Skin cancer is one of the most common types of cancer, and its incidence continues to increase. It is divided into two main categories, melanoma and non-melanoma. Treatments include surgery, radiation therapy, and chemotherapy. The relatively high mortality in melanoma and the existing recurrence rates, both for melanoma and non-melanoma, create the need for studying and developing new approaches for skin cancer management. Studies have focused on immunotherapy, photodynamic therapy, photothermal therapy, and photoimmunotherapy. Photoimmunotherapy has gained much attention due to its excellent potential outcomes. It combines the advantages of photodynamic and/or photothermal therapy with a systemic immune response, making it ideal for metastatic cancer.
- immunotherapy
- photothermal therapy
- photodynamic therapy
- melanoma
- basal cell carcinoma
- squamous cell carcinoma
1. Introduction
1.1. Immunotherapy
Cancer immunotherapy has been contributing to improved survival and quality of life for cancer patients. It consists in triggering the immune system to control and fight cancer, overcoming the mechanisms that cancer cells develop to escape immune surveillance and avoid detection and elimination [18,19][18][19]. Immunotherapy started more than 100 years ago, in New York, with Dr. Coley, who treated sarcoma patients with Coley’s toxin, a vaccine with a mixture of two bacterial toxins [20]. Currently, there are two types of immunotherapies, active and passive immunotherapy. Active immunotherapy stimulates the patient’s immune system, whereas passive immunotherapy can be with the administration of, for example, cytokines, vaccines, and antibodies [21,22,23][21][22][23]. The immune system plays a critical role in recognizing, eliminating, and controlling tumor progression. However, cancer cells develop mechanisms to avoid it, namely: (i) downmodulation of components of antigen processing and presentation machinery; (ii) an environment that promotes suppressor immune cells, such as regulatory T cells (Treg), an immunosuppressive subset of CD4+ T-cell family, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages, which are anti-inflammatory macrophages (M2- like); (iii) production of soluble factors associated with immunosuppression, such as TGF-β and IL-10; (iv) and upregulation of ligands for coinhibitory receptors that downmodulate programmed death ligand-1 (PD-L1) [24,25,26,27,28,29][24][25][26][27][28][29]. Dendritic cells (DCs) induce the differentiation of T cells to their antigen-specific effector T cells. CD4+ T cells are responsible for inducing DC maturation and for CD8+ T-cell priming. The primed cells are activated to form cytotoxic T lymphocytes, and these are responsible for releasing INF-γ and TNF-α, which will induce cytotoxicity in cancer cells. INF-γ is produced by both CD4+ and CD8+ T cells and stimulates the antitumor pro-inflammatory macrophages (M1). These tumor suppressor cells, such as cytotoxic T lymphocytes, also upregulate the release of pro-inflammatory cytokines, namely, IL-2, IL-6, IL-12, INF-γ, and TNF-α [24,30,31,32][24][30][31][32]. The higher calreticulin (CRT) exposure and release of high mobility group box 1 (HMGB-1) also act as an “eat-me” signal to induce tumor cell apoptosis [33,34,35,36][33][34][35][36].1.2. Phototherapy for Cancer Treatment
Phototherapy (PT) started 4000 years ago in ancient Egypt to treat Vitiligo, when a plant extract was boiled and then combined with sun exposure. Modern phototherapy, on the other hand, started only in the 70s, using artificial light sources [37,38,39][37][38][39]. Phototherapy is mainly divided into two categories, photodynamic therapy (PDT) and photothermal therapy (PTT). In PDT, a photosensitizer agent is irradiated by light to generate reactive oxygen species (ROS). These are highly toxic, causing cell death. PTT is based on local temperature increase, usually triggered by laser radiation. Usually, NIR lasers (650–1350 nm) are used in PTT due to their efficiency in penetrating tumors [40,41,42,43][40][41][42][43]. PTT can be divided into two categories. In the first, called mild hyperthermia, temperature increases up to 43–50 °C, leading to enhanced membrane permeability, cellular uptake, metabolic signaling disruption, and dysfunctional membrane transport. The capability of tumor cells to recover from such damages is very low. The second one is photothermal ablation (>50 °C), which destroys the cellular membrane, leading to necrotic cell death [44]. PDT has been FDA-approved for almost 40 years [40,45][40][45]. Hematopotphyrin derivative (HPD) was the first PS receiving FDA approval, nowadays Foscan®, Levulan®, Radachlorin®, Metvix®, and Photofrin® are FDA-approved PS [39,43][39][43].1.3. Photoimmunotherapy for Cancer Treatement
PT triggers immunogenic cell death (ICD) that will release tumor-specific antigens (TSAs) and damage-associated molecular patterns (DAMPs), namely CRT, HMGB-1, and ATP. This phenomenon increases the immunogenicity of the tumor microenvironment once DAMPs induce the maturation of DCs, and pro-inflammatory cytokines, such as IL-2, IL-6, IL-12, INF-γ and TNF-α, were also reported to increase. The immunostimulatory effect of PT boosts anti-tumor immunity when compared to immunotherapy alone. Although immunotherapy by itself can be effective in triggering the immune response at tumor site, it is inefficient to eradicate primary tumors [46,47,48,49,50][46][47][48][49][50]. When combining phototherapy with immunotherapy, in photoimmunotherapy (PIT), a synergy is reported to occur between them. PT directly kills the tumor cells and triggers a systemic immune response, and when in combination with immunotherapy, immunological memory is formed. Photoimmunotherapy has the advantages of phototherapy and the ability to trigger an immune response, making it ideal for treating metastatic cancer. Thereby, PIT eradicates primary tumors and, through simultaneously stimulating immune memory, it has the potential to prevent tumor recurrence and metastasis [42,46,51,52][42][46][51][52].2. Nanomaterials
2.1. Structure and Properties
In recent years, nanomaterials have gained much interest in the biomedical field, namely in drug delivery, tissue engineering, diagnosis, and theragnostics, amongst others. Nanomaterials can be divided into different categories according to their properties (e.g., size, shape, physicochemical properties, etc.). Regarding nanomaterials used for skin cancer photoimmunotherapy, the focus of this revisewarch, the main categories found are metallic, polymeric, lipid-based and 2D nanomaterials [53], as illustrated in Figure 1.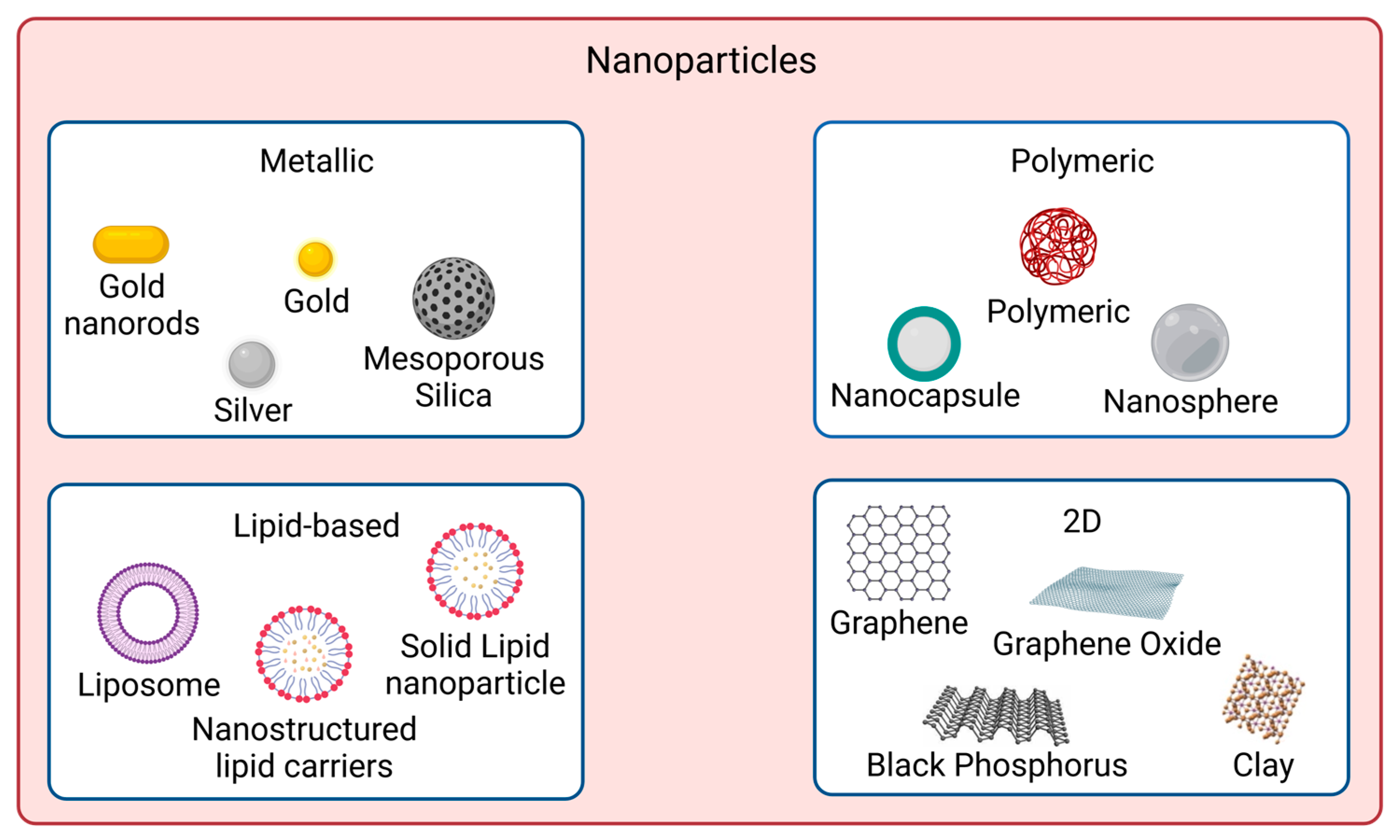
2.2. Surface Modification and Encapsulation Strategies
Nanomaterials to be used for biomedical applications should have good stability in physiological conditions and biocompatibility. Many nanoparticles agglomerate in aqueous solutions due to being hydrophobic or creating strong interparticle interactions, for instance. Covalent and non-covalent surface functionalization can be performed to enhance nanoparticles properties. However, such modifications should not affect nanomaterials photoabsorption properties if skin cancer photoimmunotherapy applications are desired. Figure 2 shows examples of such approaches.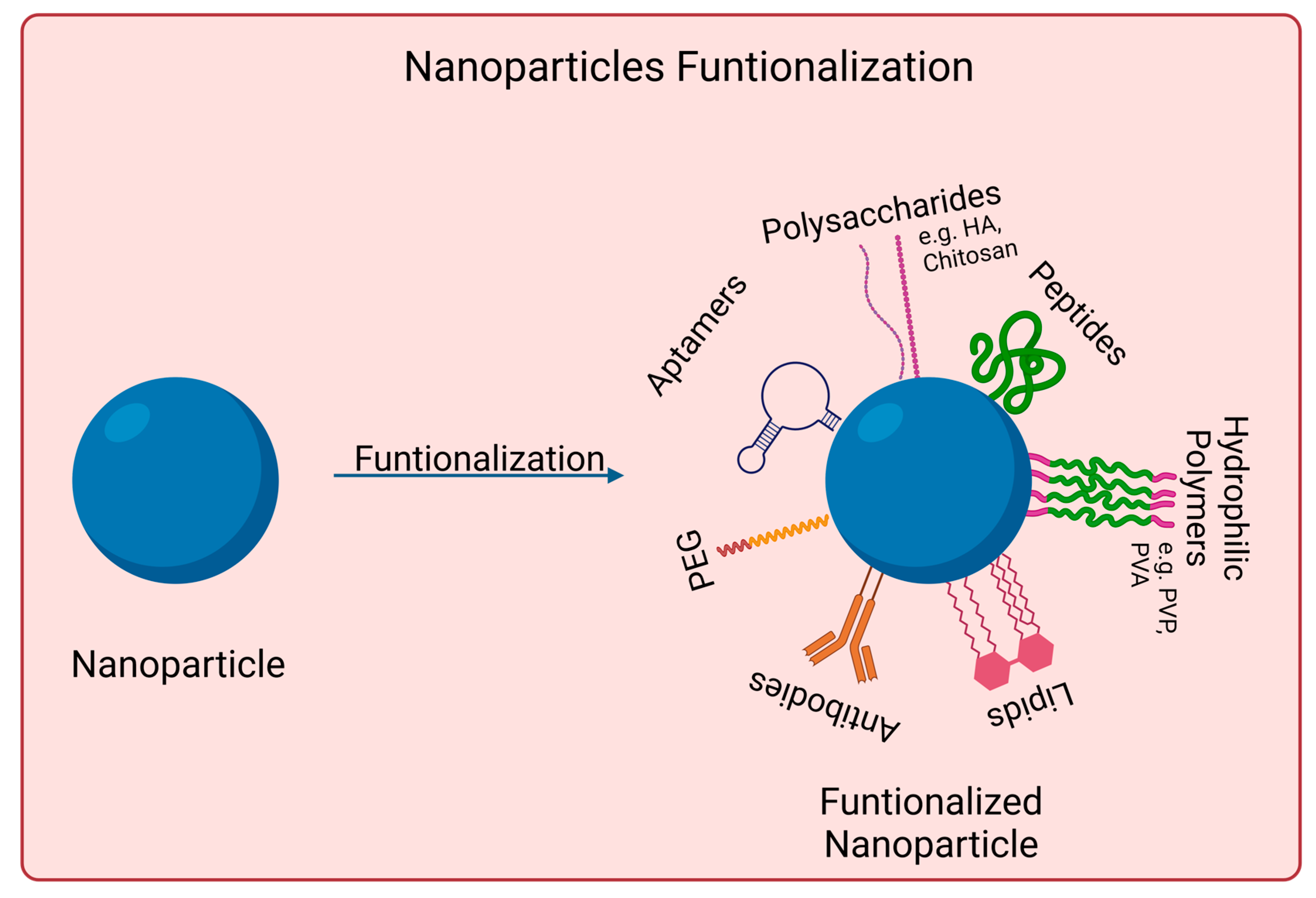
3. Skin Cancer Photoimmunotherapy Studies
Skin cancer is one of the most common cancers. Although there are already several treatment options available, there is still an urgent need to reduce mortality, recurrence rates, and side effects [3,8,91][3][8][91]. Photoimmunotherapy has gained much interest in recent years. It combines the advantages of phototherapy with enhancement of the immune response, resulting in a more effective cancer treatment approach. Figure 3 shows the mechanisms of action of photoimmunotherapy. Table 1 summarizes the state-of-the-art literature regarding nanomaterials used for skin cancer photoimmunotherapy, including their composition, size, and biological effects.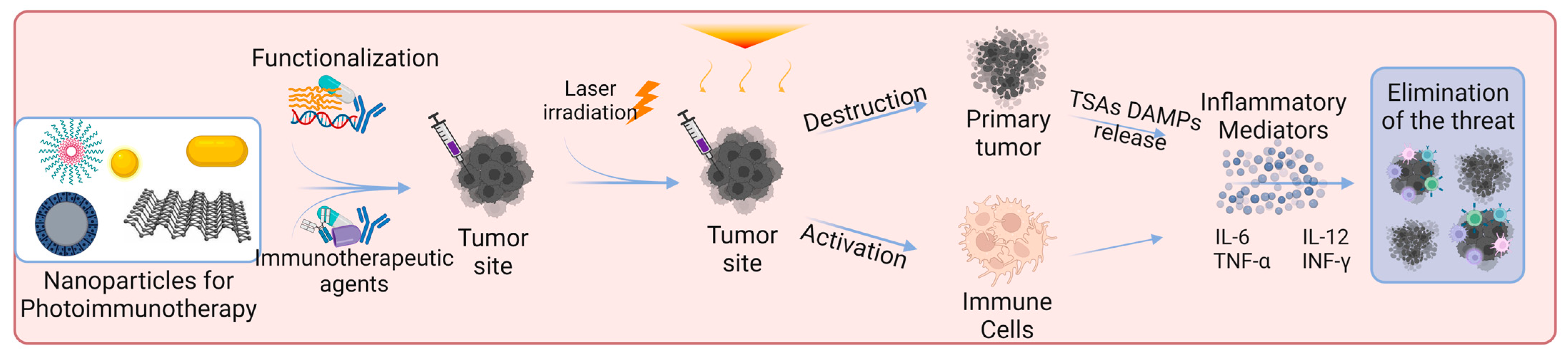
|
Nanomaterial |
Physicochemical Modifications |
Loaded Substance |
Particle Size |
In Vitro Studies |
In Vivo Studies |
Ref. |
||
|---|---|---|---|---|---|---|---|---|
|
Parameters |
Results |
Parameters |
Results |
|||||
|
Aluminum hydroxide |
BSA surface adsorption |
Chlorin e6 |
25.25 ± 2.1 nm |
B16F10 cells [Al-BSA-Ce6] = 0.1 μg mL−1 I: 660 nm, 0.8 W cm−2, 5 min |
95% cell death (MTT assay) ↑CD80 ↑TNF-α, IL-12p70, IL-1β |
C57BL/6 mice subcutaneously injected with B16F10 cells [Al-BSA-Ce6] = 5 mg kg−1 I: 660 nm, 0.8 W cm−2, 5 min |
Tumor volume of 0 mm3 at day 7 ≈63% mice survived 100 days ↑T cells tumor infiltration ↑TNF-α and INF-γ production |
[92] |
|
Black Phosphorus |
PEG electrostatic adsorption |
Imiquimod |
≈120 nm |
B16 cells [BP-PEG] = 10 μg mL−1 I: 808 nm, 3.2 W cm−2, 10 min |
45% cell viability↓ (MTT assay) ↑TNF-α, IL-6, IL-12 DCs↑ 30.8% |
C57BL/6 mice subcutaneously injected with B16 [BP] = 0.5 mg kg−1, 25 μL [R837] = 0.35 mg kg−1, 25 μL I: 808 nm, 3.2 W cm−2, 3 min |
≈10-fold tumor vol.↓ DCs↑ 45.5% ↑TNF-α, IL-6, IL-12 |
[93] |
|
Black Phosphorous |
N/A |
FKF-OVAp |
≈500 × 23 nm |
N/A |
N/A |
C57BL/6 mice subcutaneously injected with B16-OVA [FKF-OVAp] = 10 nmol per mouse [BPs] = 15.9 μg per mouse I: 808 nm, 0.5 W cm−2, 5 min |
≈3-fold tumor vol.↓ 100% survival over 60 days ↑DC activation ↑CD8+ T cells effector and central memory |
[94] |
|
Chitosan |
Cross-linking, sodium tripolyphosphate |
IDO |
220 nm |
B16 cells [ICG-NP] = 30 μg mL−1 I: 808 nm, 0.35 W cm−2, 5 min |
≈0% cell viability (CCK8 assay) ≈85% DC frequency |
C57BL/6 mice subcutaneously injected with B16F10 Drug loading: 4 and 35 μg per microneedle patch of ICG and 1-MT, respectively I: 808 nm, 0.35 W cm−2, 5 min 3 cycles at interval of 2 days |
Tumor volume of ≈0 mm3 80% survival rate without recurrence after 120 days ≈55% DC maturation level ↑CD8+ T cells in distant tumor ↑TNF-α, IL-12p70, IL-6 |
[95] |
|
Gold |
HA surface adsorption |
M2pep |
64.6 nm |
B16F10 cells [HA-AuNR/M-M2pep] = 20 μg mL−1 I: 808 nm, 1.5 W cm−2, 2 min |
40% cell viability (CCK8 assay) 35.1 ± 1.8% apoptosis (Annecin V-FITC) ↑CRT-positive cells and HMGB1 release |
C57BL/6 mice subcutaneously injected with B16F10 [AuNR] + [M2pep] = 10 + 12 mg kg−1 I: 808 nm, 1.5 W cm−2, 2 min |
≈10-fold tumor vol.↓ 67% survival rate at 45 days 3.7-fold↑ CD8+ T cells INF-γ, TNF-α↑ ≈ 6-fold |
[96] |
|
Gold |
BSA surface adsorption |
R837 |
122.1 ± 11.6 nm |
B16-F10 cells [Au] = 11.5 μg mL−1 I: 1064 nm, 1.0 W cm−2, 10 min |
Cell viability↓ to ≈27% (MTS assay) HSP70/β-Actin release ≈ 0 |
C57BL/6 mice subcutaneously injected with B16F10 [Au] = 300 μg mL−1 I: 1064 nm, 1.0 W cm−2, 10 min |
≈10-fold tumor vol.↓ TNF-α, IL-6, IL-12 ≈ 14, 9, 3 times↑ than PBS, respectively ↑CD8+ T cells infiltration |
[97] |
|
Gold |
Gold nanoparticles retained in extracellular vesicles with tumor antigens (AuNP@B16F10) |
Tumor antigens (AuNP@B16F10) |
40 nm |
N/A |
N/A |
Murine melanoma model subcutaneously injected with AuNP@DCB16F10 [Au] = 1.35 mg kg−1 I: 808 nm, 2.0 W cm−2, 1 min Cycle: 3 times, 3 days interval |
69% tumor volume↓ 50% tumor-free mice at day 19 Distant tumor inhibition ↑CD3+ and CD8+ T cells infiltration ↑INF-γ, TNF-α, IL-6 |
[98] |
|
Gold |
N/A |
SV |
50 nm |
B16-F10 cells [Au] = 60 μg mL−1 I: 808 nm, 1.5 W cm−2, 2 min |
≈12% cell viability (MTT assay) CRT expression ≈3.5-fold↑ ↑mature DCs frequency |
B16F10-bearing C57 mice I: 808 nm, 1.5 W cm−2, 2 min |
≈10-fold tumor vol.↓ ↑DC maturation CD4+ and CD8+ T cells proliferation |
[99] |
|
Hyaluronic acid |
Self-assembly of Ce6/α-linoleic acid (L-Ce6 NAs (nano-assemblies)) Fast dissolving L-Ce6 NAs in oligo-HA and micro-molding of microneedles (tips enriched with 3 µg Ce6) |
Ce6 |
≈86 nm |
B16F10 cells [Ce6] = 400 μM I: 660 nm, 200 mW cm−2, 5 min |
CRT fluorescence ↑2-fold ATP secretion ≈ 2.5 nM ↑HMGB1 release |
C57BL/6 mice subcutaneously injected with B16F10 [Ce6] = 0.12 mg kg−1 I: 660 nm, 200 mW cm−2, 4 min |
≈3-fold tumor vol.↓ ↑CD4+ and CD8+ ≈3 and 4-fold |
[100] |
|
Liposomes |
N/A |
TRP-2 |
180.4 ± 10.2 nm |
B16F10 cells [TLipIT NPs] = 100 μg mL−1 I: 808 nm, 0.75 W cm−2, 5 min |
≈12% early apoptosis (Annexin V-FITC) 37% late apoptosis ↑TNF-α, INF-γ |
C57BL/6 mice subcutaneously injected with B16F10 [TLipIT/NEs] = 100 μg mL−1 I: 808 nm, 0.75 W cm−2, 5 min |
≈10-fold tumor vol.↓ ≈33% CD80+ and CD86+ mature DCs frequency ≈49 and ≈33% CD4+ and CD8+ T lymphocytes frequency |
[101] |
|
Micelles |
N/A |
CQ IR780 |
80−90 nm |
B16 cells [C/I-Mil] = 4 μg mL−1 I: 808 nm, 1.0 W cm−2, 5 min |
20% cell viability (CCK-8 assay) Cell membrane integrity destroyed Phagocytic index ↑3.0-fold |
C57BL/6 mice subcutaneously injected with B16F10 [C/I-Mil] + [CQ/Mil] = 4 μg mL−1 + 20 μg/patch I: 808 nm, 1.0 W cm−2, 5 min |
Primary tumor suppression: 0 mm3 50% survived at least 40 days Distant tumor volume ↓3.4-fold |
[102] |
|
Micelles |
N/A |
Imiquimod |
72.0 ± 18.0 nm |
N/A |
N/A |
C57BL mice subcutaneously injected with B16 cells [IQPM] = 5 mg kg−1 I: 808 nm, 1.5 W cm−1, 5 min |
Primary tumor suppression: 0 mm3 CD8+ and CD4+ T cells ↑9.3- and 10.3-fold 2.4-fold↓ metastasis |
[103] |
|
mPEG-Pep-IDOi/ICG NPs |
N/A |
N/A |
140 nm |
B16-F10 cells [ICG] = 20 μg mL−1 I: 808 nm, 1.0 W cm−2, 5 min |
≈0% cell viability (CCK-8 assay) Induced ICD of tumor cells ≈70% CD80 and CD86↑ |
C57BL/6 mice subcutaneously injected with B16-F10 [ICG] = 4 mg kg−1 [IDOi] = 5 mg kg−1 I: 808 nm, 1.0 W cm−2, 5 min |
Primary tumor suppression: 0 mm3 CD80+ and CD86+ ↑13.5 and 12.3% ↑INF-γ, TNF-α, IL-6 |
[104] |
|
PBE |
N/A |
RSL-3 |
<100 nm |
B16-F10 cells [RSL-3] = 0.5 μg mL−1 [INF-γ] = 100 ng mL−1 I: 671 nm, 100 mW cm−2, 1 min |
30% mature DC CTR expression ↑5.0-fold |
C57BL/6 mice subcutaneously injected with B16-F10 [RSL-3] = 0.5 μg mL−1 [INF-γ] = 100 ng mL−1 I: 671 nm, 150 mW cm−2, 2 min |
≈2-fold tumor vol.↓ ≈50% survival rate ≈30% mature DC cells INF-γ secretion ↑ 4-fold |
[105] |
|
PEI-PBA |
PEG surface adsorption |
Ce6 aPDL1 |
117 ± 4.0 nm |
B16F10 cells [NC@Ce6-pH 6.0] = 7.5 μg mL−1 I: 650 nm, 20 mW cm−2, 2.5 min |
89% CRT rate ≈34% apoptosis (Annexin V-FITC) DC maturation |
B16F10 tumor-bearing mice [Ce6] = 2.5 mg kg−1 I: 650 nm, 100 mW cm−2, 10 min |
78% tumor inhibition rate ≈49% tumor infiltrating T cells DC maturation |
[106] |
|
Polydopamine |
PEI surface adsorption |
CpG oligodeoxynucleotides |
140 nm |
B16F10 cells [PPP/CpG/HA] = 200 μg mL−1 I: 808 nm, 2.0 W cm−2, 5 min |
≈5% cell viability (MTT assay) ≈60% apoptosis (Annexin V-FITC) ≈60% CD80+ DC ≈50% CD86+ DC |
C57BL/6 mice subcutaneously injected with B16F10 [PPP/CpG/HA] = 0.75 mg kg−1 I: 808 nm, 1.5 W cm−2, 5 min |
≈20-fold tumor vol.↓ Largest apoptotic cell area CD80+ DC ≈ 3% CD86+ DC ≈ 4.5% |
[107] |
|
Silicon Dioxide |
CuS loaded inside the pores PDMAEMA surface adsorption |
IL-12 gene |
157 nm |
B16F10 cells [CSP] = 34.5 μg mL−1 I: 1064 nm, 0.65 W cm−2, 5 min |
<20% cell viability (CCK-8 assay) 89% apoptotic cells (Annexin V-FITC) ↑CRT expression 66% DCs maturation |
B16F10-bearing C57BL/6 mice [CSP] = 172.4 μg per mouse I: 1064 nm, 0.65 W cm−2, 5 min |
≈3-fold tumor vol.↓ Prolonged survival DC maturation level: ≈48% 21% CD4+ and 12% CD8+ T populations |
[108] |
|
Silicon Dioxide |
Chemical synthesis of UCNP@m-SiO2@liposome NPs |
Ce6 and BSO |
≈50 nm |
B16/F10 cells [UCB] = 100 μg mL−1 I: 980 nm, 0.7 W cm−2, 10 min |
≈29% cell viability (CCK-8 assay) ≈39% apoptosis rate (Western Blot) ↑TNF-α, IL-6, INF-γ |
C57BL/6 mice subcutaneously injected with B16F10 [UCB] = 0.8 mg per mouse I: 980 nm, 0.7 W cm−2, 20 min |
≈3-fold tumor vol.↓ ↑IL-12p40, INF-γ |
[109] |
Abbreviations: ↑, increase; ↓, decrease; aPDL1, anti-programmed death-ligand 1; BSA, bovine serum albumin; B16 cells, B16 murine melanoma cell line; BSO, buthionine sulfoximine B16F10 cells, B16F10 murine melanoma cell line; B16-F10 cells, B16–F10 murine melanoma cell line; B16/F10 cells, B16/F10 murine melanoma cell line; CCK-8, cell counting kit-8; Ce6, chlorin e6; CQ, chloroquine; DC, dendritic cells; HA, hyaluronic acid; IDO, indoleamine 2,3-dioxygenase; M2pep, M2 macrophage-targeting peptide; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide; N/A, not applicable; PDMAEMA, poly((2-di- methylamino)ethyl methacrylate); PEG, polyethylene glycol; PEI, polyethyleneimine; R837, imiquimod; SV, simvastatin; TRP-2, tyrosinase-related protein 2.
References
- Linares, M.A.; Zakaria, A.; Nizran, P. Skin cancer. Prim. Care Clin. Off. Pract. 2015, 42, 645–659.
- Craythorne, E.; Al-Niami, F. Skin cancer. Medicine 2017, 45, 431–434.
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789.
- World Health Organization International Agency for Research on Cancer. GLOBOCAN 2020: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2020. Available online: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=population&mode_population=regions&population=900&populations=900&key=total&sex=0&cancer=16_17&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&nb_items=7&group_cancer=1&include_nmsc=1&include_nmsc_other=1&half_pie=0&donut=0 (accessed on 10 April 2023).
- Apalla, Z.; Nashan, D.; Weller, R.B.; Castellsagué, X. Skin Cancer: Epidemiology, Disease Burden, Pathophysiology, Diagnosis, and Therapeutic Approaches. Dermatol. Ther. 2017, 7, 5–19.
- Ahmed, B.; Qadir, M.I.; Ghafoor, S. Malignant Melanoma: Skin Cancer—Diagnosis, Prevention, and Treatment. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 291–297.
- Hogue, L.; Harvey, V.M. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol. Clin. 2019, 37, 519–526.
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42.
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of skin cancer: Update 2019. Adv. Exp. Med. Biol. 2020, 1268, 123–139.
- Kao, S.-Y.Z.; Ekwueme, D.U.; Holman, D.M.; Rim, S.H.; Thomas, C.C.; Saraiya, M. Economic burden of skin cancer treatment in the USA: An analysis of the Medical Expenditure Panel Survey Data, 2012–2018. Cancer Causes Control 2023, 34, 205–212.
- Collins, A.; Savas, J.; Doerfler, L. Nonsurgical treatments for nonmelanoma skin cancer. Dermatol. Clin. 2019, 37, 435–441.
- Qadir, M.I. Skin cancer: Etiology and management. Pak. J. Pharm. Sci. 2016, 29, 999–1003.
- Ferry, A.M.; Sarrami, S.M.; Hollier, P.C.; Gerich, C.F.; Thornton, J.F. Treatment of non-melanoma skin cancers in the absence of Mohs micrographic surgery. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3300.
- Rubin, A.I.; Chen, E.H.; Ratner, D. Basal-cell carcinoma. N. Engl. J. Med. 2005, 353, 2262–2269.
- Marzuka, A.G.; Book, S.E. Basal cell carcinoma: Pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J. Biol. Med. 2015, 88, 167–179.
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics 2019, 11, 22.
- Paliwal, S.R.; Kenwat, R.; Maiti, S.; Paliwal, R. Nanotheranostics for Cancer Therapy and Detection: State of the Art. Curr. Pharm. Des. 2020, 26, 5503–5517.
- Mohr, P.; Eggermont, A.M.M.; Hauschild, A.; Buzaid, A. Staging of cutaneous melanoma. Ann. Oncol. 2009, 20, vi14–vi21.
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H. A Review of Cancer Immunotherapy: From the Past, to the Present, to the Future. Curr. Oncol. 2020, 27, 87–97.
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489.
- Chen, J.A.; Ma, W.; Yuan, J.; Li, T. Translational Biomarkers and Rationale Strategies to Overcome Resistance to Immune Checkpoint Inhibitors in Solid Tumors. In Tumor Microenvironment; Lee, P.P., Marincola, F.M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 251–279.
- Abbott, M.; Ustoyev, Y. Cancer and the Immune System: The History and Background of Immunotherapy. Semin. Oncol. Nurs. 2019, 35, 150923.
- Aalipour, S.; Zoghi, S.; Khalili, N.; Hirbod-Mobarakeh, A.; Emens, L.A.; Rezaei, N. Specific immunotherapy in ovarian cancer: A systematic review. Immunotherapy 2016, 8, 1193–1204.
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521.
- Kuol, N.; Stojanovska, L.; Nurgali, K.; Apostolopoulos, V. The mechanisms tumor cells utilize to evade the host’s immune system. Maturitas 2017, 105, 8–15.
- Vaishampayan, P.; Curiel-Lewandrowski, C.; Dickinson, S.E. Review: PD-L1 as an emerging target in the treatment and prevention of keratinocytic skin cancer. Mol. Carcinog. 2023, 62, 52–61.
- Laureano, R.S.; Sprooten, J.; Vanmeerbeerk, I.; Borras, D.M.; Govaerts, J.; Naulaerts, S.; Berneman, Z.N.; Beuselinck, B.; Bol, K.F.; Borst, J.; et al. Trial watch: Dendritic cell (DC)-based immunotherapy for cancer. OncoImmunology 2022, 11, 2096363.
- Chen, B.-J.; Zhao, J.-W.; Zhang, D.-H.; Zheng, A.-H.; Wu, G.-Q. Immunotherapy of Cancer by Targeting Regulatory T cells. Int. Immunopharmacol. 2022, 104, 108469.
- Yang, Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J. Clin. Investig. 2015, 125, 3335–3337.
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity 2018, 49, 1148–1161.e7.
- Showalter, A.; Limaye, A.; Oyer, J.L.; Igarashi, R.; Kittipatarin, C.; Copik, A.J.; Khaled, A.R. Cytokines in immunogenic cell death: Applications for cancer immunotherapy. Cytokine 2017, 97, 123–132.
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15.
- Chiaravalli, M.; Spring, A.; Agostini, A.; Piro, G.; Carbone, C.; Tortora, G. Immunogenic Cell Death: An Emerging Target in Gastrointestinal Cancers. Cells 2022, 11, 3033.
- Zhou, J.; Wang, G.; Chen, Y.; Wang, H.; Hua, Y.; Cai, Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J. Cell. Mol. Med. 2019, 23, 4854–4865.
- Duan, X.; Chan, C.; Lin, W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem. Int. Ed. 2019, 58, 670–680.
- Dar, T.B.; Biteghe, F.A.N.; Kakar-Bhanot, R.; Aniogo, E.C.; Malindi, Z.; Akinrinmade, O.A.; Chalomie, N.E.T.; Kombe Kombe, A.J.; Aboughe Angone, S.; Ndong, J.M.N.; et al. Synergistic effects of radiotherapy and targeted immunotherapy in improving tumor treatment efficacy: A review. Clin. Transl. Oncol. 2022, 24, 2255–2271.
- van de Kerkhof, P.C.M.; de Gruijl, F.R. Phototherapy in the perspective of the chronicity of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 926–931.
- Jarrett, P.; Scragg, R. A short history of phototherapy, vitamin D and skin disease. Photochem. Photobiol. Sci. 2017, 16, 283–290.
- Shi, H.; Sadler, P.J. How promising is phototherapy for cancer? Br. J. Cancer 2020, 123, 871–873.
- Hak, A.; Ravasaheb Shinde, V.; Rengan, A.K. A review of advanced nanoformulations in phototherapy for cancer therapeutics. Photodiagn. Photodyn. Ther. 2021, 33, 102205.
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087.
- Ng, C.W.; Li, J.; Pu, K. Recent Progresses in Phototherapy-Synergized Cancer Immunotherapy. Adv. Funct. Mater. 2018, 28, 1804688.
- Zheng, Q.; Liu, X.; Zheng, Y.; Yeung, K.W.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wang, X.; Wu, S. The recent progress on metal–organic frameworks for phototherapy. Chem. Soc. Rev. 2021, 50, 5086–5125.
- Zhang, B.; Wang, Y.; Liu, J.; Zhai, G. Recent developments of phototherapy based on graphene family nanomaterials. Curr. Med. Chem. 2017, 24, 268–291.
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674.
- Liu, Y.; Zhang, L.; Chang, R.; Yan, X. Supramolecular cancer photoimmunotherapy based on precise peptide self-assembly design. Chem. Commun. 2022, 58, 2247–2258.
- Moriya, T.; Hashimoto, M.; Matsushita, H.; Masuyama, S.; Yoshida, R.; Okada, R.; Furusawa, A.; Fujimura, D.; Wakiyama, H.; Kato, T.; et al. Near-infrared photoimmunotherapy induced tumor cell death enhances tumor dendritic cell migration. Cancer Immunol. Immunother. 2022, 71, 3099–3106.
- Li, Y.; Cui, J.; Li, C.; Deng, C.; Deng, G.; Zhang, H.; An, F. Biomaterial-assisted photoimmunotherapy for synergistic suppression of cancer progression. Chin. Chem. Lett. 2023, 108180, in press.
- Zou, J.; Li, L.; Yang, Z.; Chen, X. Phototherapy meets immunotherapy: A win–win strategy to fight against cancer. Nanophotonics 2021, 10, 3229–3245.
- Wang, Y.; Wang, B.; Li, K.; Wang, M.; Xiao, H. Engineered metal and their complexes for nanomedicine-elicited cancer immunotherapy. Mater. Today Adv. 2022, 15, 100276.
- Peng, Z.; Lv, X.; Huang, S. Photoimmunotherapy: A New Paradigm in Solid Tumor Immunotherapy. Cancer Control 2022, 29, 10732748221088825.
- Guo, R.; Wang, S.; Zhao, L.; Zong, Q.; Li, T.; Ling, G.; Zhang, P. Engineered nanomaterials for synergistic photo-immunotherapy. Biomaterials 2022, 282, 121425.
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell. Physiol. 2020, 235, 1962–1972.
- Khan, S.A. Chapter 1—Metal nanoparticles toxicity: Role of physicochemical aspects. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Shah, M.R., Imran, M., Ullah, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–11.
- Shah, M.R.; Imran, M.; Ullah, S. Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2019.
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282.
- Kumar, H.; Venkatesh, N.; Bhowmik, H.; Kuila, A. Metallic nanoparticle: A review. Biomed. J. Sci. Tech. Res. 2018, 4, 3765–3775.
- Ahmad, M.Z.; Akhter, S.; Jain, G.K.; Rahman, M.; Pathan, S.A.; Ahmad, F.J.; Khar, R.K. Metallic nanoparticles: Technology overview & drug delivery applications in oncology. Expert Opin. Drug Deliv. 2010, 7, 927–942.
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615.
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B: Biointerfaces 2010, 75, 1–18.
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691.
- Castro, K.C.d.; Costa, J.M.; Campos, M.G.N. Drug-loaded polymeric nanoparticles: A review. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1–13.
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299.
- Abd Ellah, N.H.; Abouelmagd, S.A. Surface functionalization of polymeric nanoparticles for tumor drug delivery: Approaches and challenges. Expert Opin. Drug Deliv. 2017, 14, 201–214.
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731.
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109.
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508.
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638.
- Chutoprapat, R.; Kopongpanich, P.; Chan, L.W. A Mini-Review on Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Topical Delivery of Phytochemicals for the Treatment of Acne Vulgaris. Molecules 2022, 27, 3460.
- Shirodkar, R.K.; Kumar, L.; Mutalik, S.; Lewis, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Emerging Lipid Based Drug Delivery Systems. Pharm. Chem. J. 2019, 53, 440–453.
- Xu, Y.; Fourniols, T.; Labrak, Y.; Préat, V.; Beloqui, A.; des Rieux, A. Surface Modification of Lipid-Based Nanoparticles. ACS Nano 2022, 16, 7168–7196.
- Cheng, L.; Wang, X.; Gong, F.; Liu, T.; Liu, Z. 2D Nanomaterials for Cancer Theranostic Applications. Adv. Mater. 2020, 32, 1902333.
- Davis, R.; Urbanowski, R.A.; Gaharwar, A.K. 2D layered nanomaterials for therapeutics delivery. Curr. Opin. Biomed. Eng. 2021, 20, 100319.
- Mei, X.; Hu, T.; Wang, Y.; Weng, X.; Liang, R.; Wei, M. Recent advancements in two-dimensional nanomaterials for drug delivery. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1596.
- Singh, N.B.; Shukla, S.K. Chapter 3—Properties of two-dimensional nanomaterials. In Two-Dimensional Nanostructures for Biomedical Technology; Khan, R., Barua, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 73–100.
- Murali, A.; Lokhande, G.; Deo, K.A.; Brokesh, A.; Gaharwar, A.K. Emerging 2D nanomaterials for biomedical applications. Mater. Today 2021, 50, 276–302.
- Hu, T.; Mei, X.; Wang, Y.; Weng, X.; Liang, R.; Wei, M. Two-dimensional nanomaterials: Fascinating materials in biomedical field. Sci. Bull. 2019, 64, 1707–1727.
- García-Torra, V.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; Barroso, E.; Vazquez-Carrera, M.; García, M.L.; Sánchez-López, E.; Souto, E.B. State of the Art on Toxicological Mechanisms of Metal and Metal Oxide Nanoparticles and Strategies to Reduce Toxicological Risks. Toxics 2021, 9, 195.
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544.
- Pathak, Y.V. Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2019.
- Umut, E. Surface modification of nanoparticles used in biomedical applications. Mod. Surf. Eng. Treat. 2013, 20, 185–208.
- Moku, G.; Gopalsamuthiram, V.R.; Hoye, T.R.; Panyam, J. Chapter 11 – Surface Modification of Nanoparticles: Methods and Applications. In Surface Modification of Polymers; Wiley: Hoboken, NJ, USA, 2019; pp. 317–346.
- Veronese, F.M.; Mero, A. The Impact of PEGylation on Biological Therapies. BioDrugs 2008, 22, 315–329.
- Tonigold, M.; Simon, J.; Estupiñán, D.; Kokkinopoulou, M.; Reinholz, J.; Kintzel, U.; Kaltbeitzel, A.; Renz, P.; Domogalla, M.P.; Steinbrink, K.; et al. Pre-adsorption of antibodies enables targeting of nanocarriers despite a biomolecular corona. Nat. Nanotechnol. 2018, 13, 862–869.
- Lao, Y.-H.; Phua, K.K.L.; Leong, K.W. Aptamer Nanomedicine for Cancer Therapeutics: Barriers and Potential for Translation. ACS Nano 2015, 9, 2235–2254.
- Luchini, A.; Vitiello, G. Understanding the Nano-bio Interfaces: Lipid-Coatings for Inorganic Nanoparticles as Promising Strategy for Biomedical Applications. Front. Chem. 2019, 7, 343.
- Sonawane, S.H.; Bhanvase, B.A.; Sivakumar, M.; Potdar, S.B. 1—Current overview of encapsulation. In Encapsulation of Active Molecules and Their Delivery System; Sonawane, S.H., Bhanvase, B.A., Sivakumar, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–8.
- Lammari, N.; Tarhini, M.; Miladi, K.; Louaer, O.; Meniai, A.H.; Sfar, S.; Fessi, H.; Elaïssari, A. Encapsulation methods of active molecules for drug delivery. In Drug Delivery Devices and Therapeutic Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 289–306.
- Ye, C.; Chi, H. A review of recent progress in drug and protein encapsulation: Approaches, applications and challenges. Mater. Sci. Eng. C 2018, 83, 233–246.
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81.
- Gandhi, S.A.; Kampp, J. Skin cancer epidemiology, detection, and management. Med. Clin. 2015, 99, 1323–1335.
- Zhu, Y.; Xue, J.; Chen, W.; Bai, S.; Zheng, T.; He, C.; Guo, Z.; Jiang, M.; Du, G.; Sun, X. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J. Control. Release 2020, 322, 300–311.
- Wan, S.; Zhang, B.; Li, S.; He, B.; Pu, Y. Combination of PEG-decorated black phosphorus nanosheets and immunoadjuvant for photoimmunotherapy of melanoma. J. Mater. Chem. B 2020, 8, 2805–2813.
- Li, W.-H.; Wu, J.-J.; Wu, L.; Zhang, B.-D.; Hu, H.-G.; Zhao, L.; Li, Z.-B.; Yu, X.-F.; Li, Y.-M. Black phosphorous nanosheet: A novel immune-potentiating nanoadjuvant for near-infrared-improved immunotherapy. Biomaterials 2021, 273, 120788.
- Chen, M.; Quan, G.; Wen, T.; Yang, P.; Qin, W.; Mai, H.; Sun, Y.; Lu, C.; Pan, X.; Wu, C. Cold to Hot: Binary Cooperative Microneedle Array-Amplified Photoimmunotherapy for Eliciting Antitumor Immunity and the Abscopal Effect. ACS Appl. Mater. Interfaces 2020, 12, 32259–32269.
- Tian, D.; Qin, F.; Zhao, H.; Zhang, C.; Wang, H.; Liu, N.; Ai, Y. Bio-Responsive nanoparticle for tumor targeting and enhanced photo-immunotherapy. Colloids Surf. B Biointerfaces 2021, 202, 111681.
- Zhou, B.; Song, J.; Wang, M.; Wang, X.; Wang, J.; Howard, E.W.; Zhou, F.; Qu, J.; Chen, W.R. BSA-bioinspired gold nanorods loaded with immunoadjuvant for the treatment of melanoma by combined photothermal therapy and immunotherapy. Nanoscale 2018, 10, 21640–21647.
- Zhang, D.; Wu, T.; Qin, X.; Qiao, Q.; Shang, L.; Song, Q.; Yang, C.; Zhang, Z. Intracellularly Generated Immunological Gold Nanoparticles for Combinatorial Photothermal Therapy and Immunotherapy against Tumor. Nano Lett. 2019, 19, 6635–6646.
- Liu, X.; Zheng, C.; Kong, Y.; Wang, H.; Wang, L. An in situ nanoparticle recombinant strategy for the enhancement of photothermal therapy. Chin. Chem. Lett. 2022, 33, 328–333.
- Bian, Q.; Huang, L.; Xu, Y.; Wang, R.; Gu, Y.; Yuan, A.; Ma, X.; Hu, J.; Rao, Y.; Xu, D.; et al. A Facile Low-Dose Photosensitizer-Incorporated Dissolving Microneedles-Based Composite System for Eliciting Antitumor Immunity and the Abscopal Effect. ACS Nano 2021, 15, 19468–19479.
- Wu, Y.; Han, X.; Zheng, R.; Cheng, H.; Yan, J.; Wu, X.; Hu, Y.; Li, B.; Wang, Z.; Li, X. Neutrophil mediated postoperative photoimmunotherapy against melanoma skin cancer. Nanoscale 2021, 13, 14825–14836.
- Chen, M.; Yang, D.; Sun, Y.; Liu, T.; Wang, W.; Fu, J.; Wang, Q.; Bai, X.; Quan, G.; Pan, X.; et al. In Situ Self-Assembly Nanomicelle Microneedles for Enhanced Photoimmunotherapy via Autophagy Regulation Strategy. ACS Nano 2021, 15, 3387–3401.
- Le, Q.-V.; Kim, D.; Lee, J.; Shim, G.; Oh, Y.-K. Photosensitizer-Free Phototherapy with Peptide Micelle Nanoadjuvants for Cancer Vaccine against Metastasis of Melanoma. Adv. Ther. 2021, 4, 2000288.
- Liu, Y.; Lu, Y.; Zhu, X.; Li, C.; Yan, M.; Pan, J.; Ma, G. Tumor microenvironment-responsive prodrug nanoplatform via co-self-assembly of photothermal agent and IDO inhibitor for enhanced tumor penetration and cancer immunotherapy. Biomaterials 2020, 242, 119933.
- Song, R.; Li, T.; Ye, J.; Sun, F.; Hou, B.; Saeed, M.; Gao, J.; Wang, Y.; Zhu, Q.; Xu, Z.; et al. Acidity-Activatable Dynamic Nanoparticles Boosting Ferroptotic Cell Death for Immunotherapy of Cancer. Adv. Mater. 2021, 33, 2101155.
- Zang, J.; He, R.; Liu, Y.; Su, R.; Zhao, Y.; Zheng, X.; Liu, Y.; Chong, G.; Ruan, S.; Wang, H.; et al. A size/charge/targeting changeable nano-booster to realize synergistic photodynamic-immunotherapy with high safety. Chem. Eng. J. 2022, 434, 134585.
- Li, M.; Guo, R.; Wei, J.; Deng, M.; Li, J.; Tao, Y.; Li, M.; He, Q. Polydopamine-based nanoplatform for photothermal ablation with long-term immune activation against melanoma and its recurrence. Acta Biomater. 2021, 136, 546–557.
- Lin, X.; Wang, X.; Li, J.; Cai, L.; Liao, F.; Wu, M.; Zheng, D.; Zeng, Y.; Zhang, Z.; Liu, X. Localized NIR-II photo-immunotherapy through the combination of photothermal ablation and in situ generated interleukin-12 cytokine for efficiently eliminating primary and abscopal tumors. Nanoscale 2021, 13, 1745–1758.
- Li, D.; Ren, J.; Li, J.; Zhang, Y.; Lou, Y.; Zhu, J.; Liu, P.; Chen, Y.; Yu, Z.; Zhao, L.; et al. Ferroptosis-apoptosis combined anti-melanoma immunotherapy with a NIR-responsive upconverting mSiO2 photodynamic platform. Chem. Eng. J. 2021, 419, 129557.
