The term calcareous tufa, or freshwater travertine, is widely used in the scientific literature to describe carbonate deposits precipitated from cool groundwaters of meteoric origin enriched in CO2 (carbon dioxide) by percolating through organic soils.
- calcareous tufa
- climate change
- ground thermal gradient
1. Calcareous Tufa
2. Calcareous Tufa Deposition/Erosion and the CaCO3·CO2·H2O System
3. Types of Calcareous Tufa
- -
-
stromatolithic tufa, including sequences of laminae (usually 1–10 mm in thickness) formed during short depositional intervals characterized by the presence of particular encrusting microorganisms (Figure 1);
- -
-
microhermal tufa, consisting of strata lens whose fabric reveals the structure of constructing organisms (usually mosses or algae) encrusted in growth position;
- -
-
phytohermal tufa, exhibiting a layered/lensoid organization similar to microhermal tufa but larger and composed of large, encrusted plants, usually mosses, reeds, and other phanerogams (Figure 2 and Figure 3).



4. Calcareous Tufa Deposits and Landforms


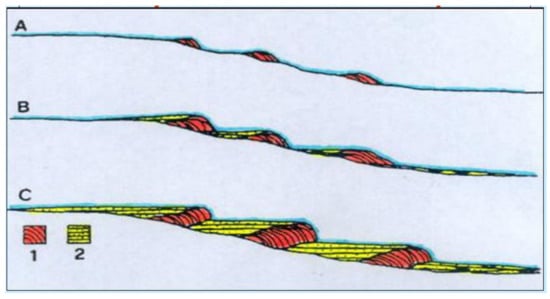

5. Factors Controlling Calcareous Tufa Deposition/Erosion
An explanatory model for the alternating periods of tufa deposition/erosion during geological times refers to the variations of thermal gradient induced in the bedrock by significant climate changes [56 (https://www.mdpi.com/2076-3417/13/7/4410#B49- applsci-13-04410)]. Due to the low thermal conductivity of bedrock [57], major climatic changes to warmer conditions, such as the rapid increase in air temperature at the Late Pleistocene-Holocene transition, induce significant thermal contrasts between the surface and the ground and reverse thermal gradients in the deep limestone aquifers. With climate warming, the infiltration water, made highly acidic when crossing the soil due to the elevated partial pressure of biogenic CO2 present therein, percolating through the progressively colder levels of the aquifer, causes relevant dissolution of CaCO3 [14 ], higher than in normal conditions. At the emergence, because of the higher surface temperatures, the groundwater loses CO2, becomes oversaturated with CaCO3 and produces tufa deposition, even at a great distance from the spring, favored by the running water turbulence, photosynthetic activity of mosses and algae, and evaporation of spray droplets. Opposite effects, such as deposition of dissolved carbonate in the upper bedrock layers and the emergence of aggressive spring waters undersaturated with CaCO3, are expected to occur with major climatic changes to cold conditions [11 ].
References
- Hynes, H.B.N. The Ecology of Running Waters; University Press: Liverpool, UK, 1978; p. 378.
- Chafetz, H.S.; Folk, R.L. Travertines: Depositional morphology and bacterial constructed constituents. J. Sediment. Petrol. 1984, 54, 289–316.
- Pentecost, A. Moss growth and travertine deposition: The significance of photosynthesis, evaporation and degassing of carbon dioxide. J. Bryol. 1996, 19, 229–234.
- Preece, R.C.; Robinson, J.E. Molluscan and ostracod faunas from Post-glacial tufaceous deposits in County Offaly. Proc. R. Ir. Acad. Sect. B Biol. Geol. Chem. Sci. 1982, 82B, 115–131.
- Krolopp, E. The importance of mollusc fauna in the study of travertine deposits. Földtany Közlöny 2003, 134, 219–225.
- Pentecost, A. Travertine; Springer-Verlag: Berlin, Germany, 2005; p. 445.
- Gandin, A.; Capezzuoli, E. Travertine versus calcareous tufa: Distinctive petrologic features and stable isotopes analysis. Alp. Mediterr. Quat. 2008, 21, 2125–2136.
- Gullentops, F.; Mullenders, W. Age et formation de dépôts de tuf calcaire Holocène en Belgique. In Proceedings of the Symposium International de Géomorphologie, Liège, Belgium, 29 June–4 July 1969.
- Goudie, A.S.; Viles, H.A.; Pentecost, A. The late-Holocene tufa decline in Europe. Holocene 1993, 3, 181–186.
- Frank, N.; Braum, M.; Hambach, U.; Mangini, A.; Wagner, G. Warm period growth of travertine during last Interglaciation in Southern Germany. Quat. Res. 2000, 54, 38–48.
- Fubelli, G.; Soligo, M.; Tuccimei, P.; Bonasera, M.; Dramis, F. Calcareous tufa deposition in connection with Late Pleistocene abrupt warming events. J. Ecol. Nat. Resour. 2021, 5, 000236.
- Schoeller, H. Les Eaux Souterraines; Masson and Cie: Paris, France, 1962; p. 642.
- Tillmans, J. Die Chemische Untersuchung von Wasser und Abwasser, 2nd ed.; Wilhelm Knapp: Halle, Germany, 1932; Volume 1, p. 252.
- Atkinson, T.C. Carbon dioxide in the atmosphere of the unsaturated zone: An important control of hardness in limestones. J. Hydrol. 1977, 35, 111–123.
- Drake, J.J. The effect of soil activity on the chemistry of carbonate groundwaters. Water Resour. Res. 1980, 16, 381–386.
- Roques, H. Observations physico-chemiques sur les eaux d alimentation de quelques concretions. Ann. Speleol. 1963, 18, 377–404.
- Moore, G.W. Introduction to the origin of limestone caves. Natl. Speleol. Soc. Bull. 1960, 22, 3–4.
- Bőgli, A. Mischungskorrosion-ein Beitrag zum Verkarstungs-problem. Erdlcunde 1964, 18, 83–92.
- Merz-Preiss, M.; Riding, R. Cyanobacterial tufa calcification in two freshwater streams: Ambient environment, chemicalthresholds and biological processes. Sediment. Geol. 1999, 126, 103–124.
- Dramis, F.; Fubelli, G.; Calderoni, G.; Esu, D. Holocene aggradation/degradation of tufa dams in northern Ethiopia and central Italy: A paleoclimatic comparison between East Africa and Mediterranean Europe. Z. Geomorphol. 2014, 58, 419–434.
- Buccino, G.; D’Argenio, B.; Ferreri, B.; Brancaccio, L.; Ferreri, M.; Panichi CStanzione, D. I travertini della bassa valle del Tanagro (Campania). Studio geomorfologico, sedimentologico e geochimico. Boll. Della Soc. Geol. Ital. 1978, 97, 617–646.
- D’Argenio, B.; Ferreri, V.; Stanzione, D.; Brancaccio, L.; Ferreri, M. I travertini di Pontecagnano (Campania) geomorfologia, sedimentologia, geochimica. Boll. Della Soc. Geol. Ital. 1983, 102, 123–136.
- Ferreri, M. Criteri di analisi di facies e classificazione dei travertini pleistocenici dell’Italia meriodionale. Rend. Acc. Scienze. Fis. Mat. 1985, 52, 121–147.
- Pedley, H.M. Classification and environmental models of cool fresh-water tufas. Sediment. Geol. 1990, 68, 143–154.
- Golubic, S.; Violante, C.; Ferreri, V.; D’Argenio, B. Algal control and early diagenesis in Quaternary travertine formation (Rocchetta a Volturno, Central Apennines). Boll. Della Soc. Paleontol. Ital. 1993, 1, 231–247.
- Riding, R. Classification of microbial carbonates. In Calcareous Algae and Stromatolites; Riding, R., Ed.; Springer: Berlin, Germany, 1991; pp. 21–51.
- Gradzinski, M.; Jach, R.; Stworzewicz, E. Origin of calcite-cemented Holocene slope breccias from the Dluga Valley (the Western Tatra Mountains). Ann. Soc. Geol. Pol. 2001, 71, 105–113.
- Choquette, P.N.; Pray, C. Geologic nomenclature and classification of porosity in sedimentary carbonates. Am. Assoc. Pet. Geol. Bull. 1970, 54, 207–250.
- Rodríguez-Berriguete, A.; Alonso-Zarza, A.M.; Martín-García, R. Diagenesis of continental carbonate country rocks underlying surficial travertine spring deposits. Quat. Int. 2017, 437, 4–14.
- Kahle, C.F. Origin of subaerial Holocene calcareous crusts: Role of algae, fungi and sparmicritization. Sedimentology 1977, 24, 413–435.
- Julia, R. Travertines. In Carbonate Depositional Environments; Scholle, P.A., Bebout, D.G., Moore, C.H., Eds.; AAPG: Tulsa, OK, USA, 1983; pp. 64–72.
- Viles, H.A.; Goudie, A.S. Tufas, travertines and allied carbonate deposits. Prog. Phys. Geogr. 1990, 14, 19–41.
- Berakhi, O.; Brancaccio, L.; Calderoni, G.; Coltorti, M.; Dramis, F.; Umer, M. The Mai Maikden sedimentary sequence: A reference point for the environmental evolution of the Highlands of Northern Ethiopia. Geomorphology 1998, 23, 127–138.
- Dramis, F.; Fubelli, G. Tufa dams in Tigray (Northern Ethiopia) as Late Pleistocene -Holocene climate proxies. In Landscapes and Landforms of Ethiopia; Billi, P., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 201–211.
- Golubic, S. Cyclic and non cyclic mechanism in the formation of travertine. Verh. Int. Ver. Limnol. 1969, 17, 956–961.
- Calderoni, G.; Cilla, G.; Dramis, F.; Esu, D.; Magnatti, M.; Materazzi, M. La deposizione di travertino nelle aree prossimali dei fiumi Esino, Potenza e Chienti durante l’Olocene antico (Appennino Centrale Marchigiano). Quaternario 1996, 9, 481–492.
- Dramis, F.; Mohamed, U.; Calderoni, G.; Mitiku, H. Holocene climate phases from buried soils in Tigray (northern Ethiopia): Comparison with lake level fluctuations in the Main Ethiopian Rift. Quat. Res. 2003, 60, 274–283.
- Fubelli, G.; Dramis, F.; Calderoni, G.; Cilla, G.; Materazzi, M.; Mazzini, I.; Soligo, M. Holocene aggradation/erosion of a tufa dam at Triponzo (Central Italy). Geogr. Fis. Din. Quat. 2013, 36, 259–266.
- Nicod, J. Facteurs physico-chimiques de 1’accumulation des formation travertineuses. Méditerranée 1986, 57, 161–164.
- Weisrock, A. Variations climatiques et périodes de sédimentation carbonatée à l’holocène. L’âge des dépôts. Méditerranée 1986, 57, 165–167.
- Pazdur, A.; Pazdur, M.F.; Starkel, L.; Szulc, J. Stable isotopes of Holocene calcareous tufa in southern Poland as paleoclimatic indicators. Quat. Res. 1988, 30, 177–189.
- Brook, G.A.; Folkoff, M.E.; Box, E.O. A world model of soil carbon dioxide. Earh Surf. Processes Landf. 1983, 8, 79–88.
- Hennig, G.J.; Grun, R.; Brunnacker, K. Speleothems, travertines and paleoclimates. Quat. Res. 1983, 20, 1–29.
- Bonifay, E. Origine et age des formations travertineuses de la Vallee de l’Huveaune entre Roquevaire et Auriol (Bouches du Rhone). Méditerranée 1986, 57, 101–104.
- Vaudour, J. Travertins holocenes et pression anthropique. Méditerranée 1986, 57, 165–167.
- Allen, E.T. The agency of algae in the deposition of travertine and silica from thermal waters. Am. J. Sci. 1934, 28, 373–389.
- Ordonez, S.; Gonzalez Martin, J.A.; Garda del Cura, M.A.; Pedley, H.M. Temperate and semi-arid tufas in the Pleistocene to recent fluvial barrage system in the Mediterranean area: The Ruidera Lakes Natural Park (Central Spain). Geomorphology 2005, 69, 332–350.
- Viles, H.A.; Taylor, M.P.; Nicoll, K.; Neumann, S. Facies evidence of hydroclimatic regime shifts in tufa depositional sequences from the arid Naukluft Mountains, Namibia. Sediment. Geol. 2007, 195, 39–53.
- Camuera, J.; Alonso-Zarza, A.M.; Rodriguez-Berriguete, A.; Melendez, A. Variations of fluvial tufa sub-environments in a tectonically active basin, Pleistocene Teruel Basin, NE Spain. Sedimentology 2012, 59, 502–526.
