. Thromb. Res. 2005, 115, 95–100.
- Shang, J.; Chen, Z.; Wang, M.; Li, Q.; Feng, W.; Wu, Y.; Wu, W.; Graziano, M.P.; Chintala, M. Zucker Diabetic Fatty rats exhibit hypercoagulability and accelerated thrombus formation in the Arterio-Venous shunt model of thrombosis. Thromb. Res. 2014, 134, 433–439.
- Millet, J.; Vaillot, M.; Theveniaux, J.; Brown, N.L. Experimental venous thrombosis induced by homologous serum in the rat. Thromb. Res. 1996, 81, 497–502.
- Deykin, D.; Wessler, S. Activation product, factor IX, serum thrombotic accelerator activity, and serum-induced thrombosis. J. Clin. Investig. 1964, 43, 160–166.
- Furugohri, T.; Fukuda, T.; Tsuji, N.; Kita, A.; Morishima, Y.; Shibano, T. Melagatran, a direct thrombin inhibitor, but not edoxaban, a direct factor Xa inhibitor, nor heparin aggravates tissue factor-induced hypercoagulation in rats. Eur. J. Pharmacol. 2012, 686, 74–80.
- Wessler, S.; Ward, K.; Ho, C. Studies in intravascular coagulation. III. The pathogenesis of serum-induced venous thrombosis. J. Clin. Investig. 1955, 34, 647–651.
- Jing, J.; Du, Z.; Wen, Z.; Jiang, B.; He, B. Dynamic changes of urinary proteins in a rat model of acute hypercoagulable state induced by tranexamic acid. J. Cell. Physiol. 2019, 234, 10809–10818.
- Weiler, H.; Lindner, V.; Kerlin, B.; Isermann, B.H.; Hendrickson, S.B.; Cooley, B.C.; Meh, D.A.; Mosesson, M.W.; Shworak, N.W.; Post, M.J. Characterization of a mouse model for thrombomodulin deficiency. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1531–1537.
- Cleuren, A.C.; van Vlijmen, B.J.; Reitsma, P.H. Transgenic mouse models of venous thrombosis: Fulfilling the expectations? In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2007; pp. 610–616.
- Folts, J.D.; Crowell, E.B., Jr.; Rowe, G.G. Platelet aggregation in partially obstructed vessels and its elimination with aspirin. Circulation 1976, 54, 365–370.
- McDonald, A.P.; Meier, T.R.; Hawley, A.E.; Thibert, J.N.; Farris, D.M.; Wrobleski, S.K.; Henke, P.K.; Wakefield, T.W.; Myers, D.D., Jr. Aging is associated with impaired thrombus resolution in a mouse model of stasis induced thrombosis. Thromb. Res. 2010, 125, 72–78.
- Wernersson, R.; Schierup, M.H.; Jørgensen, F.G.; Gorodkin, J.; Panitz, F.; Stærfeldt, H.-H.; Christensen, O.F.; Mailund, T.; Hornshøj, H.; Klein, A. Pigs in sequence space: A 0.66 X coverage pig genome survey based on shotgun sequencing. BMC Genom. 2005, 6, 1–7.
- Folts, J.; Rowe, G. Acute thrombus formation in stenosed pig coronary-arteries, causing sudden-death by ventricular-fibrillation. In Circulation; American Heart Association: Dallas, TX, USA, 1983; p. 264.
- Leach, C.M.; Thorburn, G.D. A comparative study of collagen induced thromboxane release from platelets of different species: Implications for human athero sclerosis models. Prostaglandins 1982, 24, 47–59.
- Topaz, O. Cardiovascular Thrombus: From Pathology and Clinical Presentations to imaging, Pharmacotherapy and Interventions; Academic Press: Cambridge, MA, USA, 2018.
- Steele, P.M.; Chesebro, J.H.; Stanson, A.W.; Holmes, D.R., Jr.; Dewanjee, M.K.; Badimon, L.; Fuster, V. Balloon angioplasty. Natural history of the pathophysiological response to injury in a pig model. Circ. Res. 1985, 57, 105–112.
- Schwartz, R.S.; Huber, K.C.; Murphy, J.G.; Edwards, W.D.; Camrud, A.R.; Vlietstra, R.E.; Holmes, D.R. Restenosis and the proportional neointimal response to coronary artery injury: Results in a porcine model. J. Am. Coll. Cardiol. 1992, 19, 267–274.
- Miyauchi, K.; Kasai, T.; Yokayama, T.; Aihara, K.; Kurata, T.; Kajimoto, K.; Okazaki, S.; Ishiyama, H.; Daida, H. Effectiveness of statin-eluting stent on early inflammatory response and neointimal thickness in a porcine coronary model. Circ. J. 2008, 72, 832–838.
- Kohda, N.; Tani, T.; Nakayama, S.; Adachi, T.; Marukawa, K.; Ito, R.; Ishida, K.; Matsumoto, Y.; Kimura, Y. Effect of cilostazol, a phosphodiesterase III inhibitor, on experimental thrombosis in the porcine carotid artery. Thromb. Res. 1999, 96, 261–268.
- Cohen, R.A.; Zitnay, K.M.; Haudenschild, C.C.; Cunningham, L.D. Loss of selective endothelial cell vasoactive functions caused by hypercholesterolemia in pig coronary arteries. Circ. Res. 1988, 63, 903–910.
- Hasdai, D.; Mathew, V.; Schwartz, R.S.; Holmes, D.R., Jr.; Lerman, A. The effect of basic fibroblast growth factor on coronary vascular tone in experimental hypercholesterolemia in vivo and in vitro. Coron. Artery Dis. 1997, 8, 299–304.
- Schwein, A.; Magnus, L.; Markovits, J.; Chinnadurai, P.; Autry, K.; Jenkins, L.; Barnes, R.; Vekilov, D.P.; Shah, D.; Chakfé, N. Endovascular porcine model of iliocaval venous thrombosis. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 623–630.
More
