Berberine is a plant metabolite belonging to the group of isoquinoline alkaloids with
strong biological and pharmacological activity. Currently, berberine is receiving considerable interest due to its anticancer activity based on many biochemical pathways, especially its proapoptotic and anti-inflammatory activity. Therefore, the growing number of papers on berberine demands summarizing the knowledge and research trends. The efficacy of berberine in breast and colon cancers seems to be the most promising aspect. Many papers focus on novel therapeutic strategies based on new formulations or search for new active derivatives. The activity of berberine is very important as regards sensitization and support of anticancer therapy in combination with well-known but in some cases inefficient therapeutics. Currently, the compound is being assessed in many important clinical trials and is one of the most promising and intensively examined natural agents.
- apoptosis
- metastasis
- berberine
- anticancer activity
1. Anticancer Activity of Berberine
The first study of the cytotoxic activity of berberine was published in 1986 [1][32]. Later studies demonstrated the cytotoxic activity of berberine towards many cancer cell lines such as the promyelocytic leukemia HL-60 cell line [2][3][33,34], uterine cancer HeLa cell line [4][5][35,36], lymphocytic leukemia L1210 cell line, myelomonocytic leukemia WEHI-3 cell line [6][37], myeloid leukemia K562 cell line [7][38], large intestine cancer HT29 cell line [8][9][39,40], bladder cancer BIU-87 and T24 cell lines [10][41], hepatoma HepG2 cell line, non-small cell lung cancer [11][12][20,42], Lewis lung cancer [13][43], astrocytoma G95/VGH and GBM 8401 cell lines [14][44], melanoma B16 cell line and model U937 cell line [15][45]. Berberine is cytotoxic towards cancer cell lines and this activity is dependent on the dose and time. Studies have shown many mechanisms of the anticancer activity of berberine. In the assessment of the levels of expression of a panel of 44 genes in the human colon adenocarcinoma HCA-7 cell line, berberine treatment resulted in downregulation of 33 genes differently involved in the cell cycle, differentiation and epithelial–mesenchymal transition in a time- and dose-dependent manner [16][46]. The therapeutic window of berberine in most cases is narrow and depends on the dosage and type of cells that are treated.
1.1. Cell Cycle Arrest
It has been shown that berberine in low concentrations arrests human cancer cells in the G1 phase, while high concentrations arrest the cell cycle in the G2/M phase [17][18][29,47]. Berberine has been shown to inhibit the cell cycle in the G1 phase by upregulation of the BTG2 gene (B cell translocation gene 2), which is a cell proliferation regulatory gene induced by the p53 protein. The arrest of the cell cycle in the G2/M phase is p53 independent [18][19][20][47–49]. The arrest of the cell cycle in the G0/G1 phase after exposure to berberine was reported in lymphocytic leukemia cell line L1210 [21][35] and bladder cancer cells BIU-87 and T24 cell lines [22][50]. Colon cancer cells exposed to berberine were characterized by G0/G1 phase cell cycle arrest with downregulation of the antiapoptotic gene BCL2 in a concentration-dependent manner [23][24][25][51–53]. Cyclins seem to be an important target for cell cycle arrest induced by berberine. Downregulation of cyclin D1 was observed after exposure to berberine in the G1 cell cycle phase [18][47]. Reduction of the expression of cyclin B1 by berberine and the increase in the expression of Wee1 can arrest tumor cells in the G1 and G2 phases [18][26][47,54]. G0/G1 arrest was observed in MDA-MB-231 and MCF-7 breast cancer cells after exposure to berberine, possibly due to a decrease in the level of the cell cycle regulatory protein cyclin B1. This effect was also dose dependent [18][47]. As reported by Chidambara et al., arrest of the cell cycle in the G2/M phase by berberine is dependent on the REV3 gene. Cells of the DT40 line deficient in REV3 are hypersensitive to berberine and their DNA undergoes double-strand breaks much more strongly than the DNA of wild-type cells after exposure to berberine [27][55]. Berberine-induced inhibition of the cell cycle in the G2/M phase has also been described in colorectal cancer cells of the SW480 line [28][56].
1.2. Apoptosis Induction
One of the most important and comprehensively examined processes triggered by exposure to berberine, as regards its anticancer activity, is apoptosis. Induction of a number of biochemical events i.e., a decrease in the mitochondrial membrane potential, release of cytochrome c, Bcl2 family proteins and caspase activation or PARP breakdown after exposure to berberine, confirms the proapoptotic abilities of berberine [29][57]. Berberine induces apoptosis in tumor cells, mainly via upregulation of proapoptotic genes and downregulation of antiapoptotic genes [3][30][34,58]. Changes in the gene expression in leukemic cells exposed to berberine showed that, despite low cytotoxicity of observed dosage, the compound significantly increased the expression of caspase genes CASP3, CASP8 and CASP9 and proapoptotic genes BAK1, BAX and BIK. Simultaneously, downregulation of the expression of antiapoptotic genes Toxins 2020, 12, 713 5 of 26 BCL2, BCL2L2, BNIP1 and BNIP3 was noticed. This indicates that gene regulation leads to the apoptosis caused by berberine [3][34]. In other studies, after exposure of cells from the HL-60 [2][33], U937 and B16 lines [15][45] to berberine, activation of protein caspase -3 and -9, an increase in Bax (Bcl2-associated X protein) and a decrease in the Bcl-2 protein level were reported [2][33]. Additionally, an increase in the level of important proapoptotic proteins taking part in apoptosis signaling pathways such as p53, Rb (retinoblastoma Protein), ATM (serine/threonine kinase), caspase-8, Fas Receptor (death receptor)/FasL (Fas ligand), BID (BH3 interacting-domain death agonist, a proapoptotic member of the Bcl-2 protein family) and TNF (tumor necrosis factor) was reported. On the other hand, a decrease in the level of c-IAP1 (inhibitor of apoptosis protein), XIAP (X-linked Inhibitor of apoptosis protein), Bcl-X and Survivin (antiapoptotic protein) was reported after exposure to berberine. Among other mechanisms, berberine was shown to regulate proapoptotic and antiapoptotic proteins through an increase in the level of ROS—an important agent in apoptosis regulation [31][30][32][22,58,59]. An important role in berberine-induced apoptosis is played by death receptors, known as TRAIL receptor 2. TRAIL (tumor necrosis factor related apoptosis inducing ligand) is an apoptosis-inducing ligand associated with the tumor necrosis factor. It is known that TRIAL has great potential in the treatment of cancer, as it induces apoptosis by binding to the aforementioned receptors that induce tumor cell death, i.e., the so-called “death receptors”—DR4 and DR5. TRAIL induces apoptosis selectively; however, the development of partial or complete resistance limits its use. Berberine shows synergy with TRAIL. Moreover, it sensitizes cancer cells with TRAIL resistance. In TRAIL-sensitive (MDA-MB-231) and -resistant (MDA-MB-468) human breast cancer cell lines, berberine synergized with TRAIL, but it also sensitized the resistant cells to TRAIL. The markers of the process were caspase-3 and PARP 9 Poly (ADP-ribose) polymerase 1 cleavage and p53. The berberine sensitization to TRAIL-induced apoptosis is not limited to TRAIL-resistant MDA-MB-468 breast cancer cells. Despite the moderate cytotoxic effect on breast cancer cell line 4T1 in vitro, berberine in combination with antiDR5 markedly inhibited primary growth of tumor and reduced tumor metastasis to the lungs [26][33][54,60].
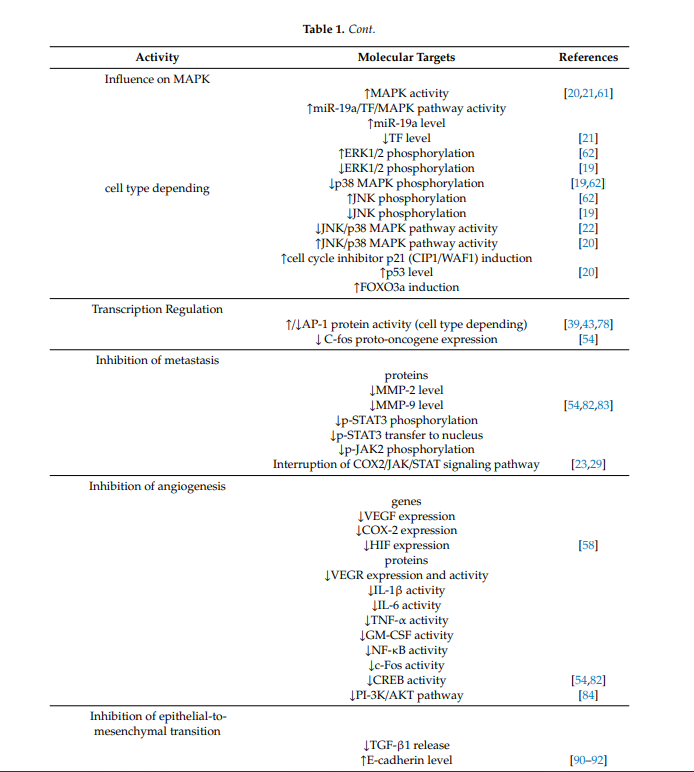
1.3. Influence on MAPK
There are many scientific reports on the effects of berberine on mitogen-activated kinases (called MAP or MAPK), which are involved in directing cellular responses to a variety of stimuli. They regulate processes that are very important in carcinogenesis, e.g., gene expression, mitosis, apoptosis, proliferation and differentiation [17][29]. Berberine has been shown to modulate mitogen-activated protein kinase signaling pathways, such as extracellular signal regulated kinase 1/2 (ERK1/2), p38 MAPK (p38 mitogen-activated protein kinases) and c-Jun N-terminal kinase (JNK) pathways. Compounds modulating these pathways are noteworthy as potential anticancer drugs. The effect depends on the type of cell. Berberine activates MAPK in human colon cancer cells [34][61], non-small cell lung cancer cells and human hepatoma cells (HepG2) [11][35][20,21]. In turn, in human HeLa cervical carcinoma cells, berberine enhances phosphorylation of JNK and ERK1/2 but inhibits phosphorylation of p38 MAPK [36][62]. In a Hong et al.’s study, berberine reduced the p38 MAPK, ERK1/2 and JNK phosphorylation in gastric cancer cells [37][19]. The JNK/p38 MAPK signaling pathway is disrupted in many types of cancer [38][63]. It was found that berberine suppressed the invasion and migration of cancer cells through blocking the JNK/p38 signaling pathway in the gastric cancer SNU-1 cell line [31][22]. One of the more precisely described phenomena in this field of berberine activity is its influence on MAPK via the impact of micro RNA inhibiting the translation of certain proteins, the dysfunction of which plays a role in formation of, e.g., non-small cells lung cancer. Abnormal levels of these proteins are correlated with the tissue factor TF, which contributes to tumor metastasis of non-small cells lung cancer. It has been shown to activate signaling cascades, including MAPK. In human lung cancer A549 cells, apoptosis through the miR-19a/TF/MAPK signaling pathway has been described after exposure to berberine [35][21]. Berberine raises the level of miR-19a and lowers the level of TF, thus activating MAPK signaling leading to apoptosis of cancer cells [35][21]. The cyclin-dependent kinase inhibitor p21 (CIP1/WAF1) is involved in the cell cycle control, cell differentiation, apoptosis and DNA replication [39][27]. It is linked with p53 and FOXO3a in the control of cancer cell growth [40][41][42][64–66]. FOXO3a (human protein Forkhead box O3) is a transcription factor from a family of transcription factors with tumor suppressor activity. It is regulated by growth factor receptor-induced activation of the phosphatidylinositol 3-kinase (PI3-K)/Akt signaling pathway. Its activation is connected with apoptosis [43][67] and cell cycle arrest [44][68] and, in various types of cells, it is associated with tumor suppression. Inhibition of FOXO3a causes tumor progression [45][69]. Zheng et al. demonstrated that, in non-small cell lung cancer, berberine inhibited proliferation and induced apoptosis by activating the p38α MAPK signaling pathway, resulting in an increase in p53 and FOXO3a and induction of the cell cycle inhibitor p21 (CIP1/WAF1) [11][20].
1.4. Trancription Regulation
Berberine also exhibits activity against the very important transcription factor-1 (AP-1), which is closely related to neoplastic transformation. AP-1 consists of complexes comprising the following families of DNA-binding proteins: Fos family (c-Fos, Fra-1, FosB and Fra-2,), Jun family (c-Jun, JunD, JunB and v-Jun), ATF/cyclic AMP-responsive element-binding (b-ATF, ATF1–4, ATF-6 and ATFx) and Maf family (c-Maf, MafA, MafB, MafG/F/K and Nrl), which play a key role in inflammation, proliferation and apoptosis. The activity of AP-1 is regulated by, e.g., growth factors, infections, cytokines, UV radiation, or cellular stress [46][70]. Extrinsic carcinogens can induce increased AP1 activity [47][71]. Many human tumors overexpress members of the Jun protein family [48][49][50][72–74]. Overexpression of these proteins has been described in aggressive forms of lymphomas [51][52][75,76] and in breast cancer [50][74]. However, increased expression of c-Fos is observed in endometrial cancer and osteosarcoma, while decreased expression of c-Fos is associated with the progression of ovarian and gastric cancer [53][77]. The role of the Fos family in tumor development is thus tissue-specific [46][70]. Studies have shown that in general AP-1 activation depends on the type of extrinsic stimulus and the cellular condition may have different effects on the cell fate. It has been shown that the AP-1 protein was inhibited in hepatoma cells of the HepG2 line after exposure to berberine [8][54][39,78]. In turn, in oral administration of berberine, spontaneous metastasis of Lewis lung cancer cells from mediastinal lymph nodes to lung parenchyma was inhibited through AP-1 protein activation [13][43]. After oral administration of berberine, decreased expression of the C-fos proto-oncogene was described [26][54]. Thus, the influence of berberine on the AP1-protein family depends on the cell type and requires further investigations.
1.5. Inhibition of Metastasis
Berberine is a potential agent to halt or prevent metastasis. It acts at several points of cancer progression. One is its strong influence on matrix metalloproteinases—important proteins involved in degradation of the barrier of extracellular matrix—the first and important step in tumor cell metastasis. Changed expression and levels of MMP activity are strongly involved in the development of many cancers. Increased MMP-2 activity is associated with poor prognosis in such types of cancer as colon, breast, melanoma, ovary, prostate and lung cancers [55][79]. Changes in MMP-2 activity may also be derived from changes in the levels of activation, inhibition and secretion or transcription of the MMP group of enzymes. MMP production in many cancers is elevated in the surrounding stromal tissue, but not in the tumor, and cases of metastasis are correlated with higher levels of MMP-2 mRNA in surrounding healthy tissue [56][80]. MMP-2 and MMP-9 can degrade type IV collagen, i.e., a major component of the basement membrane that is important for maintaining tissue organization and provides cell signaling and polarity. The degradation of the extracellular matrix allows cells to migrate from the tumor to form metastases. This is an essential step in the metastatic progression in most cancers [56][80]. Moreover, products of degradation of MMPs further promote MMP activity [57][81]. Berberine inhibits the expression of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) in a time- and concentration-dependent manner. In tests on mice, berberine was found to reduce metalloproteinase levels in plasma [58][82]. The regulation of the expression of matrix metalloproteinases by berberine proceeds through the inhibition of the transfer of p-STAT3 (signal transducer and activator of transcription 3) to the nucleus. In colon cancer cells it significantly reduces the level of JAK2 (Janus kinase 2—A protein from the Janus kinase family) and STAT3 phosphorylation. Phosphorylated molecules p-JAK2 and p-STAT3 are significantly increased in colorectal cancer cells overexpressing COX2 (cyclooxygenase-2). Overexpression of COX2 induces the activation of JAK-STAT, which increases the levels of metalloproteinases—MMP-2 and MMP-9 in colon cancer cells. Berberine markedly decreased the levels of phosphorylated JAK2 and phosphorylated STAT3 in colorectal cancer cells and effectively interrupted the COX2/JAK/STAT signaling pathway [17][59] [23,29], which was observed as a decrease in the level of metalloproteinases [26][60][54,83]. Berberine reduces protein levels of the STAT3 in nasopharyngeal carcinoma cells and blocks STAT3 activation induced by IL-6 secreted by tumor-associated fibroblasts. Similar to the Janus kinase family, the family of kinases of the transcription factors of the STAT plays an important role in the immunity process, cell division, cell death and tumor formation [61][84].
1.6. Inhibition of Angiogenesis
Another mechanism of berberine in the antiprogression process is inhibition of angiogenesis. Additionally, in this case, the influence on metalloproteinases 2 and 9 plays a crucial role. MMP-2 plays an important role in the formation of new blood vessels in tumors by supporting the migration of endothelial cells. It is crucial in the angiogenesis process, which is essential for tumor progression. An increased level of expression and higher activity of MMP-2 is observed with increased vascularization of the metastases of lung cancer in the central nervous system [62][85]. It has been shown that MMP-2 may affect tumor viability and invasiveness also by regulating lymphangiogenesis [56][80]. In contrast, MMP-9 and other metalloproteinases play a role in angiogenesis by promoting mobilization of VEGF (vascular endothelial growth factor) [56][80]. Berberine inhibits metastases by impeding angiogenesis through the effects on MMP-2 and MMP-9. However, inhibition of angiogenesis by berberine does not only affect metalloproteinases. In B16F-10 melanoma cells exposed to berberine, decreased expression of genes encoding angiogenesis-promoting factors, i.e., COX-2, HIF (hypoxia induced factor) and VEGF were reported [30][58]. Additionally, in the liver cancer HepG2 cell line and the gastric adenocarcinoma SC-M1 cell line, berberine was found to inhibit cell proliferation, migration, vascular endothelium formation and VEGF expression [63][86]. In breast cancer cells, berberine reduced the expression of VEGF and fibronectin by inhibiting the PI-3K/AKT pathway [64][84]. Inhibition of NF-κB in tumor cells is also one of the mechanisms of a berberine-induced decrease in the expression of VEGF and IL-8 [17][29]. Prevention of metastasis by inhibition of angiogenesis by berberine was confirmed in vivo [65][87]. In tests on mice, berberine reduced tumor vasculature by inhibiting the activity of factors responsible for angiogenesis, e.g., VEGF, inflammatory mediators: IL-6, IL-1β, TNF-α and a factor stimulating the formation of macrophage colonies—GM-CSF (granulocyte macrophage colony-stimulating factor). Berberine was also reported to inhibit the activity of transcription factors responsible for angiogenesis, i.e., NFκB, c-Fos, CREB (cAMP response element-binding protein) and ATF-2 (activating transcription factor 2) [26][58][54,82].
1.7. Inhibition of Epithelial-To-Mesenchymal Transition
Another facet of the antimetastatic action of berberine is its effect on E- and N-cadherin. Berberine influences the expression of the E-cadherin and N-cadherin proteins and the effect is time and dose dependent. E-cadherin and N-cadherin are closely related to the migration and invasion of cancer cells. E-kadherin is responsible for the structural integrity of epithelial cells [88]. It is a marker of Toxins 2020, 12, 713 8 of 26 TGF-β1-induced epithelial-to-mesenchymal transition. This process leads to increased cell motility. TGF-β (transforming growth factor beta 1) is a cytokine mediating progression by enhancing the epithelial-to-mesenchymal transition process. Berberine has been proven to inhibit the TGF-β1-induced epithelial-to-mesenchymal transition process and an elevated level of E-cadherin is a marker of this process [66][88]. MMP-2 activates TGF-β [67][89]; hence, the influence of berberine on the process of epithelial-to-mesenchymal transition is probably based on the above-described reduction of the level of metalloproteinase 2 growth factors [68][90] and the release of growth factors from outside the extracellular matrix, such as TGF-β [69][70][91,92]. By affecting metalloproteinases, berberine may indirectly influence the occurrence of apoptosis. For example, in human melanoma cells with expressed integrin αvβ3, the degradation of type I of collagen by MMP-2 may reveal a binding site with integrin αvβ3 in these cells. Signaling by this integrin is essential for the viability of melanoma cells and growth in the collagen matrix, thus it potentially protects melanoma cells from apoptosis [57][81]. MMP inhibitors possess high potential for improving cancer treatment by slowing the process of cancer invasion [55][79]. Phase I of clinical trials has shown that MMP inhibitors produce minimal adverse side effects. This way, berberine used as a MMP inhibitor seems to be a potential anticancer agent.
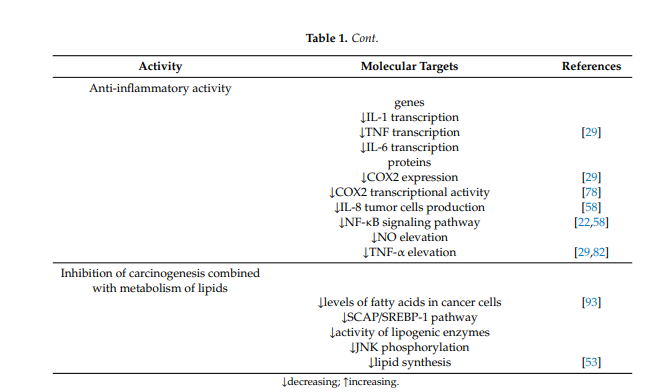
1.8. Anti-Inflammatory Activity
Anti-inflammatory activity is also one of the important effects rendering berberine a promising anticancer and protective agent. Berberine has anti-inflammatory activity in vitro and in vivo and inhibits the transcription of genes such as IL-1, TNF- and IL-6, decreasing the level of inflammatory proteins. Berberine suppresses the expression of cyclooxygenase 2 and prostaglandin E2 [17][29]. Studies have also demonstrated that berberine prevents production of IL-8 in tumor cells and blocks the NF-κb signaling pathway [30][58]. Studies have shown that berberine also inhibits the elevation of NO and TNF-α [17][58][29,82]. In colorectal cancer cells, berberine also inhibits COX-2 transcriptional activity, which is significantly increased in this type of cancer [54][78].
1.9. β-Catenin Expression
Berberine acts on β-catenin, whose mutations and overexpression are associated with many cancers, including colorectal carcinoma, breast tumors, ovarian and endometrial cancer. In colon cancer cells, the expression of its mRNA, is downregulated by berberine. Berberine efficiently inhibits the nuclear level of β-catenin by increasing adenomatous polyposis coli protein and β-catenin interactions [6][37]. It stimulates the expression of adenomatous polyposis coli protein and regulates β-catenin negatively [17][26][29,54].
1.10. Inhibition of Carcinogenesis Combined with Metabolism of Lipids
The influence on the metabolism of fats and lipids is one of the mechanisms of berberine action in the aforementioned metabolic diseases, but it also seems to be very important in malignancies, especially those of the digestive system. It has been described that berberine can act by inducing apoptosis via reduction of FABP expression and accumulation of fatty acids in gastric cancer [71][93] and by downregulation of key lipogenic enzymes in colon cancer. Berberine targets the SREBP-1 cleavage-activating protein-1/sterol receptor element-binding protein-1 (SCAP/SREBP-1) pathway driving lipogenesis, inhibits the pathway and thus results in downregulation of lipogenic enzymes. Downregulation of key lipogenic enzymes, leading to suppression of lipid synthesis, which is linked to cell proliferation through the Wnt/β-catenin pathway, has been described as one of the anticancer mechanisms of berberine [25][53]. Similarly, the influence on JNK kinases described above is important in anticancer and chemopreventive activity based on the influence on lipid metabolism [72][73][94,95].
Table 1. Molecular mechanisms of anticancer activity of berberine.
| Activity | Molecular Targets | References |
|---|---|---|
| Cell cycle arrest | ||
| Induced G0/G1 phase arrest | [18][23][24][25] | |
| ↓cyclin B1 expression | ||
| Induced G1 phase arrest | ||
| ↑geneBTG2 expression | ||
| ↑p53 protein expression | [18][19][20] | |
| ↓cyclin B1 expression | ||
| ↑Weel1 expression | [18][26] | |
| ↓cyclin D1 expression | [18] | |
| Induced G2 phase arrest | ||
| ↓cyclin B1 expression | ||
| ↑Weel1 expression | [18[26] | |
| Induced G2/M phase arrest | ||
| REV3-gene dependent | [27][28] | |
| Apoptosis induction | ||
| genes | ||
| ↑CASP3 expression | ||
| ↑CASP8 expression | ||
| ↑CASP9 expression | ||
| ↑BAX expression | ||
| ↑BAK1 expression | ||
| ↑BIK expression | ||
| ↓BNIP3 expression | ||
| ↓BNIP1 expression | ||
| ↓BCL2 expression | ||
| ↓BCL2L2 expression | [13] | |
| proteins | ||
| ↑caspase-3 expression | ||
| ↑caspase-9 expression | ||
| ↑Bax expression | [2][15] | |
| ↓Bcl-2 expression | [2] | |
| ↑p-53 expression | ||
| ↑ Rb expression | ||
| ↑ATM expression | ||
| Caspase-8 expression | ||
| ↑BID expression | ||
| ↑TNF expression | ||
| ↓c-IAP1 expression | ||
| ↓XIAP expression | ||
| ↓Bcl-X expression | ||
| ↓Survivin expression | [31][30][32] | |
| TNF-alpha receptors | ||
| ↑DR4 activation | ||
| ↑DR5 activation | [26][33] | |
| Influence on MAPK | ||
| cell type depending | ↑MAPK activity | [11][34][35] |
| ↑miR-19a/TF/MAPK pathway activity | ||
| ↑miR-19a level | ||
| ↓TF level | [35] | |
| ↑ERK1/2 phosphorylation | [36] | |
| ↓ERK1/2 phosphorylation | [37] | |
| ↓p38 MAPK phosphorylation | [36][37] | |
| ↑JNK phosphorylation | [36] | |
| ↓JNK phosphorylation | [37] | |
| ↓JNK/p38 MAPK pathway activity | [31] | |
| ↑JNK/p38 MAPK pathway activity | [11] | |
| ↑cell cycle inhibitor p21 (CIP1/WAF1) induction | ||
| ↑p53 level | [11] | |
| ↑FOXO3a induction | ||
| Transcription Regulation | ||
| ↑/↓AP-1 protein activity (cell type depending) | [8][13][54] | |
| ↓ C-fos proto-oncogene expression | [26] | |
| Inhibition of metastasis | ||
| proteins | ||
| ↓MMP-2 level | ||
| ↓MMP-9 level | [26][58][60] | |
| ↓p-STAT3 phosphorylation | ||
| ↓p-STAT3 transfer to nucleus | ||
| ↓p-JAK2 phosphorylation | ||
| Interruption of COX2/JAK/STAT signaling pathway | [17][59] | |
| Inhibition of angiogenesis | ||
| genes | ||
| ↓VEGF expression | ||
| ↓COX-2 expression | ||
| ↓HIF expression | [30] | |
| proteins | ||
| ↓VEGR expression and activity | ||
| ↓IL-1β activity | ||
| ↓IL-6 activity | ||
| ↓TNF-α activity | ||
| ↓GM-CSF activity | ||
| ↓NF-κB activity | ||
| ↓c-Fos activity | ||
| ↓CREB activity | [26][58] | |
| ↓PI-3K/AKT pathway | [61] | |
| Inhibition of epithelial-to-mesenchymal transition | ||
| ↓TGF-β1 release | ||
| ↑E-cadherin level | [68][69][70] | |
| Anti-inflammatory activity | ||
| genes | ||
| ↓IL-1 transcription | [17] | |
| ↓TNF transcription | ||
| ↓IL-6 transcription | ||
| proteins | ||
| ↓COX2 expression | [17] | |
| ↓COX2 transcriptional activity | [54] | |
| ↓IL-8 tumor cells production | [30] | |
| ↓NF-κB signaling pathway | [30][31] | |
| ↓NO elevation | ||
| ↓TNF-α elevation | [17][58] | |
| Inhibition of carcinogenesis combined with metabolism of lipids | ||
| ↓levels of fatty acids in cancer cells | [71] | |
| ↓SCAP/SREBP-1 pathway | ||
| ↓activity of lipogenic enzymes | ||
| ↓JNK phosphorylation | ||
| ↓lipid synthesis | [25] | |
↓decreasing; ↑increasing.