Nanoemulgels (NEGs) are novel drug delivery systems that aim to improve the solubility and permeability of drugs across the biological membrane. The nano-sized droplets in the nanoemulsion enhance the permeation of the formulation, along with surfactants and co-surfactants that act as permeation enhancers and can further improve permeability. The hydrogel component of NEG helps to increase the viscosity and spreadability of the formulation, making it ideal for topical application. Moreover, oils that have anti-inflammatory properties, such as eucalyptus oil, emu oil and clove oil, are used as oil phases in the preparation of the nanoemulsion, which shows a synergistic effect with active moiety and enhances its overall therapeutic profile. This leads to the creation of hydrophobic drugs that possess enhanced pharmacokinetic and pharmacodynamic properties, and simultaneously avoid systemic side effects in individuals with external inflammatory disorders. The nanoemulsion’s effective spreadability, ease of application, non-invasive administration, and subsequent ability to achieve patient compliance make it more suitable for topical application in the combat of many inflammatory disorders, such as dermatitis, psoriasis, rheumatoid arthritis, osteoarthritis and so on. Although the large-scale practical application of NEG is limited due to problems regarding its scalability and thermodynamic instability, which arise from the use of high-energy approaches during the production of the nanoemulsion, these can be resolved by the advancement of an alternative nanoemulsification technique.
- anti-inflammatory
- nanoemulsion
- nanoemulgel
- pharmacokinetics
- pharmacodynamics
1. Nanoemulgel, a Potential Carrier System
2. Nanoemulgels in Topical Delivery for Anti-Inflammatory Drugs
Anti-inflammatory drugs showcase their activity at different stages of the inflammatory cascade (Figure 1). Glucocorticoids inhibit the phospholipase enzyme that catalyzes the formation of Arachidonic acid, which is the precursor of inflammatory mediators such as prostaglandins and leukotrienes [45,46][10][11]. Non-steroidal anti-inflammatory drugs (NSAIDs) primarily inhibit cyclooxygenase 1 (COX1) and cyclooxygenase 2 (COX2), which, in turn, restrict the formation of prostaglandins, which are responsible for inflammatory reactions [47][12]. Meanwhile, many natural anti-inflammatory moieties such as curcumin and quercetin have been reported to have both COX and lipoxygenase (LOX)-inhibiting properties [14,48][13][14]. Topical delivery is an excellent approach for the management of external inflammatory conditions, because by opting for this route, we can not only bypass the first-pass metabolism, but also the gastrointestinal (GI) barriers; these are the gastric emptying time and the intestinal transit time, the enzymes present and pH changes [49,50][15][16]. Topical NSAID application has the benefit of improving the distribution of local drugs to the damaged tissues, while minimizing the risk of systemic side effects, such GI bleeding and peptic ulcers [51][17]. Additionally, oils that possess anti-inflammatory properties can be meticulously chosen as the oil phase for the formulation; this can synergistically enhance the desired outcome of the formulation. The nanosized globules of the chosen NE enhance the potency of the NEG by improving its permeability and diffusibility through the use of appropriate permeation enhancers [43][6]. Moreover, a high concentration of water in the gel can sufficiently hydrate the stratum corneum (SC) and cause its tight architecture to leak, allowing the active ingredients to readily penetrate the skin [52][18]. Before reaching the skin’s surface, the drug in the NE first traverses from the internal phase (Nanoemulsion) to the external phase (gel), and thus serves as a drug reservoir for topical delivery. When applied topically, oily globules first get liberated from the hydrogel, then permeate deep into the SC of the skin, where they deliver the drug moiety [23][19].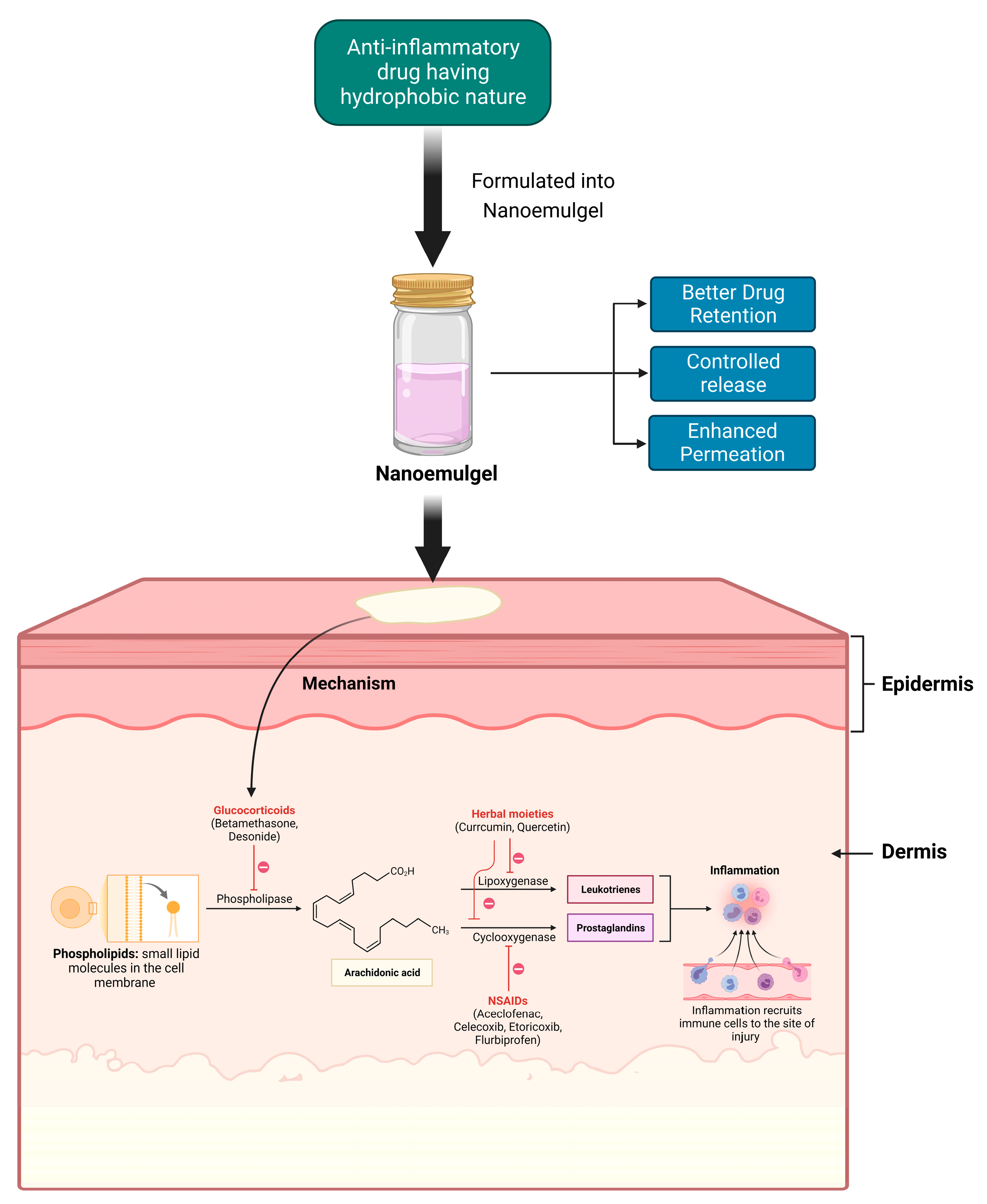
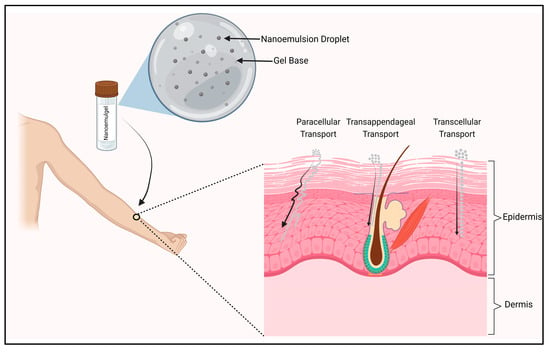
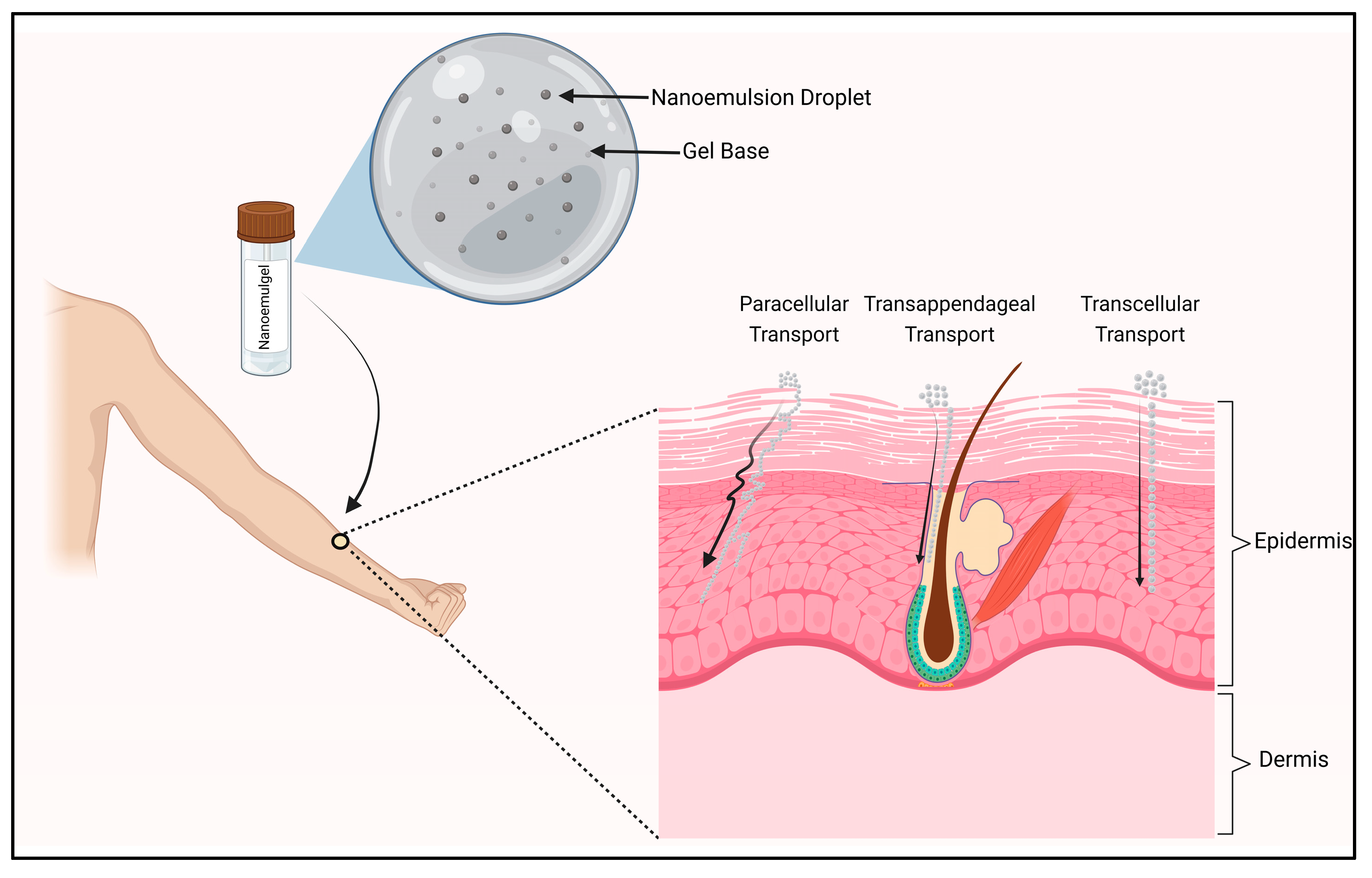
Mechanistic Approach of Nanoemulgel Delivery via Topical Route
3. Components of Nanoemulgel
3. Components of Nanoemulgel
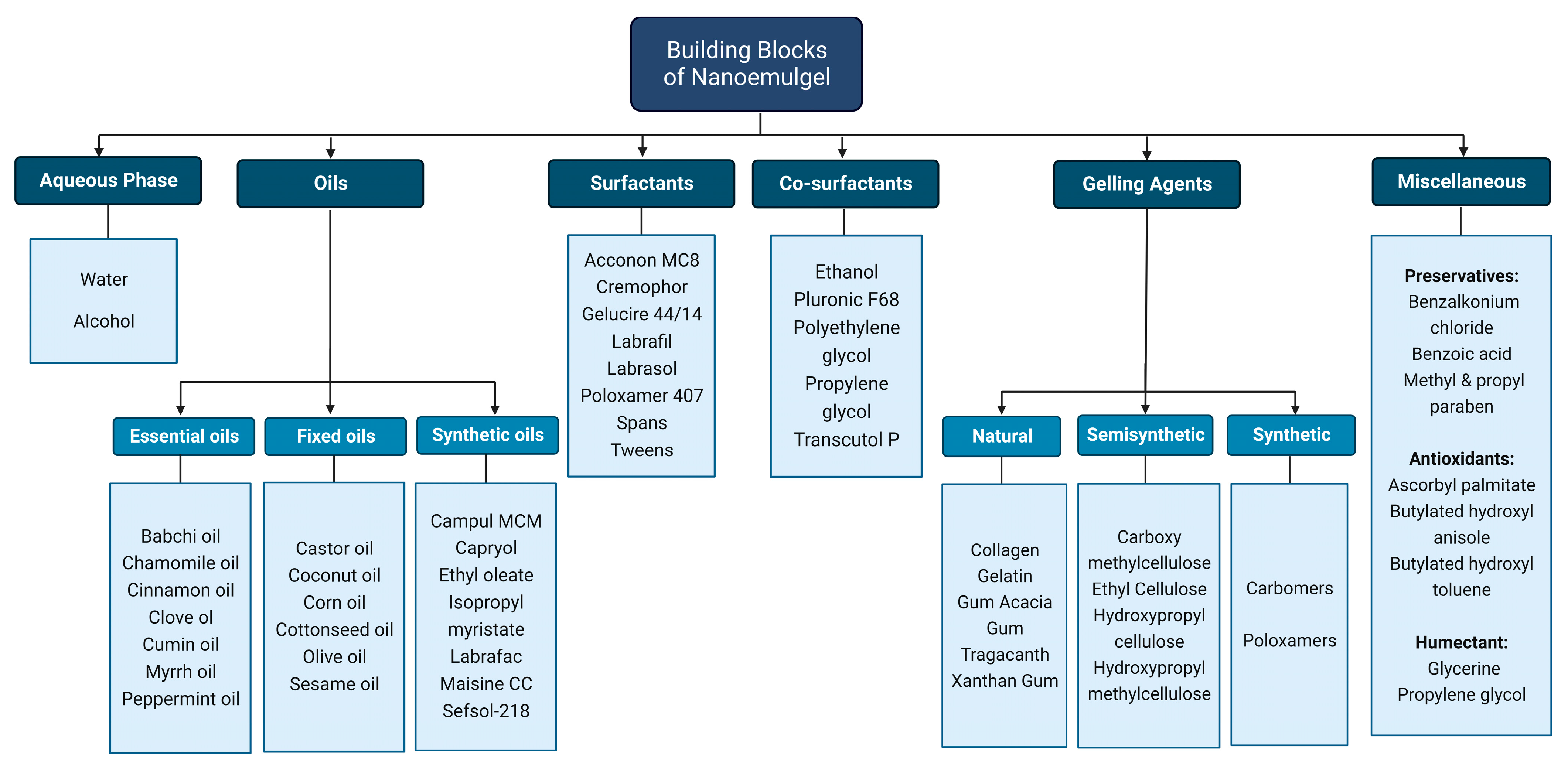
3.1. Aqueous Solvents
3.2. Oil Phase
3.3. Surfactants
Surfactants that are helpful in the emulsification process, thus named emulsifiers, can be added to stabilize the oil and water phases together [69]. They work at the interface by lowering the tension between the two phases, which is important for the development of dispersion, and they also aid in reaching the distribution equilibrium at the interface, resulting in the increased thermodynamic and kinetic stability of the emulsion [21,70]. As a result, they have good solubility in both water and oil. “The portion in which the surfactant is more soluble produces the external phase of the emulsion”, states the Bancroft rule [71]. For instance, sorbitan fatty acid esters that are oil-soluble favor the production of w/o emulsion, while tweens that are water-soluble facilitate the production of the o/w type of emulsion [39,72].3.4. Cosurfactants
3.4. Cosurfactants
3.5. Gelling Agents
3.5. Gelling Agents
Gelling agents are introduced into the formulation to transform the nanoemulsion formulation’s liquid state to gel and address the issues of low viscosity, limited spreadability, and poor retention [18][34]. A gelling ingredient, when dispersed in liquid (mostly aqueous phase), results in a weakly cohesive gelling matrix that stabilizes the formulation and promotes the optimum percutaneous drug delivery when utilized in an externally applied formulation [78][35]. The gelling agent’s thixotropic property thickens the formulation without changing its volume, aiding in the transition of the liquid to gel. Some gelling agents work by dissolving in the liquid phase and producing a colloidal mixture that may cross-polymerize to construct a minimally cohesive internal structure. Other gelling agents work by virtue of their thixotropic property, i.e., their time-dependent shear-thinning ability, which causes discrete particles to adhere or interlock to withstand strain. Gelling agents have a crucial role in determining the number of parameters, which can include bioadhesion, consistency, drug-release kinetics, extrudability, homogeneity, rheological properties, swelling index, and the spreadability of nanoemulgels. Sodium Carboxy methylcellulose (NaCMC), Carbomers and Hydroxypropyl methylcellulose (HPMC) are the most commonly used gelling agents. Plant-derived polysaccharides that act as stimuli-responsive gelling agents can be used for preparing a gel base that allows the delivery of drugs in a more targeted manner [79,80][36][37]. These kinds of polymers are able to interchange their phases, alter their rigidness, and control and sustain their drug delivery. They can be made to release the drug only if specific conditions (stimuli) are met; this includes in a ROS (Reactive Oxygen Species)-rich environment during inflammation and tissue injury [81][38], when there is a lower extracellular pH in inflamed tissue or around a tumorous microenvironment [82][39], or when temperature is increased, which acts as an indicator of the inflammatory phase in wound progression [80,83][37][40].4. Toxicity Concerns
Nanoemulgels contain components such as surfactants and cosurfactants which, are known to cause skin irritation and deterioration, cytotoxicity, and allergic reactions in some patients. Anionic surfactants, such as sodium dodecyl sulfate (SDS), primarily target the SC layer of skin, causing damage to the bilipid layer, the denaturation of protein and skin dehydration, which may lead to dermatitis in sensitive skin. Cationic surfactants cause more severe cytotoxic effects and apoptosis in normal and cancerous cells. Conversely, non-ionic surfactants are relatively less toxic and irritative to skin [140,141][41][42]. Hence, non-ionic surfactants are preferred over ionic surfactants for topical formulations. Cosurfactants such as propylene glycol, when used in an unreasonably high amount, can cause hyperosmolarity, cardiac arrhythmia, lactic acidosis and toxicity of the central nervous system. Fligner et al. reported cardiorespiratory arrest due to a high propylene glycol concentration (1059 mg/dL) in an 8-year-old child during topical silver sulfadiazine therapy [142][43]. To minimize these types of toxic effects, these kinds of components should be used in a minimum quantity. When used in the proper amount, numerous researchers have reported that the nanoemulgels did not cause skin irritation or toxicity. Abdallah et al. conducted a skin irritation study by spreading a brucine-loaded nanoemulgel over the shaved skin of rat and covered it with gauze. The rats did not show any signs of oedema or erythema, even after 24 h of application. Similarly, Bhattacharya and Prajapati conducted an acute skin irritation study of a Celecoxib-loaded nanoemulgel on rabbits and reported a 1.6 times lower skin irritation score compared to that of a standard irritant after 7 days of application. Moreover, the risk of experiencing systemic side effects caused by topically applied nanoemulgels cannot be ignored due to the possibility of the formulation reaching the blood stream by virtue of its enhanced permeation and nano size range, although extensive research needs to be conducted to reach conclusive findings regarding nanoemulgel toxicity. Nano formulations such as polymeric nanoparticles are associated with oxidative stress and cytotoxicity, lipid-based nanoparticles have shown hypersensitivity and cardiopulmonary distress, while tiny rare-earth fluoride nanoparticles can encourage tumor growth via electrical dipole interactions, which are inversely proportion to the size [143,144][44][45].References
- Ojha, B.; Jain, V.K.; Gupta, S.; Talegaonkar, S.; Jain, K. Nanoemulgel: A promising novel formulation for treatment of skin ailments. Polym. Bull. 2022, 79, 4441–4465.
- Rai, V.K.; Sharma, A.; Thakur, A. Quality Control of Nanoemulsion: By PDCA Cycle and 7QC Tools. Curr. Drug Deliv. 2021, 18, 1244–1255.
- Indrati, O.; Martien, R.; Rohman, A.; Nugroho, A.K. Development of Nanoemulsion-based Hydrogel Containing Andrographolide: Physical Properties and Stability Evaluation. J. Pharm. Bioallied. Sci. 2020, 12, S816–S820.
- Kass, L.E.; Nguyen, J. Nanocarrier-hydrogel composite delivery systems for precision drug release. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1756.
- Liu, Y.; Weng, P.; Liu, Y.; Wu, Z.; Wang, L.; Liu, L. Citrus pectin research advances: Derived as a biomaterial in the construction and applications of micro/nano-delivery systems. Food Hydrocoll. 2022, 133, 107910.
- Aithal, G.C.; Narayan, R.; Nayak, U.Y. Nanoemulgel: A Promising Phase in Drug Delivery. Curr. Pharm. Des. 2020, 26, 279–291.
- Sengupta, P.; Chatterjee, B. Potential and future scope of nanoemulgel formulation for topical delivery of lipophilic drugs. Int. J. Pharm. 2017, 526, 353–365.
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent Update on Nanoemulgel as Topical Drug Delivery System. J. Pharm. Sci. 2017, 106, 1736–1751.
- Nazneen, S.; Juber, A.; Badruddeen; Mohammad Irfan, K.; Usama, A.; Muhammad, A.; Mohammad, A.; Tanmay, U. Nanoemulgel: For Promising Topical and Systemic Delivery. In Drug Development Life Cycle; Juber, A., Badruddeen, Mohammad, A., Mohammad Irfan, K., Eds.; IntechOpen: Rijeka, Croatia, 2022; pp. 1–20.
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 986–1000.
- van der Velden, V.H. Glucocorticoids: Mechanisms of action and anti-inflammatory potential in asthma. Mediat. Inflamm. 1998, 7, 229–237.
- Vane, J.R.; Botting, R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998, 47, S78–S87.
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167.
- Rao, C.V. Regulation of COX and LOX by curcumin. Adv. Exp. Med. Biol. 2007, 595, 213–226.
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and Transdermal Drug Delivery: From Simple Potions to Smart Technologies. Curr. Drug Deliv. 2019, 16, 444–460.
- Viswanathan, P.; Muralidaran, Y.; Ragavan, G. Chapter 7—Challenges in oral drug delivery: A nano-based strategy to overcome. In Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 173–201.
- Heyneman, C.A.; Lawless-Liday, C.; Wall, G.C. Oral versus Topical NSAIDs in Rheumatic Diseases. Drugs 2000, 60, 555–574.
- Permawati, M.; Anwar, E.; Arsianti, A.; Bahtiar, A. Anti-inflammatory Activity of Nanoemulgel formulated from Ageratum conyzoides (L.) L. and Oldenlandia corymbosa L. Extracts in Rats. J. Nat. Remedies 2019, 19, 124–134.
- Mukherjee, P.K.; Harwansh, R.K.; Bhattacharyya, S. Chapter 10—Bioavailability of Herbal Products: Approach Toward Improved Pharmacokinetics. In Evidence-Based Validation of Herbal Medicine; Mukherjee, P.K., Ed.; Elsevier: Boston, MA, USA, 2015; pp. 217–245.
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin Barriers in Dermal Drug Delivery: Which Barriers Have to Be Overcome and How Can We Measure Them? Pharmaceutics 2020, 12, 684.
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554.
- Zoabi, A.; Touitou, E.; Margulis, K. Recent Advances in Nanomaterials for Dermal and Transdermal Applications. Colloids Interfaces 2021, 5, 18.
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 10.
- Wiechers, J.W. The barrier function of the skin in relation to percutaneous absorption of drugs. Pharm. Weekbl. 1989, 11, 185–198.
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics 2023, 15, 164.
- Akdeniz, M.; Gabriel, S.; Lichterfeld-Kottner, A.; Blume-Peytavi, U.; Kottner, J. Transepidermal water loss in healthy adults: A systematic review and meta-analysis update. Br. J. Derm. 2018, 179, 1049–1055.
- Vandamme, T.F. Microemulsions as ocular drug delivery systems: Recent developments and future challenges. Prog. Retin. Eye Res. 2002, 21, 15–34.
- Goodarzi, F.; Zendehboudi, S. A comprehensive review on emulsions and emulsion stability in chemical and energy industries. Can. J. Chem. Eng. 2019, 97, 281–309.
- Sapra, B.; Thatai, P.; Bhandari, S.; Sood, J.; Jindal, M.; Tiwary, A. A critical appraisal of microemulsions for drug delivery: Part I. Ther. Deliv. 2013, 4, 1547–1564.
- Ruckenstein, E. Microemulsions, macroemulsions, and the Bancroft rule. Langmuir 1996, 12, 6351–6353.
- Wang, J.; Shi, A.; Agyei, D.; Wang, Q. Formulation of water-in-oil-in-water (W/O/W) emulsions containing trans-resveratrol. RSC Adv. 2017, 7, 35917–35927.
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion components screening and selection: A technical note. AAPS PharmSciTech 2009, 10, 69–76.
- Syed, H.K.; Peh, K.K. Identification of phases of various oil, surfactant/ co-surfactants and water system by ternary phase diagram. Acta Pol. Pharm. 2014, 71, 301–309.
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 2020, 7, 100027.
- Kar, M.; Chourasiya, Y.; Maheshwari, R.; Tekade, R.K. Chapter 2—Current Developments in Excipient Science: Implication of Quantitative Selection of Each Excipient in Product Development. In Basic Fundamentals of Drug Delivery; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 29–83.
- Zhang, Y.; Dong, L.; Liu, L.; Wu, Z.; Pan, D.; Liu, L. Recent Advances of Stimuli-Responsive Polysaccharide Hydrogels in Delivery Systems: A Review. J. Agric. Food Chem. 2022, 70, 6300–6316.
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive hydrogels: Fabrication and biomedical applications. VIEW 2022, 3, 20200112.
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox. Signal. 2014, 20, 1126–1167.
- Chen, X.; Jaiswal, A.; Costliow, Z.; Herbst, P.; Creasey, E.A.; Oshiro-Rapley, N.; Daly, M.J.; Carey, K.L.; Graham, D.B.; Xavier, R.J. pH sensing controls tissue inflammation by modulating cellular metabolism and endo-lysosomal function of immune cells. Nat. Immunol. 2022, 23, 1063–1075.
- Jose, M.; Bronckaers, A.; Kumar, R.S.N.; Reenaers, D.; Vandenryt, T.; Thoelen, R.; Deferme, W. Stretchable printed device for the simultaneous sensing of temperature and strain validated in a mouse wound healing model. Sci. Rep. 2022, 12, 10138.
- Cserháti, T.; Forgács, E.; Oros, G. Biological activity and environmental impact of anionic surfactants. Environ. Int. 2002, 28, 337–348.
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Chemistry, Toxicity and Remediation. In Pollutant Diseases, Remediation and Recycling; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Springer International Publishing: Cham, Switzerland, 2013; pp. 277–320.
- Lim, T.Y.; Poole, R.L.; Pageler, N.M. Propylene Glycol Toxicity in Children. J. Pediatr. Pharmacol. Ther. 2014, 19, 277–282.
- Sharma, S.; Parveen, R.; Chatterji, B.P. Toxicology of Nanoparticles in Drug Delivery. Curr. Pathobiol. Rep. 2021, 9, 133–144.
- Semashko, V.V.; Pudovkin, M.S.; Cefalas, A.-C.; Zelenikhin, P.V.; Gavriil, V.E.; Nizamutdinov, A.S.; Kollia, Z.; Ferraro, A.; Sarantopoulou, E. Tiny Rare-Earth Fluoride Nanoparticles Activate Tumour Cell Growth via Electrical Polar Interactions. Nanoscale Res. Lett. 2018, 13, 370.
