Corn silk (CS) is an agro-by-product from corn cultivation. It is used in folk medicines in some countries, besides being commercialized as health-promoting supplements and beverages. Unlike CS-derived natural products, their bioactive peptides, particularly antioxidant peptides, are understudied.
- antioxidant
- in silico
- in vitro
- mechanism
- molecular docking
- peptide
- purification
- Stigma maydis
1. Introduction
Image by M Ameen from Pixabay
2. Purification of 1-h Trypsin Hydrolysate by Ultrafiltration
1-h Trypsin hydrolysate (T1H) was separated by ultrafiltration (UF), yielding two UF fractions. The >3 kDa fraction exhibited stronger antioxidant activity than the <3 kDa fraction based on their EC50 for 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt radical cation (ABTS•+) (28.4 and 45.6 µg dry mass (DM)/mL, respectively) and H2O2 (174.5 and 461.7 µg DM/mL, respectively) scavenging activities (Figure 1C,D). Thiobarbituric acid reactive species (TBARS) value of the negative control increased from 0.7 to 1.6 µM malondialdehyde equivalents after incubation of the lecithin liposomes from 24 to 48 h, indicating an increase in lipid peroxidation. As revealed by the TBARS values, treatment with 0.5 mg DM/mL of >3 kDa and <3 kDa fractions inhibited lipid peroxidation by 22% and 16% after 24 h, and by 64% and 50% after 48 h, respectively (Figure 1E). Our results are concordant with previous observations on the antioxidant activities of the UF fractions of fennel seed hydrolysate [10]. In theirs and this study, the superiority of the >3 kDa fraction in scavenging radicals relative to the <3 kDa fraction was observed. This may be attributed, in part, to the presence of large compounds, such as long peptides, partially degraded proteins, or other components with antioxidant properties in the >3 kDa fraction. In fact, the protein content of the >3 kDa fraction was 8-fold higher than that of the <3 kDa fraction (Figure 1A). By contrast, the <3 kDa fraction was peptide-rich, with peptide content 5-fold greater than the >3 kDa fraction (Figure 1B).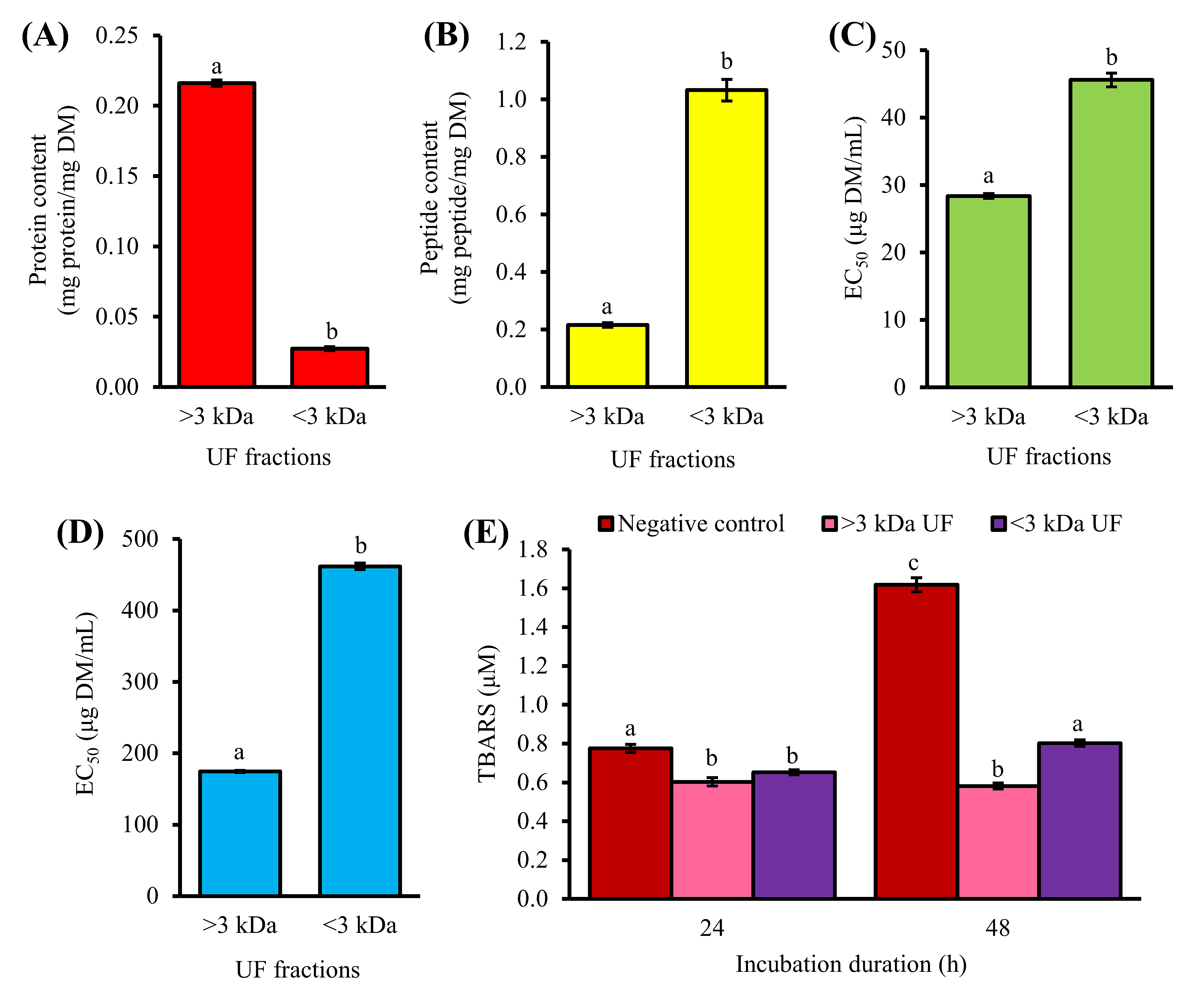
3. Purification of <3 kDa Fraction by Gel Filtration Chromatography
Purification by gel filtration chromatography (GFC) resulted in three pooled fractions: GF-I, GF-II, and GF-III (Figure 2A). Among the three, GF-I exhibited the strongest effects in ABTS•+ scavenging activity (Figure 2C). GF-I possibly comprised more non-aromatic peptide residues with radical scavenging activity, such as Leu and Pro [12] than the other two fractions. Meanwhile, GF-III showed the highest H2O2 scavenging activity among the three pooled fractions (Figure 2D). All three pooled GFC fractions could dampen the time-dependent increase in lipid peroxidation in the liposome model at 0.1 mg peptide/mL, with 26–35% inhibition of TBARS formation after 48 h (Figure 2E), although their activities were significantly lower than that of GSH (57% inhibition after 48 h). The peptide content of GF-III (0.68 mg peptide/mg DM) was 3.5-fold greater than those of GF-I and GF-II (Figure 2B). When compared to GF-III, 3.5-fold higher DM of GF-I and GF-II was required to achieve the standardized peptide concentration used for evaluating the lipid peroxidation inhibitory activity depicted in Figure 2E. It can be anticipated that when expressed in terms of DM, the lipid peroxidation inhibitory activity of GF-III may exceed that of GF-I. Thus, we also analyzed the lipid peroxidation inhibitory activity of the three pooled fractions at 0.5 mg DM/mL. As expected, among the three fractions, GF-III showed the strongest inhibition of TBARS formation, with 26% and 51% inhibition after 24 and 48 h, respectively (Figure 2F). The antioxidant activity of GF-III could be owing to its relatively high absorbance at 280 nm (Figure 2A), which suggests an abundance of aromatic amino acid residues (e.g., Phe, Tyr, and Trp) in the pooled fraction. In keeping with this study, a previous study on Chinese chestnut also found that the GFC fraction with the most prominent absorbance at 280 nm had the highest antioxidant activity among all GFC fractions [13].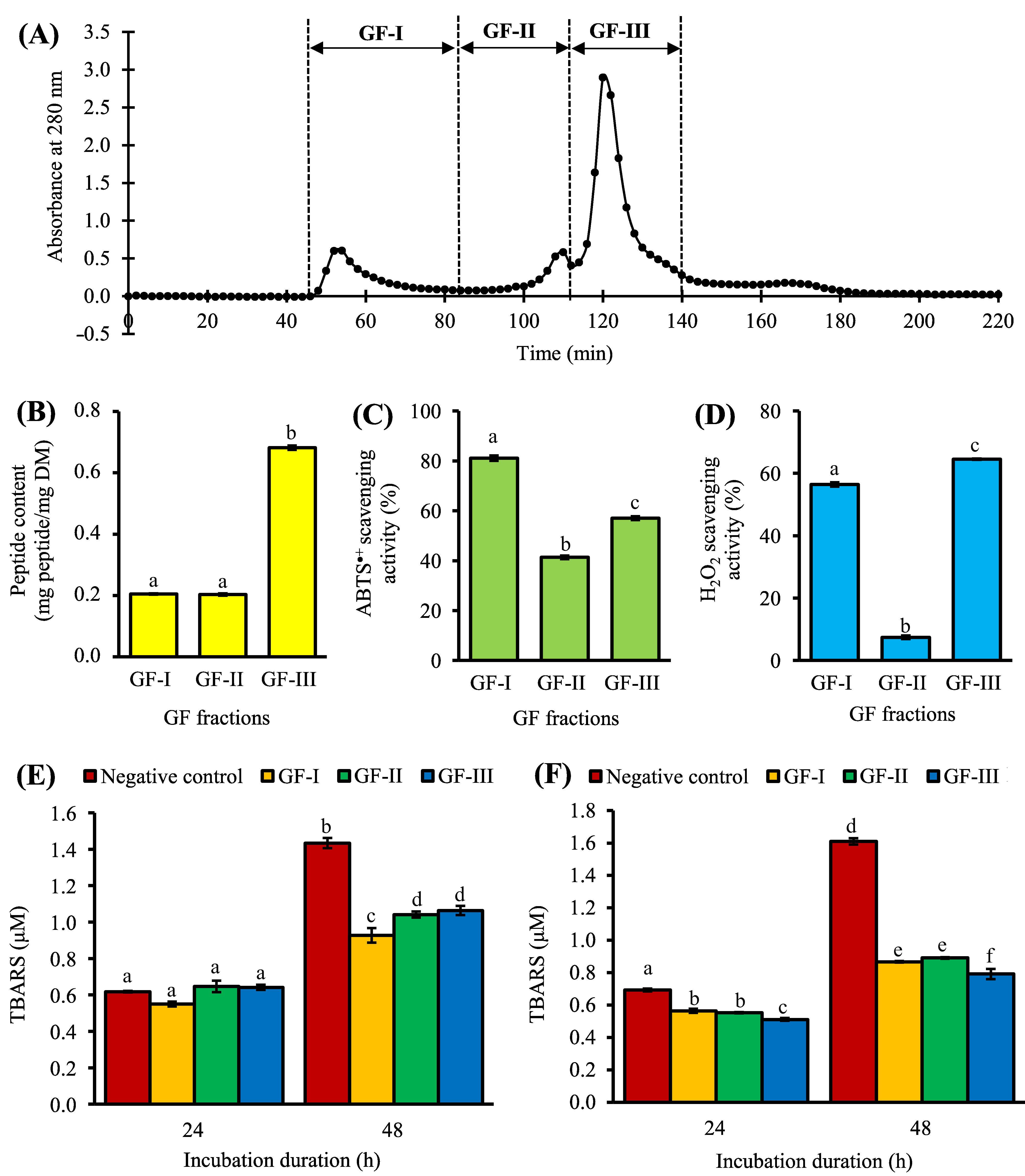
4. Purification of GF-III by Strong-Cation-Exchange Solid-Phase Extraction
GF-III was further purified by strong-cation-exchange solid-phase extraction (SCX-SPE), producing six SPE fractions. As shown in Figure 3A, most of the peptide constituents of GF-III were found in 50 mM KCl fraction (0.9 mg peptide/mL), which was 22–118 times greater than the other five SPE fractions. The 50 mM KCl fraction showed relatively low or no ABTS•+ and H2O2 scavenging activities at the peptide concentrations tested (Figure 3B,C). Our result suggests that SCX-SPE has partitioned most of the non-antioxidant peptides and/or peptides with weak antioxidant activity into the 50 mM KCl fraction.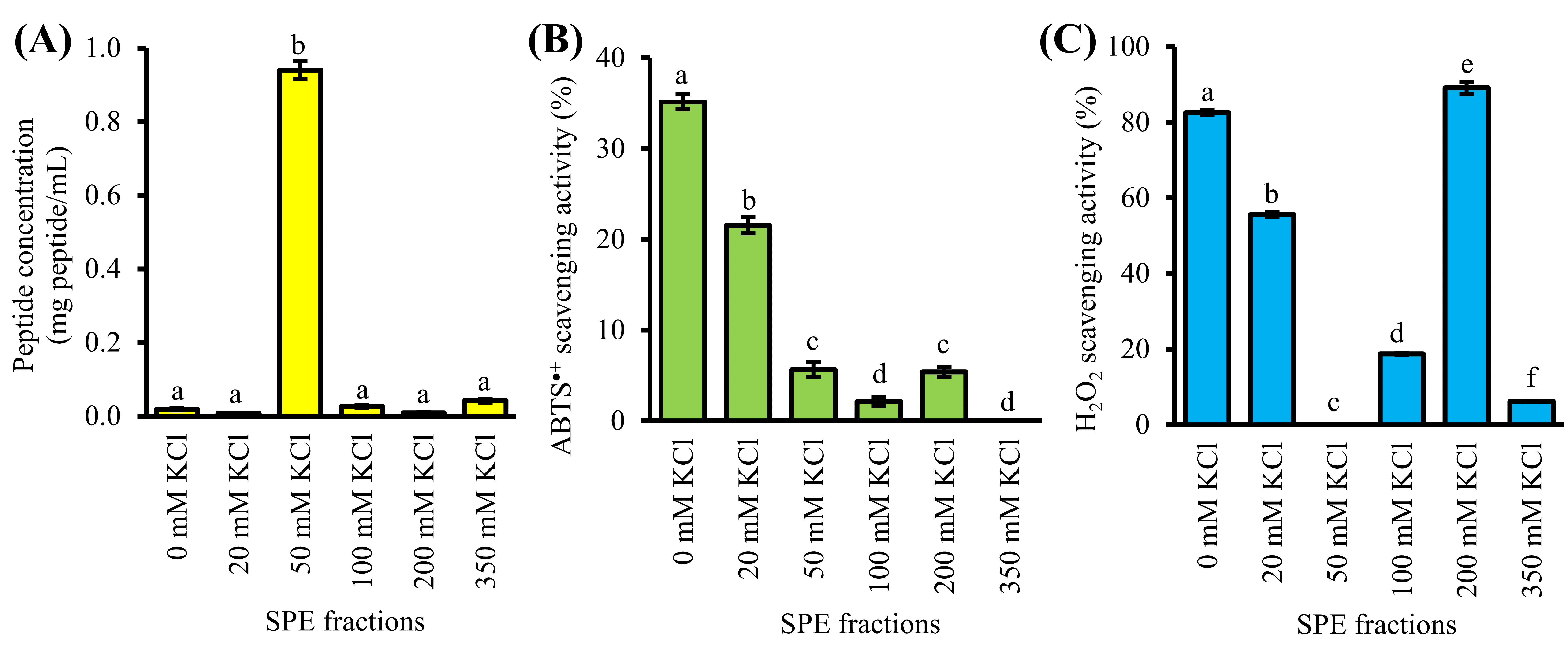
5. Identification and Characterization of Antioxidant Peptides
Liquid chromatography-tandem mass spectrometry analysis identified 29 peptide sequences comprising 6–14 residues (633.33 to 1517.81 Da) from the 0, 20, and 200 mM KCl fractions (Table 1). This range of peptide masses agrees with the observation that the molecular masses of food-derived antioxidant peptides commonly range between 500–1800 Da [12]. Twenty-three of the 29 peptides contain 11–56% aliphatic amino acid residues (Table 1). Such residues are responsible for the thermal stability of proteins [17]. Two thermal-stable antioxidant peptides WAFAPA and MYPGLA that were identified from the blue-spotted stingray, for instance, are composed of 50% and 33% of aliphatic residues, respectively [18]. Based on the comparison of the aliphatic index, 10 of the 29 peptides were likely superior to both WAFAPA and MYPGLA in terms of thermal stability (Table 1). The discovery of such peptides also supports our previous observation of the thermal stability of T1H, the protein hydrolysate from which the 29 peptides were purified. T1H retained its radical scavenging and ferric reducing activity at temperatures up to 100 °C [4]. These CS peptides that are likely to be thermal-stable can thus be utilized as alternatives for food additives to address the concerns regarding the food processing heat treatment.| SPE Fractions | Peptides | Measured m/z [M + 2H]2 |
Molecular Mass (Da) a |
Aromatic Residues (%) b |
Basic Residues (%) b |
Hydrophobic Residues (%) b |
Aliphatic Residues |
|---|
| Peptides | SPE Fractions | Binding Affinity (kcal/mol) |
Peptide Residues Interacting with ABTS•+ a | (%) | b | Aliphatic Index | b | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Interaction | ||||||||||
| 0 mM KCl | KRYFKR | 449.28 | |||||||||
| MCFHHHFHK | 20 mM KCl | 896.57 | 33 | 67 | 33 | −4.8 | 0 | 0 | |||
| - | Phe3, His6, Phe7 | PRVRVAGR | 455.79 | 909.58 | 0 | 38 | 63 | 38 | 85 | ||
| VHFNKGKKR | 0 mM KCl | −4.7 | Lys7, Arg9 | Val1, His2, Gly6, Lys7, Arg9 | PVVWAAKR | 463.79 | 925.57 | 13 | 25 | 75 | |
| PVVWAAKR | 0 mM KCl | −4.7 | 50 | 98 | |||||||
| Arg8 (2) | Val2, Trp4, Ala5, Ala6, Arg8 | QVASGPLQR | 478.28 | 954.55 | FMFFVYK0 | 11 | 56 | 20 mM KCl | −4.4 | Lys733 | 87 |
| Phe1, Phe3, Phe4, Lys7 | MAPRTPRK | 478.78 | 955.57 | 0 | 38 | 50 | 13 | 13 | |||
| NKVVKLMR | 494.31 | 986.62 | 0 | 38 | 50 | 38 | 121 | ||||
| KVPLAVFSR | 508.82 | 1015.64 | 11 | 22 | 67 | 44 | 119 | ||||
| LKKGSPLKR | 513.84 | 1025.69 | 0 | 44 | 44 | 22 | 87 | ||||
| FQLKPVFR | 517.82 | 1033.63 | 25 | 25 | 63 | 25 | 85 | ||||
| THAVKGVVHK | 538.34 | 1074.67 | 20 | 40 | 50 | 40 | 97 | ||||
| YTWKFKGR | 543.31 | 1084.61 | 38 | 38 | 50 | 0 | 0 | ||||
| ARVPQQSYR | 552.80 | 1103.61 | 11 | 22 | 44 | 22 | 43 | ||||
| VHFNKGKKR | 557.34 | 1112.69 | 22 | 56 | 33 | 11 | 32 | ||||
| TAPLSSKALKR | 586.37 | 1170.73 | 0 | 27 | 45 | 36 | 89 | ||||
| FSCPLVMKGPNGLR | 759.91 | 1517.81 | 7 | 14 | 71 | 21 | 76 | ||||
| 20 mM KCl | RHGSGR | 335.18 | 668.37 | 17 | 50 | 33 | 0 | 0 | |||
| NMVPGR | 337.17 | 672.34 | 0 | 17 | 67 | 17 | 48 | ||||
| FMFFVYK | 491.25 | 980.50 | 57 | 14 | 86 | 14 | 41 | ||||
| Peptides | SPE Fractions | FRS Scores |
|---|---|---|
| MCFHHHFHK | 20 mM KCl | 0.68068 |
| VGPWQK * | - | 0.52254 |
| MYPGLA * | - | 0.49386 |
| NLEGYR | 200 mM KCl | 0.48158 |
| AGFPLGK |
| Peptides a | Basic Residues | Mutant Peptides | Binding Affinity (kcal/mol) |
|---|---|---|---|
| 200 mM KCl | |||
| FSCPLVMKGPNGLR | 0.44866 | ||
| 0 mM KCl | −4.2 | Arg14 (2) | Leu5, Lys8, Gly9, Pro10, Gly12, Arg14 |
| NLEGYR | |||
| Arg6 | |||
| NLEGYA | |||
| −3.7 | |||
| RHGSGR | |||
| Arg1 | AHGSGR | −3.8 | |
| Arg6 | RHGSGA | −4.2 | |
| DFPGAK | Lys6 | DFPGAA | −3.4 |
7. Molecular Docking of Peptides on Keap1
Food-derived bioactive peptides, in addition to scavenging free radicals, can confer cellular protection by modulating the gene expression and activities of antioxidant and oxidant enzymes [5]. Given this, we conducted a docking-based screening experiment to unravel the potential of the 29 CS peptides identified in this study in interacting with cellular protein targets that can regulate the endogenous oxidant status: Keap1, MPO, and XO. Soy-derived DEQIPSHPPR was predicted in molecular docking study to interact stably with Keap1, in keeping with its demonstrated ability to disrupt Keap1-Nrf2 binding and increase Nrf2 levels in the nucleus [6]. Hence, DEQIPSHPPR was used as a reference peptide for comparison with CS peptides. Our docking results show that 13 of the 29 CS peptides could bind to Keap1 similarly or more stably than DEQIPSHPPR (Table S2 (could be found in https://www.mdpi.com/2076-3921/10/11/1822#supplementary)). Further in silico screening for low toxicity and allergenicity as well as high cell-penetrating potential narrowed down the 13 CS peptides to five, namely NDGPSR, NLEGYR, NMVPGR, SSPATGGSLR, and NANSLAGPQR (Table 5). Screening based on these parameters allows the search for CS peptides that might be able to cross the cell membrane barrier and block the Keap1-Nrf2 interaction in cells with minimal or no harmful effects. Unlike the five CS peptides, the reference peptide DEQIPSHPPR may elicit allergy (Table 5), hence it is less desirable for the application of functional food ingredients.| Peptides | Toxicity | Allergenicity | CPP Prediction |
|---|---|---|---|
| MCFHHHFHK | His6 | MCFHHAFHK | −4.1 |
| NDGPSR | Non-toxin | Probable non-allergen |
| Peptides | Binding Affinity (kcal/mol) |
Interaction with Keap1 a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Interaction | Salt Bridge | |||||||||||
| CPP | |||||||||||||
| NLEGYR | −8.7 | Arg415, Arg483, Ser508, Gln530, Ser555 | |||||||||||
| VHFNKGKKR | His2 | VAFNKGKKR | −4.8 | ||||||||||
| Tyr334 | , Ser363, Gly364, Leu365, Ala366, | Arg415 | , Ile416, Gly417, Gly462, Phe478, Arg483, Ser508, Gly509, Ala510, Tyr525, Gln530, Ser555, Ala556, Leu557, Tyr572, Phe577, Ser602, Gly603, Val604 | Arg415 | NLEGYR | Non-toxin | Probable non-allergen | CPP | Lys7 | VHFNKGAKR | −5.0 | ||
| NMVPGR | Non-toxin | Probable non-allergen | CPP | Arg9 | VHFNKGKKA | −4.4 | |||||||
| SSPATGGSLR | Non-toxin | Probable non-allergen | CPP | PVVWAAKR | Arg8 | PVVWAAKA | −4.3 | FMFFVYK | NMVPGR | 20 mM KCl | −4.1 | Asn1, Arg6 (2) | Asn1, Pro4, Gly5, Arg6 |
| NANSLAGPQR | Non-toxin | Probable non-allergen | CPP | FMFFVYK20 mM KCl | 0.44397 | ||||||||
| Lys7 | FMFFVYA | −4.3 | |||||||||||
| KRYFKR | Non-toxin | Probable non-allergen | NMVPGR | NLEGYR20 mM KCl | 200 mM KCl | −4.1 | - | Tyr5, Arg6 | |||||
| CPP | FSCPLVMKGPNGLR | Lys8 | FSCPLVMAGPNGLR | −4.2 | |||||||||
| RHGSGR | Non-toxin | Probable non-allergen | |||||||||||
| NANSLAGPQR | −8.2 | Arg415 (3), Val418, Val465, Arg483 | Ser363, Gly364, Leu365, Arg380, Asn382, Asn414, Arg415, Ile416, Gly417, Ile461, Gly462, Val463, Val465, Phe478, Arg483, Ser508, Gly509, Tyr525, Gln530, Ser555, Ala556, Ile559, Phe577, Gly603 | - | |||||||||
| NMVPGR | −8.1 | Ser363, Leu365, Asn382, Ser602 | Tyr334, Ser363, Gly364, Leu365, Ala366, Asn382, Arg415, Ile416, Ile461, Gly462, Ser508, Gly509, Ala510, Tyr525, Gln530, Ser555, Ala556, Ser602 | - | |||||||||
| SSPATGGSLR | −8.1 | Ser363, Arg380, Asn414, Arg415, Ser431, Ser602 | Tyr334, Gly364, Leu365, Arg380, Asn382, Asn414, Arg415, Ile416, Ser431, Gly433, His436, Gly462, Phe478, Arg483, Ser508, Gly509, Ala556, Ser602, Gly603 | - | 0.44319 | ||||||||
| CPP | PVVWAAKR | RHGSGR | 20 mM KCl | −3.9 | Arg1, Arg6 | Arg1, Gly5, Arg6 | |||||||
| Arg14 | |||||||||||||
| NDGPSR | −8.0 | Arg415 (2), Ala510 | Tyr334, Gly364, Leu365, Arg415, Ile461, Gly462, Phe478, Ser508, Gly509, Tyr525, Ala556, Ser602, Gly603, Val604 | - | FSCPLVMKGPNGLA | ||||||||
| DEQIPSHPPR * | −8.0 | Tyr334, Asn414, Arg415 (4), Ser431, Arg483 (3), Ser555 | Tyr334, Ser363, Arg380 | 0 mM KCl | 0.43744 | ||||||||
| −4.7 | DFPGAK | 200 mM KCl | 0.43574 | ||||||||||
| AGFPLGK | 200 mM KCl | −3.7 | - | Phe3, Pro4, Leu5 | FPLPSF * | - | 0.43352 | ||||||
| DFPGAK | 200 mM KCl | −3.6 | - | Pro3, Gly4, Lys6 | FSCPLVMKGPNGLR | 0 mM KCl | |||||||
| FPLPSF * | - | −4.6 | Phe1, Ser5 | Phe1, Pro2, Leu3, Pro4, Ser5 | |||||||||
| WAFAPA * | |||||||||||||
| , Asn382, Asn414, | Arg415 | YETLNR | Non-toxin | Probable non-allergen | Non-CPP | ||||||||
| AGFPLGK | Non-toxin | Probable non-allergen | Non-CPP | ||||||||||
| KVPLAVFSR | Non-toxin | Probable non-allergen | Non-CPP0.41864 | ||||||||||
| - | −4.3 | - | Trp1, Ala4, Pro5 | ||||||||||
| TAGASLVAR | Non-toxin | Probable allergen | Non-CPP | WAFAPA * | - | 0.41519 | |||||||
| YTWKFKGR | Non-toxin | Probable allergen | CPP | RHGSGR | 20 mM KCl | 0.41088 | |||||||
| VGPWQK * | - | −3.9 | - | Pro3, Trp4, Lys6 | |||||||||
| AMQQDK | Non-toxin | Probable allergen | CPP | VHFNKGKKR | 0 mM KCl | MYPGLA * | -0.41055 | ||||||
| −3.8 | Pro3 | NANSLAGPQR | |||||||||||
| MPPKSTR | Non-toxin | Probable allergen | CPP | 200 mM KCl | 0.40415 | ||||||||
| PVVWAAKR | Non-toxin | Probable allergen | CPP | QVASGPLQR | 0 mM KCl | ||||||||
| DFPGAK | Non-toxin | Probable allergen | 0.32957 | ||||||||||
| NMVPGR | Arg6 | NMVPGA | −3.1 | Pro3, Leu5, Ala6 | 0.40213 | Non-CPP | |||||||
| FMFFVYK | Non-toxin | Probable allergen | Non-CPP | ||||||||||
| QVASGPLQR | MCFHHHFHK | 612.27 | 1222.53 | 67 | 56 | 44 | 0 | 0 | |||||
| 200 mM KCl | DFPGAK | 317.66 | 633.33 | 17 | 17 | 67 | 17 | 17 | |||||
| NDGPSR | 323.15 | 644.29 | 0 | 17 | 33 | 0 | 0 | ||||||
| AGFPLGK | 345.20 | 688.41 | 14 | 14 | 86 | ||||||||
| MAPRTPRK | 0 mM KCl | 0.39973 | |||||||||||
| NDGPSR | 200 mM KCl | Non-toxin0.38760 | |||||||||||
| Probable allergen | Non-CPP | KRYFKR | 0 mM KCl | 0.38352 | |||||||||
| YETLNR | 200 mM KCl | 0.37938 | |||||||||||
| DEQIPSHPPR * | FQLKPVFR | 0 mM KCl | 0.37599 | 29 | 70 | ||||||||
| AMQQDK | 360.66 | 719.32 | 0 | 17 | 33 | 17 | 17 | ||||||
| NLEGYR | 376.19 | 750.38 | 17 | 17 | 50 | 17 | 65 | ||||||
| ARVPQQSYR | 0 mM KCl | 0.37580 | |||||||||||
| YTWKFKGR | 0 mM KCl | 0.36769 | |||||||||||
| AMQQDK | 200 mM KCl | 0.36324 | YETLNR | 398.20 | 794.41 | 17 | 17 | 33 | 17 | 65 | |||
| MPPKSTR | 408.72 | 815.43 | 0 | 29 | 43 | 0 | 0 | ||||||
| TAGASLVAR | 423.25 | 844.49 | 0 | 11 | 67 | 56 | 109 | ||||||
| SSPATGGSLR | 200 mM KCl | 0.35382 | |||||||||||
| THAVKGVVHK | 0 mM KCl | 0.35200 | |||||||||||
| MPPKSTR | 200 mM KCl | 0.33529SSPATGGSLR | 466.74 | 931.49 | 0 | 10 | 50 | 20 | 49 | ||||
| NANSLAGPQR | 514.27 | 1026.55 | 0 | 10 | 50 | 30 | 59 | ||||||
| PRVRVAGR |
| 0 mM KCl |
| 0.32698 |
| KVPLAVFSR |
| 0 mM KCl |
| 0.32525 |
| TAGASLVAR |
| 200 mM KCl |
| 0.32285 |
| TAPLSSKALKR |
| 0 mM KCl |
| 0.29320 |
| NKVVKLMR |
| 0 mM KCl |
| 0.27437 |
6. Molecular Docking between CS Peptides and ABTS•+
Docking simulation was performed to clarify the interactions between ABTS•+ and the 10 CS peptides with the best FRS scores. All seven peptides originating from the 0 and 20 mM KCl fractions had higher binding affinities towards ABTS•+ than the two peptides (AGFPLGK and DFPGAK) from the 200 mM KCl fraction (Table 3). Five of the seven peptides (from 0 and 20 mM KCl fractions) were also stronger than NLEGYR (from 200 mM KCl fraction) in binding to ABTS•+. The overall trend is in accordance with the relative levels of in vitro ABTS•+ scavenging activity of the three SPE fractions (Figure 3B). Notably, the binding affinities of all seven peptides from the 0 and 20 mM KCl fractions were more negative than that of reference peptides MYPGLA. The binding energy of three peptides MCFHHHFHK, VHFNKGKKR, and PVVWAAKR was up to 21% more negative than all four reference peptides (Table 3). Taken together, the seven peptides originating from the 0 and 20 mM KCl fractions could bind to ABTS•+ similarly or more stably than could the four reference peptides. Peptides that bind stably to free radicals can neutralize them. For instance, FPLPSF that was predicted to bind to ABTS•+ has been experimentally demonstrated to quench ABTS•+ in vitro [20]. Furthermore, our prediction of WAFAPA binding to ABTS| Non-toxin | |||
| Probable allergen | |||
| Non-CPP | |||
| DTETGVPT * | |||
| Non-toxin | |||
| Probable non-allergen | |||
| Non-CPP | |||
| VPY * | |||
| Non-toxin | |||
| Probable allergen | |||
| CPP | |||
| ACECD * | Non-toxin | Probable allergen | CPP |
| , Ser431, Gly433, His436, Gly462, Phe478, |
| Arg483 | |||
| , Ser508, Gly509, | |||
| Tyr525 | |||
| , Ser555, Ala556, | |||
| Tyr572 | |||
| , | |||
| Phe577 | , Ser602 | Arg483 | (2) |
| LKKGSPLKR | |||
| 0 mM KCl |
8. Molecular Docking of Peptides on MPO
In this study, CS peptides were compared with the soy tripeptide VPY and false abalone-derived DTETGVPT in their affinity towards MPO. Oral administration of VPY was shown to induce a 4-fold reduction in the MPO activity of mice [22]. Meanwhile, DTETGVPT is a potential inhibitor of MPO, as revealed by molecular docking simulation [23]. Our results revealed that 12 of the 29 CS peptides had up to 23% stronger affinity to MPO when compared with DTETGVPT, but were all weaker than VPY (Table S2). Five of the 12 potential MPO-binding CS peptides, namely NDGPSR, NLEGYR, NMVPGR, KRYFKR, and RHGSGR, were found to have low toxicity, low allergenicity, and cell-penetrating potential (Table 5). The five CS peptides would thus have an advantage over the reference peptide VPY (probable allergen) and DTETGVPT (non-cell-penetrating peptide) (Table 5) in the context of the application as food additives or functional food ingredients.
The interactions between the five CS peptides and MPO active site residues were highly similar to those between the reference peptides (VPY and DTETGVPT) and MPO (Table 7). With the exemption of NLEGYR, CS peptides and reference peptides were all forming only the hydrophobic interaction with the key residues in MPO active site. Likewise, similar to the reference peptides, each CS peptide could interact with 4–5 of the seven key residues in the active site of MPO. These key residues contributed to the stability of the interaction between 7-benzyl-1H-[1,2,3]triazolo[4,5-b]pyridin-5-amine, the bound inhibitor in the MPO crystal, and the active site of MPO [24]. As observed in the two reference peptides, all the five CS peptides could bind directly to the heme moiety of MPO (Table 7). Blockage of the heme pocket of MPO could preclude the access of substrates to the MPO active site, suppressing the enzyme’s action-driving ROS production [25].
Table 7. Binding affinities and types of interactions between myeloperoxidase (MPO) and five corn silk peptides predicted as non-toxic, non-allergenic and cell-penetrating peptides, in comparison with two reference peptides.
| Peptides | Binding Affinity (kcal/mol) |
Interaction with MPO a | ||
|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Interaction | Salt Bridge | ||
| NMVPGR | -6.6 | - | Phe99, Thr100, Glu102, Glu116, Pro145, Phe147, Leu216, Pro220, Arg239, Glu242, Phe366, Phe407, Met411, Arg424, Hec606 | - |
| NLEGYR | -6.5 | His95 | His95, Phe99, Glu102, Glu116, Pro145, Phe146, Phe147, Pro220, Thr238, Arg239, Glu242, Phe407, Val410, Met411, Leu420, Hec606 | - |
| NDGPSR | -6.3 | Glu102 | Phe99, Glu102, Glu116, Pro145, Phe146, Phe147, Pro220, Thr238, Arg239, Glu242, Phe366, Phe407, Met411, Leu415, Leu420, Hec606 | - |
| RHGSGR | -6.2 | Thr100, Thr238 | Phe99, Thr100, Glu102, Pro145, Phe146, Phe147, Leu216, Pro220, Thr238, Arg239, Glu242, Phe366, Phe407, Met411, Leu415, Hec606 | Glu102 (5) |
| KRYFKR | -5.5 | Thr100, Thr238 | His95, Phe99, Thr100, Glu102, Glu116, Pro145, Phe147, Pro220, Thr238, Arg239, Glu242, Phe366, Phe407, Val410, Met411, Leu415, Leu420, Hec606 | Glu102 (2) |
| VPY * | -7.4 | - | His95, Phe99, Thr100, Glu102, Pro220, Thr238, Arg239, Glu242, Phe366, Hec606 | - |
| DTETGVPT * | -5.5 | Thr238 | Phe99, Thr100, Glu102, Phe147, Pro220, Thr238, Arg239, Glu242, Phe366, Phe407, Met411, Leu415, Leu420, Hec606 | - |
* Indicates reference peptides. a Number in brackets indicates the number of interactions. Residues in bold are key residues (Gln91, His95, Phe99, Arg239, His336, Phe366, and Phe407) in the active site of MPO [24].
9. Molecular Docking of Peptides on XO
Only 14 of the 29 CS peptides were docked onto XO successfully, yielding negative values for binding affinity (Table S2). Docking of NDGPSR and DFPGAK onto XO involved the lowest binding energy, thus the strongest affinity, among the 14 peptides. Furthermore, NDGPSR and DFPGAK were predicted to bind equally stably to XO as was ACECD, the reference peptide. ACECD, an XO inhibitory peptide derived from Skipjack tuna hydrolysate, was reported to exert its inhibition by binding to the active site of XO [26]. In light of the predicted allergenicity and non-cell-penetrating potential of DFPGAK (Table 5), we proceeded to analyze the intermolecular interactions in only the NDGPSR-XO docked model.
As shown in Table 8, our LigPlot+ analysis revealed that NDGPSR could interact with catalytically critical residues (Glu802 and Arg880), substrate binding-residue (Phe914, Phe1009, and Thr1010), and the residues associated with the extended solvent-accessible channel leading to the molybdenum active center (Leu873, Val1011, Phe1013, and Leu1014) [27]. Most of such interactions are hydrophobic in nature. The dominance of hydrophobic interactions was also observed in our ACECD-XO docked model (Table 8). Meanwhile, NDGPSR could form hydrophobic interaction with Glu1261, an amino acid around the active center of XO, and contribute to the catalytic reaction of XO [26]. Moreover, as observed in ACECD, NDGPSR could also interact with Leu648 and Phe649 through hydrophobic interactions. The two residues are at the gate of the aforementioned solvent-accessible channel [27]. Interactions with Leu648 and Phe649 were reported to enhance the potency of quercetin as an XO inhibitor [27]. Altogether, our results suggest that NDGPSR can potentially occupy the catalytic center of XO, hindering the entry of XO substrates, thereby suppressing XO activity and ROS generation. In addition to their protective role in cells, antioxidant peptides with XO inhibitory activity are useful in reducing milk-fat oxidation in the dairy industry [28]. Thus, NDGPSR may also be developed into a potent antioxidant for the formulation of dairy products and other oxidation-prone foods.
Table 8. Binding affinity and types of interactions between xanthine oxidase (XO) and corn silk peptide NDGPSR, which was predicted to be a non-toxic, non-allergenic, and cell-penetrating peptide, in comparison with a reference peptide.
| Peptides | Binding Affinity (kcal/mol) |
Interaction with XO a | ||
|---|---|---|---|---|
| Hydrogen Bond | Hydrophobic Interaction | Salt Bridge | ||
| NDGPSR | -5.2 | Ser876, Thr1010, Val1011 | Leu648, Phe649, Gly799, Glu802, Leu873, His875, Ser876, Arg880, Phe914, Phe1009, Thr1010, Val1011, Pro1012, Phe1013, Leu1014, Ala1078, Ala1079, Glu1261 | His875, Glu1261 (2) |
| ACECD * | -5.2 | His875, Ser876 | Leu648, Phe649, Glu802, Leu873, His875, Ser876, Glu879, Phe914, Phe1009, Thr1010, Val1011, Pro1012, Phe1013, Leu1014 | - |
* Indicates reference peptide. a Number in brackets indicates the number of interactions. Residues in bold are key residues (Glu802, Leu873, Arg880, Phe914, Phe1009, Thr1010, Val1011, Phe1013, and Leu1014) in the active site of XO [27].
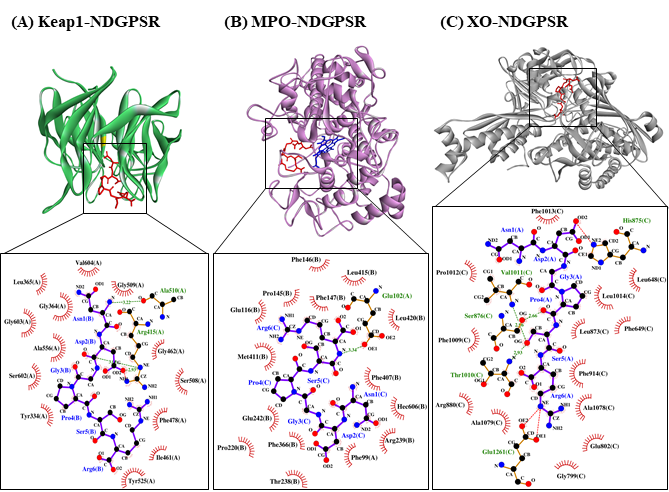
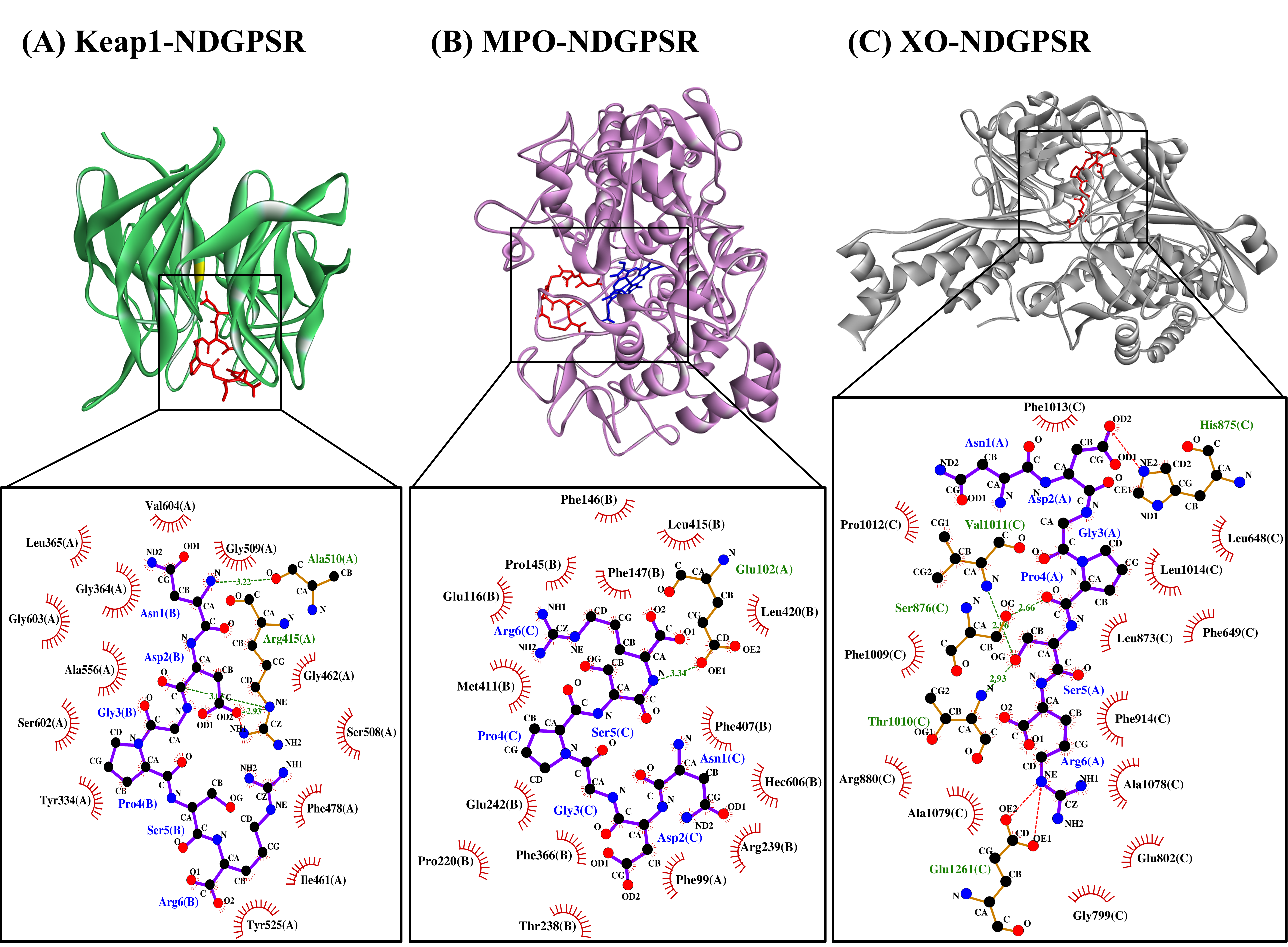
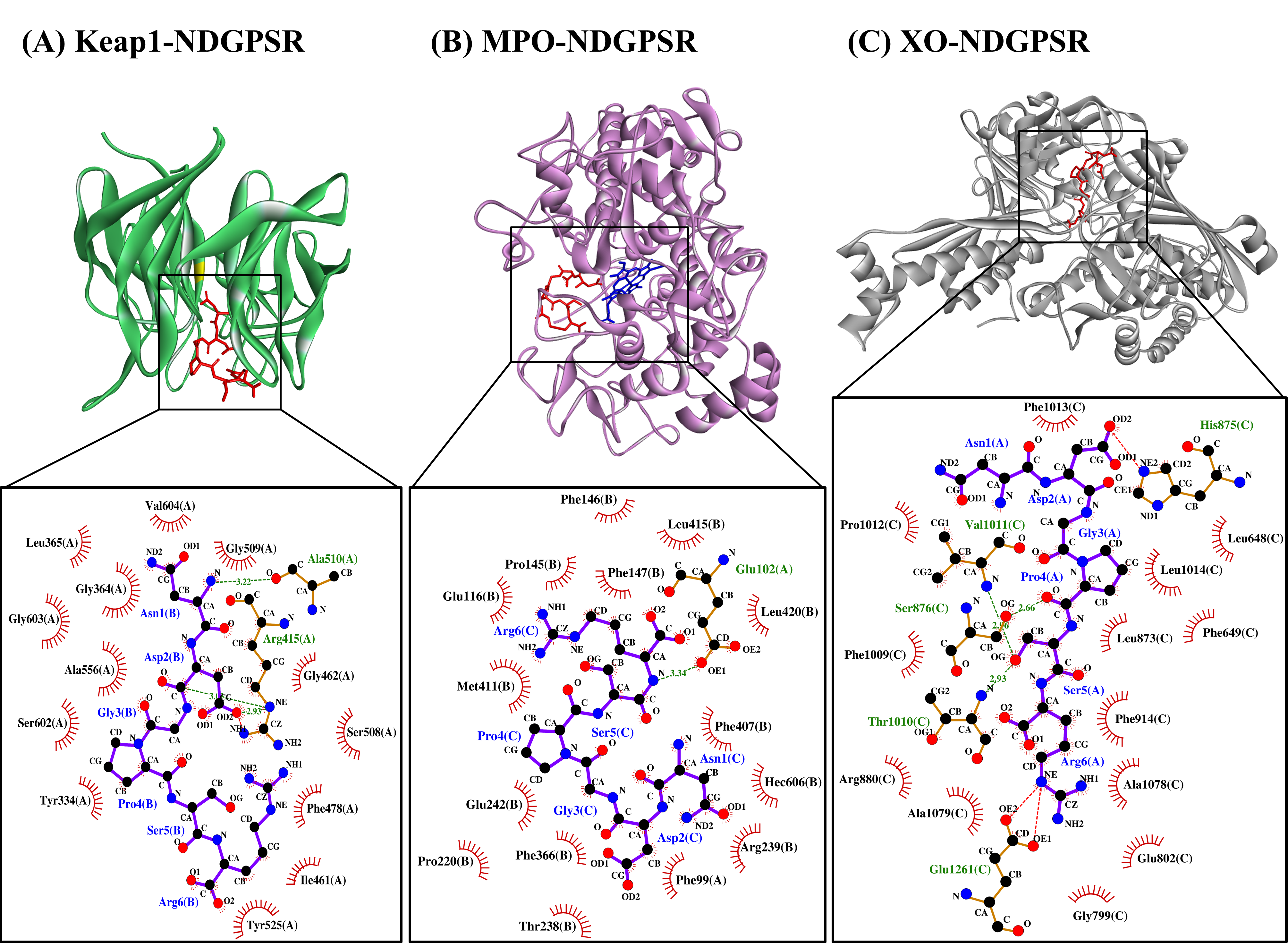
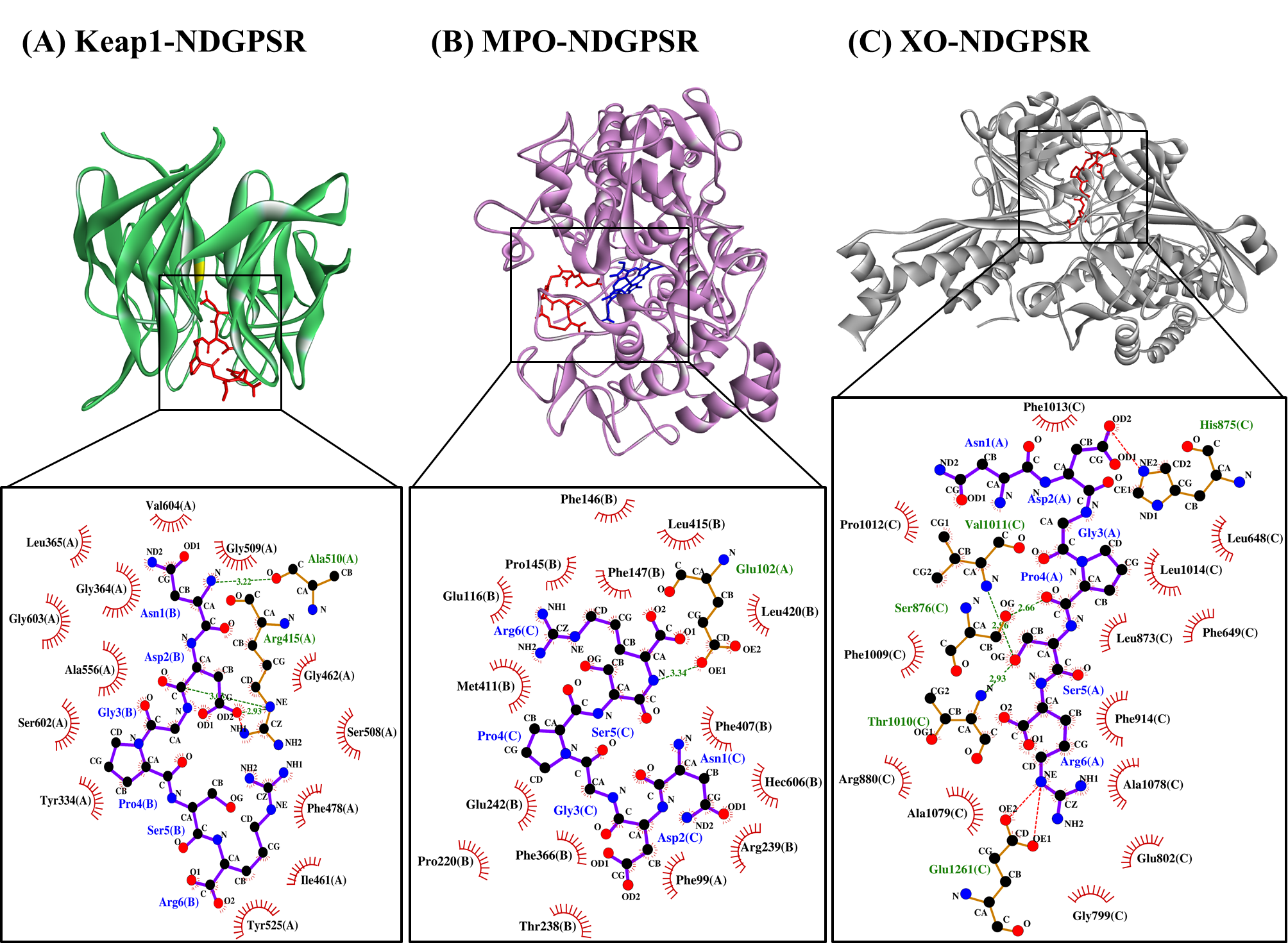
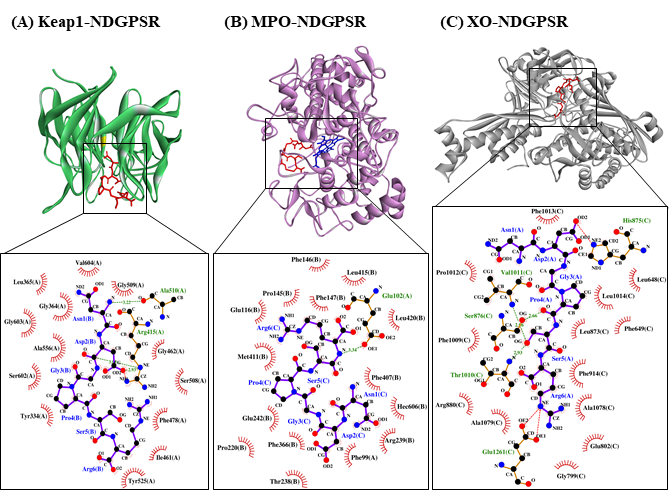
Our in silico analysis revealed three multifunctional peptides that are also non-toxic, non-allergenic, and have cell-penetrating potential, namely NDGPSR, NLEGYR, and NMVPGR. Among the three, NDGPSR could be a potential inhibitor of Keap1-Nrf2 interaction, MPO, and XO. Models of NDGPSR docked to the three protein targets are shown in Figure 4. Moreover, NLEGYR and NMVPGR could be potential dual-function inhibitors of Keap1-Nrf2 interaction and MPO (Table S2). Antioxidant peptides possessing multiple functionalities likely have greater versatility and commercial value when compared to other antioxidant peptides [29]. In this context, the three multifunctional peptides, with their safety and cell-penetrating properties, are desirable candidates for the future development of functional food ingredients and/or health supplements.
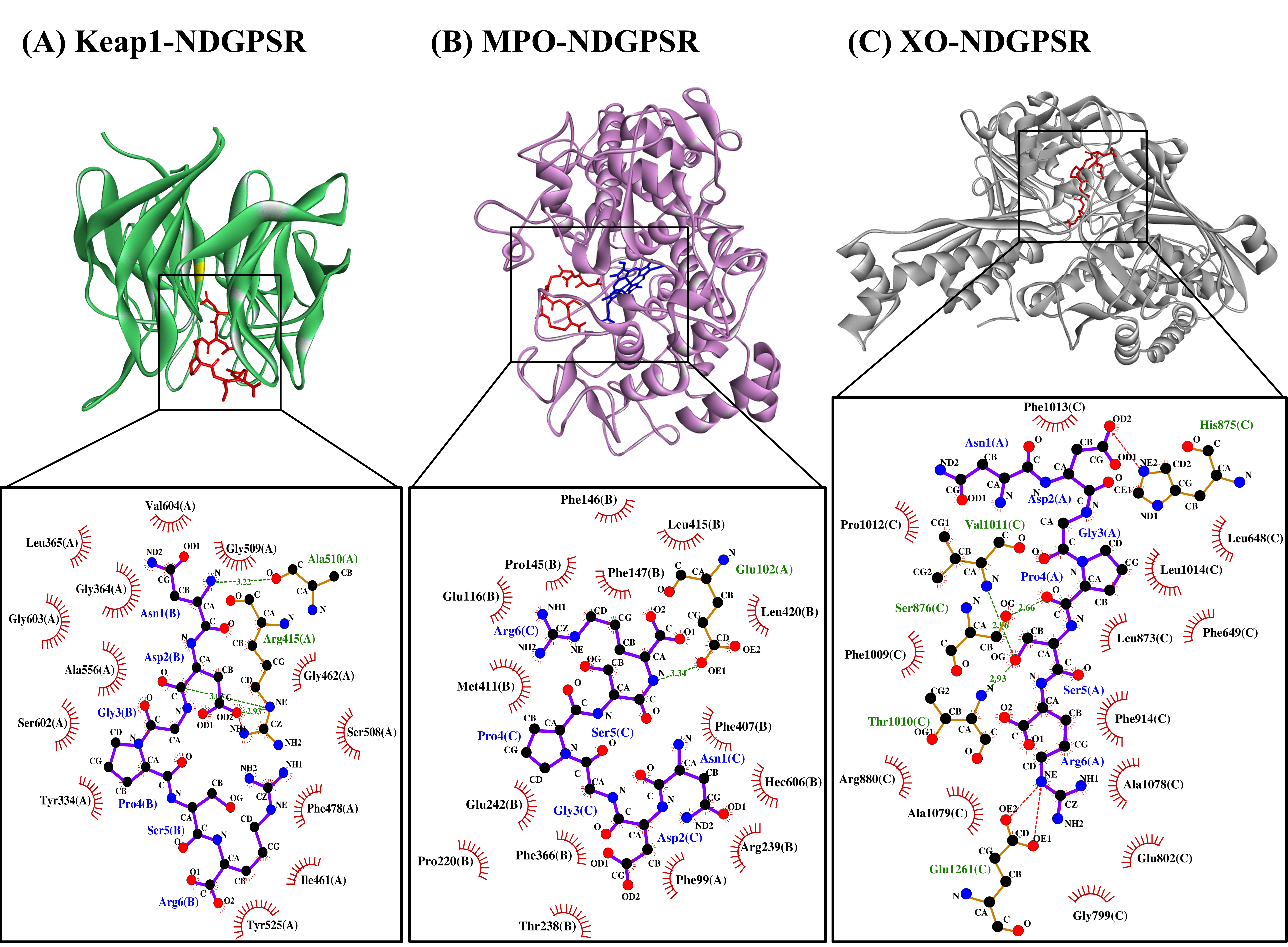
Figure 4. The docked models of NDGPSR interacting with (A) Keap1, (B) MPO, and (C) XO in 3D (top) and 2D (bottom) diagrams. In the 3D diagrams, NDGPSR is displayed in red; the heme moiety of MPO in (B) is displayed in blue. In the 2D diagrams, bonds of proteins are in orange, whereas those of peptides are in purple. Hydrophobic interactions, hydrogen bonds, and salt bridges are represented in red spoked arcs, green dashed lines, and red dashed lines, respectively.
10. Conclusions
In this study, 29 potential antioxidant peptides were purified and identified, for the first time, from a CS hydrolysate. The prevalence of aromatic and basic residues, in addition to binding affinity to ABTS·+, as revealed by molecular docking simulation, may account for the antioxidant activities of the peptides. Our in silico study also unraveled the potential of the peptides as inhibitors of Keap1-Nrf2 interaction, MPO and XO. NDGPSR stood out among the 29 peptides for its concurrent affinities towards the three protein targets, besides being predicted as non-toxic, non-allergenic, and having cell-penetrating potential. Taken together, our findings highlight the potential of CS as a source of antioxidant peptides with desirable properties for future applications in functional food and drug discovery. Future investigations by using in vitro and in vivo models are warranted for more in-depth exploration of CS-derived antioxidant peptides highlighted in this study.

References
- Hasanudin, K.; Hashim, P.; Mustafa, S. Corn silk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules 2012, 17, 9697–9715.
- Li, C.-C.; Lee, Y.-C.; Lo, H.-Y.; Huang, Y.-W.; Hsiang, C.-Y.; Ho, T.-Y. Antihypertensive effects of corn silk extract and its novel bioactive constituent in spontaneously hypertensive rats: The involvement of angiotensin-converting enzyme inhibition. Molecules 2019, 24, 1886.
- Ho, T.-Y.; Li, C.-C.; Lo, H.-Y.; Chen, F.-Y.; Hsiang, C.-Y. Corn silk extract and its bioactive peptide ameliorated lipopolysaccharide-induced inflammation in mice via the nuclear factor-κB signaling pathway. J. Agric. Food Chem. 2017, 65, 759–768.
- Chai, T.-T.; Ang, S.-Y.; Goh, K.; Lee, Y.-H.; Ngoo, J.-M.; Teh, L.-K.; Wong, F.-C. Trypsin-hydrolyzed corn silk proteins: Antioxidant activities, in vitro gastrointestinal and thermal stability, and hematoprotective effects. eFood 2020, 1, 156–164.
- Wong, F.-C.; Xiao, J.; Wang, S.; Ee, K.-Y.; Chai, T.-T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57.
- Tonolo, F.; Moretto, L.; Grinzato, A.; Fiorese, F.; Folda, A.; Scalcon, V.; Ferro, S.; Arrigoni, G.; Bellamio, M.; Feller, E.; et al. Fermented soy-derived bioactive peptides selected by a molecular docking approach show antioxidant properties involving the Keap1/Nrf2 pathway. Antioxidants 2020, 9, 1306.
- Winkel, A.F.; Engel, C.K.; Margerie, D.; Kannt, A.; Szillat, H.; Glombik, H.; Kallus, C.; Ruf, S.; Güssregen, S.; Riedel, J.; et al. Characterization of RA839, a noncovalent small molecule binder to Keap1 and selective activator of Nrf2 signaling. J. Biol. Chem. 2015, 290, 28446–28455.
- Deng, Z.; Cui, C.; Wang, Y.; Ni, J.; Zheng, L.; Wei, H.-K.; Peng, J. FSGHF3 and peptides, prepared from fish skin gelatin, exert a protective effect on DSS-induced colitis via the Nrf2 pathway. Food Funct. 2020, 11, 414–423.
- Thaha, A.; Wang, B.-S.; Chang, Y.-W.; Hsia, S.-M.; Huang, T.-C.; Shiau, C.-Y.; Hwang, D.-F.; Chen, T.-Y. Food-derived bioactive peptides with antioxidative capacity, xanthine oxidase and tyrosinase inhibitory activity. Processes 2021, 9, 747.
- Sharmila, M.D.; Chai, T.-T.; Wong, F.-C. Antioxidant and protein protection potentials of fennel seed-derived protein hydrolysates and peptides. Mod. Food Sci. Technol. 2019, 35, 22–29.
- Hu, R.; Dunmire, K.M.; Truelock, C.N.; Paulk, C.B.; Aldrich, G.; Li, Y. Antioxidant performances of corn gluten meal and DDGS protein hydrolysates in food, pet food, and feed systems. J. Sci. Food Agric. 2020, 2, 100030.
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: A review. Mar. Drugs 2017, 15, 42.
- Feng, Y.-X.; Ruan, G.-R.; Jin, F.; Xu, J.; Wang, F.-J. Purification, identification, and synthesis of five novel antioxidant peptides from Chinese chestnut (Castanea mollissima Blume) protein hydrolysates. LWT 2018, 92, 40–46.
- Wang, X.; Zheng, X.; Kopparapu, N.; Cong, W.; Deng, Y.; Sun, X.; Liu, X. Purification and evaluation of a novel antioxidant peptide from corn protein hydrolysate. Process Biochem. 2014, 49, 1562–1569.
- Chai, T.-T.; Xiao, J.; Mohana Dass, S.; Teoh, J.-Y.; Ee, K.-Y.; Ng, W.-J.; Wong, F.-C. Identification of antioxidant peptides derived from tropical jackfruit seed and investigation of the stability profiles. Food Chem. 2021, 340, 127876.
- He, R.; Ju, X.; Yuan, J.; Wang, L.; Girgih, A.T.; Aluko, R.E. Antioxidant activities of rapeseed peptides produced by solid state fermentation. Food Res. Int. 2012, 49, 432–438.
- Osorio, D.; Rondón-Villarreal, P.; Torres, R. Peptides: A package for data mining of antimicrobial peptides. R J 2015, 7, 4–14.
- Wong, F.-C.; Xiao, J.; Ong, M.G.-L.; Pang, M.-J.; Wong, S.-J.; Teh, L.-K.; Chai, T.-T. Identification and characterization of antioxidant peptides from hydrolysate of blue-spotted stingray and their stability against thermal, pH and simulated gastrointestinal digestion treatments. Food Chem. 2019, 271, 614–622.
- Mishra, M.; Nagarajan, K. Assessment of antioxidant influence of short series peptides using hydrogen peroxide scavenging assay and superoxide radical scavenging activity. World J. Pharm. Pharm. Sci. 2018, 7, 1064–1070.
- Chen, T.; Chen, Z.; Wang, H.; Chen, X.; Yang, J.; Han, A.; Lin, D.-H.; Hong, J. Underlying action mechanism of a novel antioxidant peptide derived from Allium tuberosum Rottler protein hydrolysates and its protective effects on hydrogen peroxide induced cell injury. J. Funct. Foods 2018, 40, 606–613.
- Olsen, T.H.; Yesiltas, B.; Marin, F.I.; Pertseva, M.; García-Moreno, P.J.; Gregersen, S.; Overgaard, M.T.; Jacobsen, C.; Lund, O.; Hansen, E.B.; et al. AnOxPePred: Using deep learning for the prediction of antioxidative properties of peptides. Sci. Rep. 2020, 10, 21471.
- Kovacs-Nolan, J.; Zhang, H.; Ibuki, M.; Nakamori, T.; Yoshiura, K.; Turner, P.V.; Matsui, T.; Mine, Y. The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim. Biophys. Acta 2012, 1820, 1753–1763.
- He, S.; Zhang, Y.; Sun, H.; Du, M.; Qiu, J.; Tang, M.; Sun, X.; Zhu, B. Antioxidative peptides from proteolytic hydrolysates of false abalone (Volutharpa ampullacea perryi): Characterization, identification, and molecular docking. Mar. Drugs 2019, 17, 116.
- Shaw, S.A.; Vokits, B.P.; Dilger, A.K.; Viet, A.; Clark, C.G.; Abell, L.M.; Locke, G.A.; Duke, G.; Kopcho, L.M.; Dongre, A.; et al. Discovery and structure activity relationships of 7-benzyl triazolopyridines as stable, selective, and reversible inhibitors of myeloperoxidase. Bioorg. Med. Chem. 2020, 28, 115723.
- Zhang, Y.; He, S.; Bonneil, É.; Simpson, B.K. Generation of antioxidative peptides from Atlantic sea cucumber using alcalase versus trypsin: In vitro activity, de novo sequencing, and in silico docking for in vivo function prediction. Food Chem. 2020, 306, 125581.
- Zhong, H.; Abdullah; Zhang, Y.; Deng, L.; Zhao, M.; Tang, J.; Zhang, H.; Feng, F.; Wang, J. Exploring the potential of novel xanthine oxidase inhibitory peptide (ACECD) derived from Skipjack tuna hydrolysates using affinity-ultrafiltration coupled with HPLC-MALDI-TOF/TOF-MS. Food Chem. 2021, 347, 129068.
- Cao, H.; Pauff, J.M.; Hille, R. X-ray crystal structure of a xanthine oxidase complex with the flavonoid inhibitor quercetin. J. Nat. Prod. 2014, 77, 1693–1699.
- Thaha, A.; Wang, B.-S.; Chang, Y.-W.; Hsia, S.-M.; Huang, T.-C.; Shiau, C.-Y.; Hwang, D.-F.; Chen, T.-Y. Food-derived bioactive peptides with antioxidative capacity, xanthine oxidase and tyrosinase inhibitory activity. Processes 2021, 9, 747.
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: A review. Mar. Drugs 2017, 15, 42.