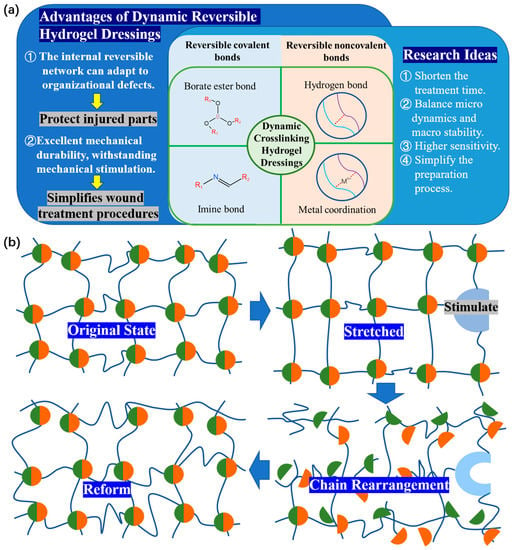
1. Dynamic cross-linked hydrogel dressing动态交联水凝胶敷料
Traditional dressings lack the benefits of self-healing and injectability during the clinical process [1]. Due to the strong rigidity and nondynamic nature of the skeleton, hydrogel is prone to deformation or damage caused by external mechanical forces, resulting in a shortened service life and making it difficult to maintain full contact with the wound, especially near joints, resulting in an unsatisfactory treatment effect [2][3]. For example, traditional dressings attached to fingers do not fit to finger wounds well and easily deform and fall off due to the frequent bending of the fingers [4]. Injectable hydrogels can fill irregular wound areas and promote in situ tissue regeneration, and self-healing hydrogels can withstand external mechanical forces, thus extending their service life [5]. So, to promote a favorable environment for wound repair, functional hydrogel dressings should have improved resistance to mechanical injury and debridement capability [6] (Figure 1a).传统敷料在临床过程中缺乏自愈和可注射性的优点[33]。由于骨架刚性强且非动态性,水凝胶容易因外力而变形或损坏,导致使用寿命缩短,难以与伤口保持完全接触,尤其是关节附近,导致治疗效果不理想[34,35]。例如,附着在手指上的传统敷料不能很好地贴合手指伤口,并且由于手指经常弯曲而容易变形和脱落[36]。可注射水凝胶可以填充不规则的伤口区域并促进原位组织再生,自修复水凝胶可以承受外部机械力,从而延长其使用寿命[37]。因此,为了促进伤口修复的有利环境,功能性水凝胶敷料应具有更高的抗机械损伤能力和清创能力[38](图3a)。
Figure 1图3. (
(
a) The research idea and(
)动态可逆水凝胶敷料的自愈和可注射性的研究思路和(
b) principle of self-healing and injectability of dynamic reversible gel dressing.
)原理。
Hydrogels with self-healing and injectable properties can be prepared together through noncovalent cross-linking or dynamic covalent cross-linking [7]可以通过非共价交联或动态共价交联连接在一起来制备具有自愈和可注射特性的水凝胶[39](
Figure 1图3b)
. According to the research and development achievements of dynamic reversible gel dressing, team Zhang proposed some research ideas for the further development of dynamic hydrogel [8]。根据动态可逆水凝胶敷料的研发成果,张团队为动态水凝胶的进一步发展提出了一些研究思路[40]:(<>)
Need to reduce the time required for dynamic application of wound treatment.需要减少动态应用伤口治疗所需的时间。(<>)
To achieve a better balance between micro dynamics and macro stability.(<>)Higher sensitivity is needed to characterize the dynamic characteristics of hydrogels in real time.(<>)The preparation process of hydrogel should be simplified to promote the wide application of dynamic hydrogel in basic research and clinical transformation. These ideas point out the direction for further development of functional dynamic reversible gel dressings and wound repair.要在微观动态和宏观稳定之间取得更好的平衡。(<>)需要获得更高的灵敏度来实时表征水凝胶的动态特性。(<>)要简化水凝胶制备工艺,促进动态水凝胶在基础研究和临床转化中的广泛应用。这些想法为进一步开发功能性动态可逆凝胶敷料和伤口修复指明了方向。
When discussing the general expectations and design principles of the performance of dynamic hydrogel dressings, the balance between the macro stability and micro level dynamics of hydrogel dressings and the application requirements of dressings should be considered. In practice, the most common reversible covalent bond is the imine bond. The borate ester bond, a special dynamic covalent bond, can exchange bonds within a few seconds at room temperature [9]. Metal coordination bond and hydrogen bond are two typical examples of reversible noncovalent bond. This will serve as the starting point for this section, introducing the success of current research in this field.
在讨论动态水凝胶敷料性能的一般期望和设计原则时,应考虑水凝胶敷料的宏观稳定性和微观水平动力学与敷料应用要求之间的平衡。在实践中,最常见的可逆共价键是亚胺键。还有硼酸酯键,特殊的动态共价键,在室温下可以在几秒钟内发生键交换反应[41]。金属配位键和氢键是可逆非共价键的两个最典型的例子。这将作为本节的起点,介绍该领域当前研究的成功。
2. Reversible covalent bond可逆共价键
Reversible covalent bond is usually reversible, which means that bond exchange can occur. According to a large number of studies, hydrogel dressings can be restored through dynamic reversible covalent bond [10]. Proved its potential for self repair and injectability.可逆共价键通常是可逆的,这意味着可以发生键交换。根据大量研究,水凝胶敷料可以通过动态可逆共价键恢复[42],证明了其自我修复和注射性的潜力。
2.1. Imine bond
2.1. 亚胺键
Since Schiff's first successful preparation of imine bonds in 自1864
, researchers have conducted in-depth research on imine bonds and accumulated a wealth of research experience [11]. The imine bond has dynamic reversible properties. The dynamic reversible covalent bond between these functional groups is the main driving force for the self-healing of hydrogels, rather than the simple physical adhesion between the broken hydrogel interfaces [12]. The hydrogel dressings dynamically crosslinked by the imine bond have excellent injectability and efficient self-healing ability [13].年希夫首次成功制备亚胺键以来,研究人员对亚胺键进行了深入研究,积累了大量的研究经验[43]。亚胺键具有动态可逆性质。这些官能团之间的动态可逆共价键是水凝胶自愈的主要驱动力,而不是断裂的水凝胶界面之间的简单物理粘附[44]。通过亚胺键动态交联的水凝胶敷料具有出色的注射性和高效的自愈能力[45]。
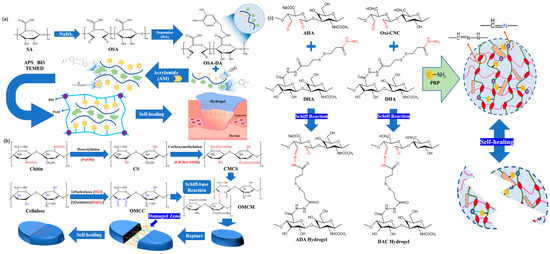
In early research, 在早期的研究中,Chen
et al. [14] used dopamine grafted oxidized sodium alginate (等[46]使用多巴胺接枝氧化海藻酸钠(OSA-DA
), polyacrylamide (),聚丙烯酰胺(PAM
) and dopamine hydrochloride (DA) as main raw materials to prepare )和盐酸多巴胺(DA)作为主要原料制备OSA-DA-PAM
hydrogel dressing. Hydrogel dressings are synthesized by the alkali reaction formed between the aldehyde group of the dynamic covalent crosslinking 水凝胶敷料。通过动态共价交联OSA-DA
chain and the amino group of the PAM ch链的醛基和PAM链的氨基之间形成的碱反应来合成水凝胶敷料(图4a
in (Figure 2a). The resulting hydrogel can effectively self repair without any external stimulation. However, the main weakness of this study is its failure to address injectable issues. We need to find other preparation methods that excel in both self-healing and injectability.)。由此产生的水凝胶可以在没有任何外部刺激的情况下有效地自我修复。然而,这项研究的主要弱点是未能解决注射性问题。需要找到在自愈和注射性方面都出色的其他制备方法。
Figure 2图4. (a) Synthesis process and structure diagram of OSA-DA-PAM hydrogel. 水凝胶的合成工艺和结构图。(b) 基于希夫碱反应和自愈过程方案制备OMCM hydrogel was prepared based on Schiff base reaction and self-healing process. 水凝胶。(c) Schematic diagram of AHA/DHA/oxygen CNC hydrogel.氧数控水凝胶示意图。
In view of the problems encountered in previous studies, 鉴于先前研究中遇到的问题,Yin
et al.等人 [47] [15]发表了一篇论文,其中描述了使用来自菠萝皮 published(PP) a paper describing a hydrogel dressing constructed by amino crosslinking of aldehyde group of oxidized microcrystalline cellulose (OMCC) from的氧化微晶纤维素 (OMCC) 的醛基与来自猴头菇残基 pineapple(HER) peel (PP) and carboxymethyl chitosan (CMCS) from Hericium erinaceus residue (HER), And constructed using Schiff base reaction (Figure的羧甲基壳聚糖 (CMCS) 的氨基交联构建的水凝胶敷料,并使用希夫碱反应构建(图 2) Due to the continuous rupture and regeneration of the imine bond, the researchers divided the network composed of the hydrogel dressing into two parts, and it took 4由于亚胺键的不断破裂和再生,研究人员将水凝胶敷料组成的网络分为两部分,需要5 h
ours to achieve self-repair. For the requirements of wound dressing, the self-healing time of this product is too long. Dynamic covalent cross-linking can be achieved in the hydrogel dressing by using specific products or components that promote the formation and exchange of reversible covalent bond. These dynamic covalent cross-linking interactions help hydrogel dressing Its self-healing and injectable properties. On the other hand, the covalently crosslinked hydrogel dressing is not blocked during the injection process, showing good injection performance. These two features help alleviate pain and accelerate the patient's healing process [16]. Similarly, by using the imine bond, 才能实现自我修复。对于伤口敷料的要求,本品的自愈时间太长。动态共价交联可以在水凝胶敷料中通过使用促进可逆共价键形成和交换的特定产品或组分来实现。这些动态共价交联相互作用有助于水凝胶敷料的自愈和可注射特性。另一方面,共价交联的水凝胶敷料在注射过程中不被堵塞,显示出良好的注射性能。这两个特征有助于减轻疼痛并加速患者的愈合过程[48]。同样,通过使用亚胺键,Li
et al. [17] produced a self-repairing composite hydrogel dressing consisting of aldehyde modified sodium hyaluronate (等人[49]产生了由醛改性透明质酸钠(AHA
), hydrazine modified sodium hyaluronate (),肼改性透明质酸钠(ADA
) and aldehyde modified cellulose nanocrystals (o)和醛改性纤维素纳米晶体(oxi-CNC
) (Figure 2)组成的自修复水凝胶敷料(图4c
). When these hydrogel dressings are divided into two pieces, they can self-repair and reconnect into a cohesive fragment after about )。当这些水凝胶敷料被分成两块时,它们可以自我修复并在大约4
hours. However, the self-healing time of these two projects is still not optimal.小时后重新连接成一个有凝聚力的碎片。然而,这两个项目的自我修复时间仍然不是最佳的。
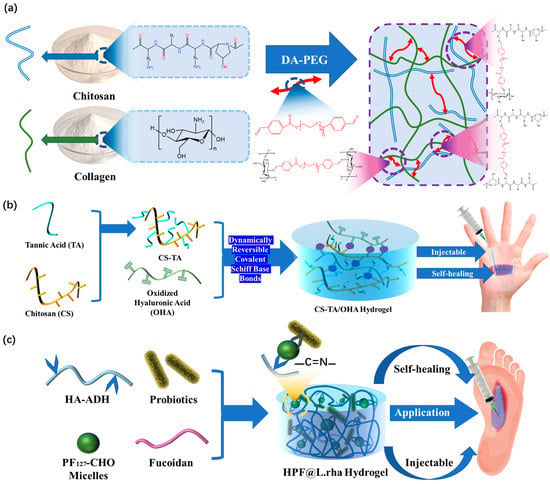
In order to improve the injectivity and self-healing ability of hydrogel dressings, 为了提高水凝胶敷料的注射能力和自愈能力,Ding
et al. [18] proved that collagen (等[50]证明胶原蛋白(CoL
) )-
chitosan (CS) composite hydrogels can be applied to multi-functional wound dressings in the medical field. In their work, dibenzaldehyde modified 壳聚糖(CS)复合水凝胶可应用于医疗领域的多功能伤口敷料。在他们的工作中,二苯甲醛改性PEG
2000(DA-PEG)
is a long-distance crosslinking agent introduced into a mixed 是一种长距离交联剂,被引入混合的COL-CS
network 网络中,并用作动态交联剂(图5a
nd used as a dynamic crosslinking agent (Figure 3a). These properties help to dynamically decouple and recouple imine connections, resulting in superior self-healing hydrogel dressings. With this preparation technology, the self-healing time of functional hydrogel dressing can be significantly reduced while maintaining good injectability. This is a key stage in the application of imine bond in hydrogel dressings.)。这些特性有助于动态解耦和重新耦合亚胺连接,从而产生卓越的自修复水凝胶敷料。借助这种制备技术,功能性水凝胶敷料的自愈时间可以显着减少,同时仍保持良好的注射性。这是在水凝胶敷料中应用亚胺键的关键阶段。
Figure 35. (a) Construction mechanism of COL-CS hydrogel. (b) The construction mechanism and functional mechanism of injectable and self-healing HPF@L.rha hydrogel dressings. (c) Design strategy and application method of CS-TA/OHA composite hydrogel dressings.
Previous research on the injectability and self-healing ability of hydrogels did not address the restoration of the original hydrogel’s mechanical properties. Exceptional performance of both properties is not only necessary to integrate the hydrogel dressing into a whole, but also to restore its excellent mechanical properties, such as its good tensile properties
[19][51]. In order to achieve leapfrogging improvements, Mei et al.
[20][52] modified hyaluronic acid (HA) with adipic hydrazide through acetylation to synthesize hyaluronic acid adipic hydrazide (HA-ADH). (Pluronic F
127)-CHO and HA-ADH form dynamic covalent cross-links through Schiff base reaction interactions in the interventricular or physiological conditions (
Figure 35b). The formation of the dynamic imine connection is what gives the dressing its self-healing property. It can bear high stress and can easily reform at low strain levels; meanwhile, the self-healing time is significantly reduced. In addition, other studies have further improved the performance of hydrogel dressings constructed by imine bonds. Using the dynamic reversible characteristics of Schiff base bonds between chitosan (CS), tannic acid (TA), and oxidized hyaluronic acid (OHA)
[21][25][53], Liu’s hydrogel dressings were able to achieve traceless repair in a rather short time, which has a very short self-healing time compared to recent reports (
Figure 35c). This excellent treatment time is a noteworthy achievement for dynamic reversible gel dressings prepared by imine bonds. It quickly achieves untraceable repair and has good injectability.
2.2. Boric Acid Ester Bonds
Boric acid ester bonds are a special type of dynamic covalent bond that may change dynamically and reversibly at room temperature
[22][54]. The bond exchange and repair times are also substantially shorter than those for imine bonds
[23][55], which enhances their capacity for self-healing.
This hydrogel material is based on a dynamically reversible borate ester bond, and borax is used as a catalyst and dynamic crosslinking agent, expanding the design, formulation, and development possibilities and making it suitable for new hydrogel systems
[24][56]. This prepared hydrogel is favorable for applications in wound dressings. More importantly, it provides a novel idea that is superior to the imine bond. The excellent self-healing ability of the borate ester bond has been demonstrated in the field of wound repair.
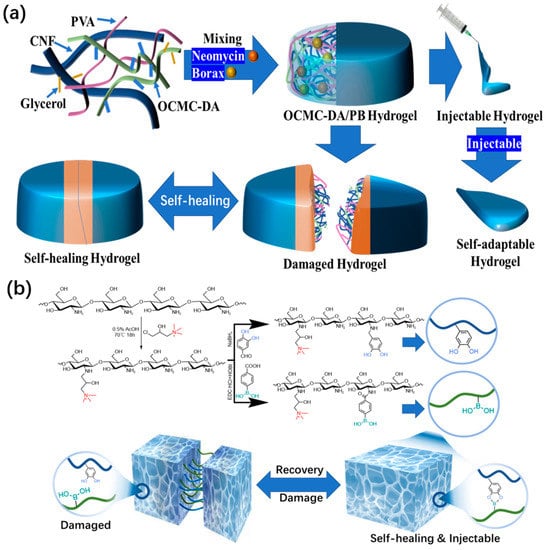
In order to demonstrate the advantages of borate bonds in the preparation of self-healing functional hydrogel dressing, Zhong et al.
[25][57] used dopamine-grafted oxidized carboxymethyl cellulose (OCMC-DA) and cellulose nanofibers (CNF) to build a dynamic reversible borate ester bond to produce a hydrogel dressing that could quickly complete self-healing in 12 s and had certain injectability (
Figure 46a). This type of excellent hydrogel dressing also accomplishes the function of degradation. The same research group has other achievements in the research of hydrogel dressings
[26][58]. Through the dynamic covalent bond between boric acid and a catechol group, the quaternized chitosan can be loaded with epigallocatechin-3-gallate (EGCG), which can be used to meet the requirement of rapid self-healing (
Figure 46b). Within 5 min, a hydrogel dressing that has been cut can be automatically reintegrated into one piece. If the injury is just a scratch, the scratch will vanish entirely from the healing interface after 20 s, realizing complete recovery. The adaptability and durability of this hydrogel as a wound dressing are significantly enhanced by its strong self-healing capacity. Compared to the aforementioned hydrogel dressings, its injectable properties are superior. Moreover, this hydrogel dressing also has antioxidant properties and a powerful bactericidal effect.
Figure 46. (a) Schematic illustration and microscopic structure of OCMC-DA/PB hydrogel and an application diagram of a self-healing and injectable hydrogel. (b) Schematic diagram of QCS-PC hydrogel preparation and the application of self-healing and injectable properties.
Other research groups have studied this type of hydrogel dressings. Deng et al.
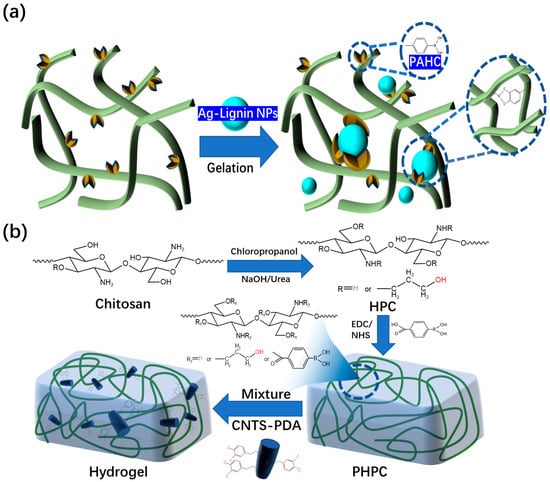
[27] [59] found that hydroxypropyl cellulose and phenylboronic acid-modified hydrogels could be constructed through dynamic borate bonds (
Figure 57a). The prepared hydrogel dressing has excellent self-healing performance. The PAHC (phenylboric acid-modified hydroxypropyl cellulose) hydrogel completely healed after 10 min, which proves that it has excellent self-healing ability. Through continuous research, the research team has made preliminary achievements in the research and development of self-healing and injectable dynamic reversible gel dressings. However, the self-healing time of these dressings still needs to be improved. In 2022, Deng’s group
[28] [60] grafted 4-carboxylphenylboronic acid onto the molecular chain of hydroxypropyl chitosan (HPC) through amidation. The dynamic borate ester bonds are formed between phenylboronic acid and the catechol structure of polydopamine (PDA)-modified carbon nanotubes (
Figure 57b). The final product has excellent self-healing ability compared to the other self-healing hydrogel dressing products mentioned above. The new hydrogel dressing developed by Deng’s group in 2022 can heal itself immediately. The new product developed by this team is more suitable for wound repair than other self-healing hydrogel dressings mentioned previously. This is because the self-healing time of the dressing has been significantly reduced compared to its previous iterations.
Figure 57. (a) Microstructure of hydrogels composed of PAHC and lignin-reduced Ag NPs. (b) Preparation mechanism of PHPC-CNT hydrogel dressing and its microscopic model.
3. Reversible Noncovalent Bonds
Dynamic reversible hydrogel dressings can also be composed of reversible noncovalent bonds, such as metal coordination bonds and hydrogen bonds. These interactions are usually always in the dynamic state of fracture and reorganization, which can achieve rapid and repeated recovery
[29][61].
3.1. Metal Coordination Bond
Hydrogels with self-healing properties were prepared by forming cross-linking points based on ligand–metal coordination bonds on the polymer skeleton. By choosing different ligands and metal ions, a variety of hydrogels with different mechanical strengths can be made to satisfy the self-healing requirements of various biomedical applications
[30][62].
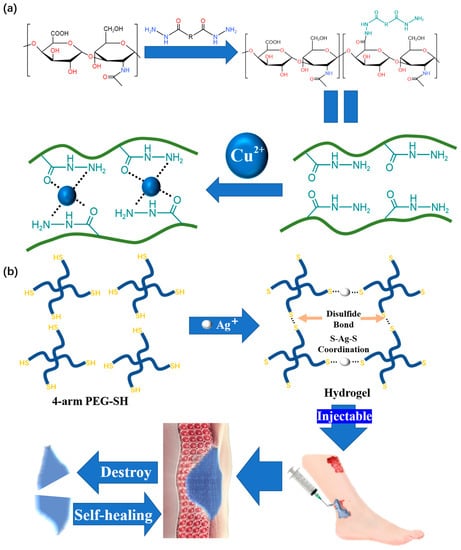
Copper ions are a common material used for metal coordination crosslinking in reams of research. Using hydrazide group as ligand and Cu
2+ as coordination center, Qian et al.
[31][63] developed a copper–hydrazide-coordinated, multifunctional hyaluronan hydrogel, and the hyaluronic acid (HA)-Cu hydrogel shows practical versatility, including excellent self-healing and injectability (
Figure 68a). Self-healing is achieved after 0.5 h, and the self-healing hydrogel can even be stretched during this time. From the perspective of hydrogel restoration, the HA-Cu hydrogel also has good injectability. Most importantly, this product is not restricted to only reacting in acidic conditions. This is the first report on hydrogels prepared by aliphatic hydrazine–metal coordination crosslinking for wound treatment.
Figure 68. (a) The fabrication of HA hydrogels through hydrazide–metal coordination crosslinking and its microstructure. (b) Schematic illustration of the self-healing Ag(I)-thiol (Au–S) coordinative hydrogel and its self-healing and injectable applications.
We should also note that silver is frequently used for metal coordination crosslinking. Compared with Cu, Ag is more suitable for wound treatment due to its wider antibacterial spectrum and stronger antibacterial ability
[32][64]. The hydrogel developed by Chen
[33][65], which can be used for wound treatment, is prepared by the coordination crosslinking of dobby thiopenyl glycol (SH-PEG) and silver nitrate (AgNO
3) (
Figure 68b). Due to the dynamic nature of the Ag-S coordination bond, the obtained coordination hydrogels have good self-healing ability and injectability. At room temperature, the hydrogel shows excellent self-healing performance by restoring its original state from an incision within 15 min. A unique feature of the prepared hydrogel is its ability to have its mechanical strength adjusted, which is useful for adapting to various wound conditions.
Fe
3+ is very common, and the raw materials are cheap. In addition, Fe
3+ can also be converted into Fe
2+ in an acidic environment
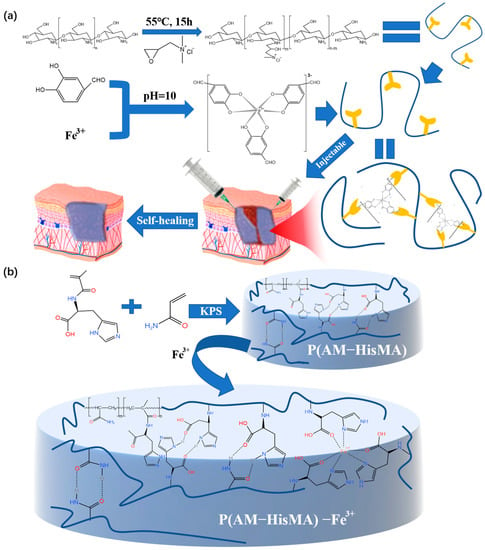
[34][66] (human skin is generally weakly acidic), which is helpful for the blood supply in the human body during wound healing. Moreover, this crosslinking method is environmentally friendly, simple, and universal. Based on the application of metal coordination bonds to prepare hydrogel dressings, the double dynamic bond crosslinked self-healing hydrogel prepared by Liang’s team
[35] [67] used the tricomplex coordination between the catechol groups from Fe
3+ and protocatechualdehyde (PA) to produce hydrogels with excellent self-healing performance and injectability (
Figure 79a). The resulting hydrogel dressing is intelligent and the wound repair efficiency is improved. The performance test revealed that the hydrogel could be fully repaired after 30 min of room temperature exposure and that its tensile capacity could be restored after an additional hour. It was proven that the reversible non-covalent cross-linked hydrogel dressing prepared by metal coordination has a certain self-healing ability, but the healing efficiency still needs to be improved.
Figure 79. (a) The process of preparing double-dynamic-bond crosslinked hydrogels and the application of the product in medicine. (b) The preparation and the network structure of the P(AM-HisMA) −Fe3+ hydrogel dressing.
Wound repair requires fast self-healing of the dressing in order to better repair the wound. Therefore, after using histidine methacrylamide (HisMA) and acrylamide (AM) to form hydrogel, Zhang
[36][68] added Fe
3+ ions into a P(AM-HisMA) hydrogel to prepare a P(AM-HisMA)-Fe
3+ hydrogel dressing with good injectability and self-healing ability (
Figure 79b). It is worth noting that the self-healing speed is greatly improved compared to the hydrogel without Fe
3+. P(AM-HisMA)-Fe
3+ hydrogel completely heals in 5 min; however, the P(AM-HisMA) hydrogels without Fe
3+ crosslinking take 24 h to achieve self-healing. Since its capacity for self-healing is an important reference scheme for this kind of hydrogel dressing, it merits special attention.
3.2. Hydrogen Bond
Another type of dynamic noncovalent bond, the hydrogen bond
[37][69], is a noncovalent bond that can be destroyed at high temperature and reformed at low temperature. Therefore, hydrogen bonds can be used to prepare self-healing hydrogel dressings.
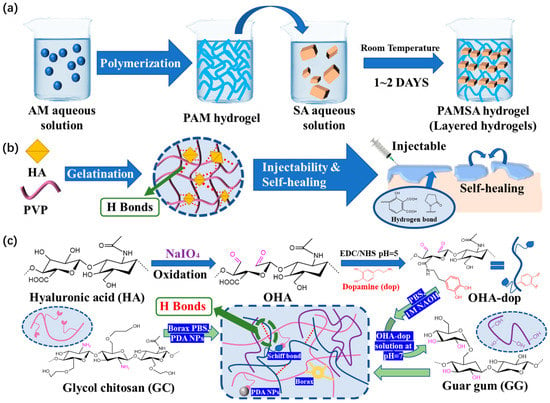
In order to improve the self-healing ability and injectability of functional hydrogel dressings, Zhao
[38][70] developed a simple and fast method to prepare a new hydrogel. This hydrogel is composed of sodium alginate (SA) and polyacrylamide (PAM). Systematic characterization revealed the formation mechanism of a layered structure through hydrogen bonding (
Figure 810a). In addition, it takes 0.5 h to achieve self-healing. Hydrogen bonds allow for the recovery of the mechanical properties of hydrogels with or without the presence of water.
Figure 810. (a) Schematic diagram of PAMSA hydrogel preparation based on PAM and SA. (b) Schematic diagram of HPC hydrogel crosslinked by HA in an ultrafast process and the possible hydrogen bonding structure of HPC hydrogels. (c) The preparation and microscopic schematic diagrams of the OHAdop/GG + GC/borax hydrogel and the OHAdop/GG + GC/borax/PDA hydrogel.
There are also better research results on the self-healing ability and injectability of hydrogel dressings. Among dynamic crosslinking hydrogel dressings with these properties, the humic acid/polyvinylpyrrolidone (PVP) complex hydrogel dressings prepared by Yu et al.
[39] [71] are driven by the hydrogen bonds between humic acid and PVP, and these bonds are easy to form and adjust (
Figure 810b). The dynamic reversible hydrogen bonds distributed in the hydrogel dressing promoted the formation of a dynamic cross-linking network. The viscosity of the humic acid/PVP complex hydrogel significantly decreases as the shear rate rises, improving its injectability. At the same time, this hydrogel is endowed with self-healing performance that can achieve complete self-repair in 10 min.
Because the force of hydrogen bonds is generally weaker than some of the dynamic reversible bonds discussed above, hydrogen bonds often do not appear alone; rather, hydrogels are constructed combing hydrogen bonds with other dynamic cross-linking bonds. Guo et al.
[72] [40] designed and prepared a polysaccharide-based adhesive hydrogel, in which dynamic hydrogen bonding between catechol-modified oxidized hyaluronic acid (OHAdop) or hydroxide groups in guar gum led to reversible crosslinking (
Figure 810c). As soon as the two parts come into contact, hydrogen interaction occurs, causing the fragments to self-heal and unite to form a single hydrogel that can be easily combined with other parts. This hydrogel dressing heals itself extremely quickly. It has high application value and competitiveness in the market.