You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Xingxing Wang and Version 2 by Camila Xu.
Remaining useful life prediction is of great significance for battery safety and maintenance. The remaining useful life prediction method, based on a physical model, has wide applicability and high prediction accuracy, which is the research hotspot of the next generation battery life prediction method. An electrochemical model is a model built by simulating the electrochemical reaction process of a battery. It describes the laws of the cell from the point of view of internal physical and chemical processes including kinetic parameters, mass conversion processes, thermodynamic properties, mechanical, thermal, and electrical properties of materials.
- lithium-ion battery
- residual life
- physical model
- prediction method
1. Introduction
Lithium-ion batteries have the advantages of high energy density, high output power, no pollution, no memory effect, small self-discharge, etc., and has become the main battery type of current new energy vehicles [1]. Common lithium-ion batteries on the market include lithium cobaltate, lithium manganate, and lithium iron carbonate batteries [2]. Lithium cobalt-acid batteries are the most widely used and have high specific energy, but the production of raw materials is more expensive; lithium manganate batteries have excellent multiplier performance and stability, but low energy density and poor safety [3]; lithium iron carbonate batteries have high energy density and good cycling performance, but poor low-temperature discharge performance [4]. There are many factors to measure the performance of the above lithium-ion batteries, and battery life is one of the key indicators and an important factor to be considered by users when purchasing electric vehicles [5]. During long-term use, the lithium-ion battery undergoes a series of electrochemical reactions and physical changes that degrade the performance and capacity until the end of its life [6].
State of health (SOH) estimation and remaining useful life (RUL) prediction are the basic problems of battery health management [7]. SOH represents the aging degree of lithium-ion batteries, which is mostly defined as the percentage of the capacity released by the battery from the full charge state to the cut-off voltage and the rated capacity of the battery under certain conditions [8]. RUL is a parameter that characterizes the deterioration of the battery to obtain the operating time from the beginning of the prediction to the end of the battery life [9]. The accurate prediction and estimation of SOH and RUL can effectively judge their future working capacity and identify problems in time to avoid unnecessary problems and losses [10].
RUL prediction methods based on the model from the perspective of the first principles are given a full explanation of the cell aging process, applicable to almost all of the conditions and operation mode. The analysis of a battery control strategy is also more detailed and accurate than other methods, which should be paid attention to and further developed in the process of RUL prediction research [11].
RUL refers to the number of charge point cycles a battery can perform before its life ends. There are many factors that affect the battery life, from the physical level including temperature, charging current, charging voltage, battery structure, etc. [12] and on the chemical level including the electrode material, electrolyte, battery resistance, etc. [13]. Generally, when the capacity of a battery is lower than 80% of the factory capacity, the battery is considered to be invalid. A common RUL definition is shown in Formula (1) below:
where CEOL represents the number of charging and discharging cycles that can be carried out when the service life terminates, and Ci represents the number of charging and discharging cycles at the current moment [14].
In this papentryr, the existing model-based lithium-ion battery life prediction methods at home and abroad were divided into three categories: the electrochemical model-based method, equivalent circuit-based method, and empirical model based method [15][16][15,16], as shown in Figure 1.
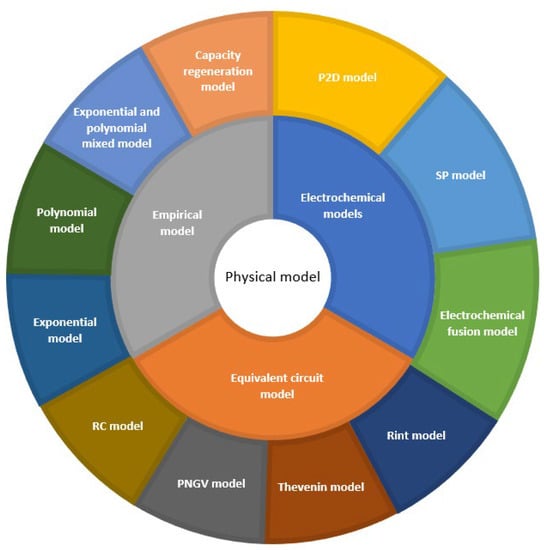

Figure 1.
Battery life prediction method based on the physical model.
2. Methods Based on Electrochemical Models
An electrochemical model is a model built by simulating the electrochemical reaction process of a battery [17]. It describes the laws of the cell from the point of view of internal physical and chemical processes including kinetic parameters, mass conversion processes, thermodynamic properties, mechanical, thermal, and electrical properties of materials [18]. It can provide a reference for battery research and development, so electrochemical models are often used for the analysis of battery principles and for battery research and development [19]. Electrochemical models include pseudo two-dimensional (P2D), single particle (SP) models, and coupled electrochemical models developed based on both.2.1. P2D Model
The P2D model is shown in Figure 2. The battery was divided into three areas: negative electrode, diaphragm, and positive electrode [20]. Several governing equations were established in the P2D model to describe the diffusion of lithium ions in solid particles, mass transfer in the liquid phase, and the electrochemical reaction on the particle surface. Among these control equations, the most important is the Butler–Volmer kinetic response equation, as shown in Equation (2):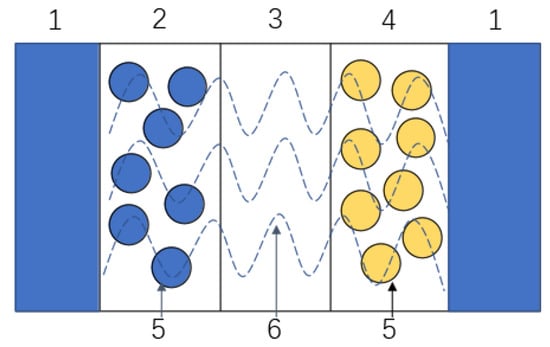
Figure 2. Structural diagram of the electrochemical model of a lithium-ion battery. (1) Liquid collector. (2). Negative electrode. (3) Diaphragm. (4) Positive electrode. (5) Active material. (6) Electrolyte.
2.2. SP Model
Compared with other electrochemical models, the single-particle model is widely used because of its simple construction and fast prediction speed [29]. In the literature, the SP model was simplified to degrade to improve the calculation speed and prediction accuracy of the model [30][31][30,31]. At the same time, various factors that may affect the battery were taken into account in the model, and internal indicators were selectively extracted to track the battery health status [32][33][32,33]. Sadabadi et al. [34] proposed a RUL prediction algorithm based on single particle model parameter estimation. The algorithm uses the moles of circulating lithium and battery resistance as indicators of the state of health, and uses the derived composite SOH metric to design particle filter-based RUL predictions [35], where the greater the SOH-related estimates, the more accurate the RUL estimate. Based on the SP model, Li et al. [36] proposed an improved simplified model, obtained physical parameters with the aging mechanism, and selected new health indicators by analyzing the relationship between the physical parameters and battery health status [37], which is helpful in accurately predicting the remaining life of the battery, and has important theoretical significance and practical value for improving the level of battery management technology. Prasad et al. [38] simplified the model by two key parameters, battery resistance, and cathode solid-phase diffusion time [39]. The model uses the gradient parameter real-time update method under the excitation of the HPPC current [40]. Within 200 s, the estimated value of the total battery resistance and diffusion time could converge to the optimal value within 99%. The calculation speed was fast, and can be used for the online estimation of the remaining life of the car battery. Deng et al. [23] employed a sequence of polynomial functions to simulate the electrolyte phase concentration profile, the solid phase concentration profile, and the nonuniform reaction flow profile, respectively, to simplify the P2D model. The accuracy of the second- and third-order polynomial estimates for the reaction fluxes was compared, and the higher order’s more robust correctness was proven. The created model was simulated using a variety of constant flow rates, mixing pulses, and driving times, and the results were compared to the P2D model and the original SP model. The findings revealed that the proposed model captures the cell properties adequately while significantly reducing the computational complexity.2.3. Electrochemical Coupling Model
The above electrochemical models can be optimized in their respective fields to compensate for deficiencies to a certain extent, but some deficiencies are inherent to the models themselves and cannot be eliminated. In this case, the integration with other models is needed to improve the accuracy. The P2D model is an isothermal model without considering the coupling relationship between heat generation and chemical reaction. In the actual charging and discharging process, the battery will inevitably generate heat. Kuangke et al. [41] put forward a kind of large capacity motor such as the lithium-ion battery electrochemistry-thermal coupling efficient modeling method through the classification of the parameters, and the measured/identification parameters for precise measurement and parameter identification, using pulse charging experiments under different temperature calibration solid phase diffusion coefficient of the DS and reaction rate constant k, further established the battery heat production model. The simulation data of the voltage and temperature agreed well with the test data, and the parameters of the model were more accurate at room temperature. The average voltage error was less than 10 mV, and the average temperature error was less than 1.1 °C. The established model had good accuracy and adaptability. Wang et al. [42] also developed an electrochemical-thermal model utilizing P2D and detailed how some parameters varied with temperature. The proportional mean square variance between the model’s anticipated and observed residual life was only 0.625%, which compensated for the simulation error caused by the temperature change. Zhang et al. [43] discovered that the P2D model’s impedance parameters were only consistent with the actual battery at low frequencies. It obtained high simulation precision in the wide bandwidth range from 10 mHz to 1 kHz by integrating the P2D model with the EIS model. Under the sinusoidal electrical excitation of 5 Hz, 10 Hz, and 20 Hz, the root mean square error of the improved model decreased by 24.8%, 30.6%, and 33.0%, respectively, when compared to the P2D model. Li et al. [36] found that the existing SP model did not consider the degradation mechanism, and proposed a new prediction model combining the capacity degradation model and SP model. This model could quickly predict the capacity attenuation and voltage distribution changes with the number of cycles and temperature. Compared with the experimental results, the root mean square error of the model prediction was 0.0103. A coupled electrochemical-thermal coupling (ECT) model of LiCoO batteries was proposed by Li et al. [44] to describe their charging and discharging behavior. Calculations of heat generation, conduction, and dissipation were added to the simplified electrochemical model by means of a total set thermal analysis and a rational reduction and recombination of the cell mechanism parameters to reduce the estimation complexity. Specifically designed identification conditions were used to obtain the mechanism parameters based on the excitation response analysis. The applicability of the model under different operating conditions was verified by simulations. The simulation results of the end voltage and surface temperature were in good agreement with the actual experimental measurements at lower C-rates and dynamic load currents. Jiang et al. [20] created a one-dimensional (1D) electricity generation-three-dimensional (3D) thermal connection model to investigate the heat transfer mechanism of hexagonal Li-ion cells cooled on various exterior surfaces. The simulation parameters studied were the forced convection cooling coefficient, h, the thermal diffusion surface area, and the dimension of the cell. The variation in temperature of the prismatic cells with conduction force cooling on tiny side surfaces was found to be more uniform than that of prismatic cells with massive front area cooling. The highest temperature differential of the prismatic cell with tiny side surface cooling was kept constant at h = 100 W/m2 K as the cell size rose. Furthermore, the influence of operating temperature on the capacity degradation of Li-ion batteries during cycling was examined. The greater the operating temperature, the faster the parasitic lithium/solvent reduction process, which resulted in the depletion of lithium ions and enhanced the rate of capacity degradation throughout cycling.2.4. Comparison of Electrochemical Models
In order to more clearly show the advantages and disadvantages of the prediction methods based on electrochemical models, the prediction methods are summarized. In this papentryr, “★“ was used to indicate the accuracy and complexity of the prediction methods. The results are shown in Table 1.Table 1.
Electrochemical model.
| Model | Advantages | Disadvantages | Prediction Accuracy | Complexity |
|---|---|---|---|---|
| P2D model | It can describe the internal dynamic behavior of the battery, and has the advantages of accurate model and high calculation accuracy. | There are many parameters in the model and the calculation is complicated and the efficiency is low. | High ★★★★☆ |
High ★★★★☆ |
| SP model | The modeling complexity is low and the calculation accuracy is high. | The physical properties of electrolyte are ignored and the problem of order reduction is not considered. | Higher ★★★★★ |
Lower ★★☆☆☆ |
| Electrochemical fusion model | Strong robustness and high prediction accuracy. | The model is complicated due to the large amount of calculation and many optimization parameters. | High ★★★★☆ |
High ★★★★☆ |
