You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Heng Yin.
Due to their unique characteristics, nanoparticles are increasingly used in agricultural production through foliage spraying and soil application. The use of nanoparticles can improve the efficiency of agricultural chemicals and reduce the pollution caused by the use of agricultural chemicals.
- nanoparticles
- uptake
- transport
- physiological activity
1. Introduction
Nowadays, due to the advantages of nanoparticles as nano-carriers and nano-pesticides, such as their small size, ease of use, capacity for long-term storage, and improvement of the efficiency of agricultural chemicals, the application of nanoparticles in agriculture is considerably more extensive [63][1]. The interaction between nanoparticles and plants has beneficial and harmful effects on the plant’s physiological morphology, plant development and yield of crops. The effects of nanoparticles on plants are related to plant species, use methods, dosage and concentration of nanoparticles.
2. Nanoparticles Promote Plant Development and Yield
The use of agricultural nanoparticles has improved the quality of plant products better than traditional pesticides, which has been proven by many researchers. Nanoparticles play an important role in plant growth and improving plant quality by increasing nutrient content, improving photosynthetic activity and metabolism [78][2] (Figure 1). Zinc oxide nanoparticles have been confirmed to participate in the synthesis and photosynthesis of plant chlorophyll and the formation of starch, thus increasing the concentration of soluble carbohydrates [79][3]. The use of ZnO nanoparticles can improve the antioxidant activity and chlorophyll content of cotton, increase the number and weight of cotton bolls per plant, and improve cotton fiber quality parameters such as uniformity and fiber strength [80][4]. ZnO NPs act on tomato plants and improve tomato yield by increasing the absorption of nutrients (phosphorus and zinc) [81][5]. Fe3O4 NPs can improve plant biomass and productivity by increasing the content of protein, nutrients, and carbohydrates in plants [81,82][5][6]. Sharifi et al. used Fe3O4 NPs to act on corn plants, and Armin et al. used Fe3O4 NPs to act on wheat plants. All their results confirmed the above conclusions [83,84][7][8]. In addition, when Fe3O4 NPs were used to treat wheat at the tillering stage, the number of wheat seeds increased significantly. Gao et al. found that TiO2 NP increased the biomass accumulation of spinach by 60% [85][9]. In addition, CeO2 NPs promoted stem elongation at 1–10 mg/L, and fruit weight significantly increased at 10 mg/L [86][10].
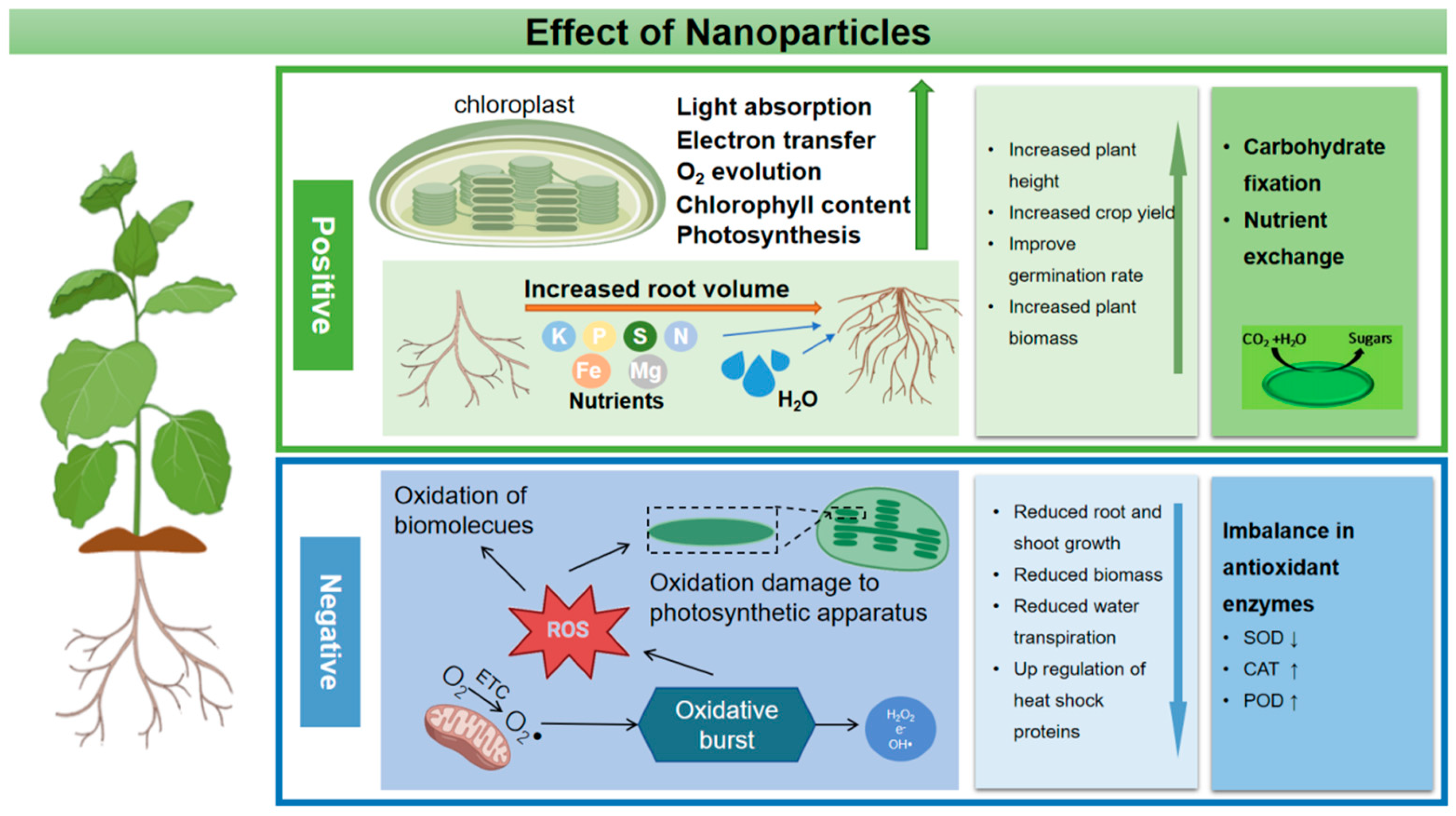
Figure 1.
A schematic diagram of the physiological activity of NPs in plants.
As an efficient environment-friendly photocatalyst, TiO2 nanoparticles have been proven to be able to improve light absorption by improving the energy conversion of the light system, and have antibacterial activity after surface chemical modification, which can reduce the half-life of pesticides and promote seed germination and seedling growth [87][11]. In 2013, it was proven that TiO2 nanoparticles could improve the photosynthetic efficiency of spinach. In recent studies, it was shown that TiO2 NPs could promote wheat growth and increase wheat yield. At the same time, experiments have proven that these improvements are due to the fact that titanium dioxide nanoparticles promote cycling and linear phosphorylation to improve photosynthetic activity, thus increasing the supply of photosynthates and in turn increasing plant yield. Spraying TiO2 NPs on the leaves improves the dry matter yield of barley because nanoparticles improve the photoreduction activity [88][12]. In addition, under the joint action of ZnO NPs and Si NPs, the yield, weight and sugar content of mango fruit increased [89][13]. The promotion effects of different kinds of nanoparticles on plant growth and seed germination are listed in Table 1.
Table 1.
Summary of the representative of nanoparticles that promote plant development and yield.
| Nanoparticle | Size (nm) | Concentration | Plants | Positive Effect | Reference |
|---|---|---|---|---|---|
| ZnO | 18 | Fresh soil with 6 mg/kg soil | Triticum aestivum Landmark | Leaf chlorophyll levels and shoot height increased | [90][14] |
| 30 | 0 and 500 mg/kg soil with and without organic P (0, 20 and 50 mg/kg) |
Maize | ZnO NMs increased root dry weight | [91][15] | |
| <100 | 50, 100 and 150 mg/L | Mangifera indica Linn | Improved the total yield (fruit number and weight per tree) the combined application of NPs resulted in an increase in fruit yield, average fruit weight, length, width, TSS, sugars and displayed the lowest acidity percentage | [89][13] | |
| 12–24 | 1000 and 2000 mg/L | Capsicum chinense | NPs promoted plant growth, increased number and average weight of the fruits, fruit quality, capsaicin and dihydrocapsaicin content at low doses |
[92][16] | |
| TiO2 | 20 | 25–750 mg/L | Oryza sativa | Enhanced accumulation of palmitic acid, amino acids and glycerol in rice grain, improved shoot growth and phosphorus concentration in whole plant and grains | [93][17] |
| 32 | 10, 100,1000 mg/L | Triticum aseivum | Enhanced growth of lateral roots and biomass with concurrent uptake of titanium | [94][18] | |
| 27 | 250, 500, 750 mg/L | Cannabls sativa | Increased potassium and phosporus in cucumber fruits | [73][19] | |
| Cu | 50 | 50–500 mg/L | Solanum lycopersicum | Enhanced lycopene, vitamin-C in tomato fruits, number of fruits and fruit firmness | [95][20] |
| SiO2 | 4–10 | 5 mM | Oryza sativa | Increased grain yield and weight | [96][21] |
| Si | 25, 50 mg/L | lentil | Promote seed germination, seedling vigor and biomass | [97][22] | |
| CeO2 | 15–30 | 200, 500 mg/L | Arabidopsis thaliana | Increased the root elongation, root and shoot growth | [98][23] |
3. Nanoparticles Alleviate Plant Abiotic Stress
Plant abiotic stress is the main problem that plants face in the process of growth due to drought, salt and heavy metal elements, which lead to the reduction of crop yield due to plant growth retardation. In order to alleviate these stresses, plants have evolved different defense mechanisms through physiological pathways. The application of nanoparticles can help plants to alleviate abiotic stresses. Due to environmental, climatic and other factors, the yield and biomass of crops in arid areas are significantly reduced compared with a normal environment. Sun et al. applied zinc oxide nanoparticles to treat corn and found that nanoparticles can improve the photosynthetic rate and chlorophyll content of plants under drought stress, which proved that nanoparticles have a mitigating effect on plant drought stress [59][24].
Inappropriate salinity in plant growth environments will lead to plant nutrition imbalance and slow plant growth [99][25]. The application of nanoparticles in agriculture can help improve the activity of enzymes involved in the salt tolerance mechanism in plants. The study showed that ZnO NPs alleviated the salt stress of cotton [100][26] and wheat [101][27]. The application of SiO2 NPs on the leaves increased the elasticity and expansion of the cell wall of cucumber during the growth period and increased the accumulation of nitrogen and phosphorus elements in the leaves by reducing the loss of leaching process, thus reducing the content of Na, alleviated the salt stress on cucumber plants [66][28].
The existence of heavy metals is more harmful to plant growth, which will affect plant morphology, inhibit plant growth and stimulate plants to produce oxidative stress. In order to resist heavy metal stress, nanoparticles can improve the ability of antioxidant systems by regulating the concentration of heavy metal ions in soil, reducing the expression of heavy metal ion transport genes in plants, stimulate the production of defense substances (such as organic acids, root exudates and phytochelatin) to cope with the stress of heavy metal ions in plants [102][29].
Sharifan et al. used ZnO NPs and heavy metals Pb2+ and Cd2+ as the hydroponic culture system to culture lettuce (Lactuca sativa L. var. Longifolia), and detected the content of Pb and Cd in plant tissue. It was found that ZnO NPs significantly reduced the accumulation of Pb and Cd in the root of lettuce, which were 81% and 49%, respectively. Pb and Cd in the shoots of lettuce decreased by 44% and 30% [103][30], respectively. Yan et al. treated rice with ZnO NPs, proving that zinc oxide nanoparticles can improve rice growth and reduce the accumulation of arsenic in rice. The best effect was found when the concentration of ZnO NPs was 100mg/L. Compared with the control group, the concentration of As in the bud and root of rice seedlings decreased by 40.7% and 31.6%, respectively. Se and Si NPs reduced the absorption and accumulation of Cd and Pb in rice, thus reducing the lethal effect of heavy metals on plants. At the same time, the use of SiO2 NPs in leaves helps to increase chlorophyll content and reduce the accumulation of Cd in rice [104][31].
4. Toxicity of Nanoparticles to Plants
In addition to many beneficial effects, the toxicity of nanoparticles in plants cannot be ignored. The toxicity of nanoparticles can damage plants in a variety of ways, for example, by stimulating plant oxidative stress, resulting in physical damage to plants, such as stomatal closure due to the aggregation of nanoparticles [6][32].
4.1. Inhibitory Effect of Nanoparticles on Plant Growth
When nanoparticles are sprayed on plants through leaves at high concentrations, a large number of NPs will gather on the surface of leaves, which results in stomatal blockage and hinders the gas exchange and photosynthesis of plants (Figure 1). Some studies have shown that when the concentration of Zn NPs and Cu NPs exceeds the critical value, the plant growth rate will slow down and the leaves will turn yellow.
CuO NPs are toxic to H. sativum, and will reduce the photosynthetic rate of plants and inhibit the growth of roots and stems [105][33]. At the same time, nanoparticles transform in plants result in damage to cell structure and reduce the absorption and transportation of nutrients [106][34].
For polymer-based nanoparticles, the current research mainly discusses their toxic effects on plants in the process of application. For example, after treating the seeds of Lepidiumsativum with plastic particles with a size of 50 and 500 nm for 8 h, the germination rates decreased by 56% and 46%, respectively [51][35]. When ryegrass was exposed to PLA nanoparticles for 30 days, the germination rate decreased by 6% [107][36]. In contrast, the germination rate of wheat seeds and onions treated with PS NPs for 72 h had no effect [107,108][36][37]. Therefore, long-term exposure of plants to polymer nanoparticles will reduce the germination rate, which is mainly due to the reduction of nutrient absorption of plants due to the blockage of pores by nanoparticles.
In addition to the effects of polymer nanoparticles on plant seed germination, other studies have reported the effects on plant seedling growth. The length of the onion root decreased by 41.5% after polystyrene nanoparticles (50 nm) acted on the onion root for 72 h [109,110][38][39]. The length of the Lemna minor root also decreased under the effect of polyethylene nanoparticles. In Arabidopsis thaliana treated with polystyrene nanoparticles with different surface charges (PS-NH2, PS-SO3H), the fresh weight of Arabidopsis thaliana decreased by 50% on average. The plant length decreased by 15%, the root length decreased by 30% on average, and the higher the concentration of nanoparticles, the more obvious the inhibition of Arabidopsis thaliana seedlings is [49][40]. A better understanding of the effects of nanoparticles on plants can help assess their toxicity (Table 2).
Table 2.
Summary of the inhibitory effects of nanoparticles on plants.
| Nanoparticle | Size (nm) | Concentration | Plants | Negative Impact | Reference |
|---|---|---|---|---|---|
| ZnO | <50 | 500 ppm | Glycine max | Inhibition of root elongation, cell viability and biomass, generation of superoxides, reduced biomass of foliage, alteration of gene expression | [111][41] |
| 90 | 400–3200 mg/kg | Zea mays | Increased production of superoxide anions and superoxide dismutase activity decreased mineral nutrient acquisition, decreased photosynthesis and root activity | [112][42] | |
| 30–40 | 0.02–2 g/L | Zea mays | Negative effect on seed germination and seedling growth | [113][43] | |
| 10 | 250, 500, 750 mg/kg | Medicago sativa | Reduced root biomass up to 80% | [114][44] | |
| 64, 80 | 20–200 mg/L | Vigna angularis | Disrupted plant physiology of plant, enhanced oxidative stress and reduced photosynthetic pigment | [115][45] | |
| Ag | 10 | 10–50 mg/kg | Lysopersicon esculentum | Induced reactive oxidative stress that reduced photosynthesis, CO2 assimilation and fruit yield | [116][46] |
| 10 | 0.001–10,000 mg/L | Allium cepa | Root growth was inhibited | [117][47] | |
| 10 | 25, 50, 75 and 100 μM | Allium cepa | Strongly reduced the root growth, induced mitotic index, induced ROS formation, caused oxidative DNA damage in higher concentrations | [118][48] | |
| 5–10 | 300–900 ppm | Lupinus termis | Decreased germination percentage, reduced the root and shoot elongation, root and shoot fresh weights, total chlorophyll, total protein content and sugar content | [119][49] | |
| CuO | 40–80 | 500, 1000, 2000 mg/L | Zea mays, Oryza sativa | 95% and 97% inhibition in root length of maize and rice at 2000 mgL−1 | [120][50] |
| 25–55 | 50–500 mg/L | Brassica rapa | Synthesis of photosynthetic pigments and sugar was decreased | [121][51] | |
| 43 | 50–1000 mg/kg | Oryza sativa | Low water uptake by root and aerial parts; grain production was considerably reduced | [122][52] | |
| TiO2 | 20 ± 5 | 50, 200 mg/L | Oryza sativa | Reduced grain yield and biomass | [123][53] |
| 25 | 250–1000 mg/L | Cannabls sativa, Brassica oleracea var. capitata, Avena sativa, Zea mays, Lactuca sativa, Allium cepa |
Inhibition of root growth for oat, corn, cabbage, lettuce and reduction of soybean and cucumber germination | [124][54] | |
| 5–15 | 0.02–2 g/L | Zea mays | Inhibition of germination, root and shoot growth | [113][43] | |
| 20 | 100–500 mg/L | Oryza sativa | Reduced biomass and altered antioxidant defense | [125][55] | |
| Al2O3 | <50 | 10–1000 mg/L | Sinapis alba | At all concentrations, seed germination was affected negatively | [126][56] |
| 13 | 5, 25, 59 mg/L | Triticum aseivum | H2O2 content was increased, reduced production of photosynthetic pigment and anthocyanin | [127][57] | |
| Au | 2–21 | 5, 8, 10 mg/L | Hordeum vulgare | Leaves became yellow and necrotic, the roots colored dark brown and decreased fresh plant biomass, leaf and root lengths | [128][58] |
| 3.5, 18 | 48 ppm | Nicotiana xanthi | Caused biotoxicity and observed leaf necrotic lesions | [6][32] |
4.2. Genotoxicity and Oxidative Stress Damage of Nanoparticles in Plants
The transport and accumulation of nanoparticles in plants will induce phytotoxicity and the interaction between nanoparticles and plants will lead to increased production of plant ROS, resulting in oxidative damage and genetic toxicity (Figure 1) [129,130][59][60]. Similarly to all aerobic organisms, plant cells activate ROS in response to environmental changes [131][61]. When ROS levels exceed defense mechanisms, cells are placed in a state of “oxidative stress”, causing unlimited damage to proteins, nucleic acids and lipids in cell membranes and inducing oxidative stress [132][62]. Plants can protect cells from the toxicity of reactive oxygen species (ROS) through antioxidant enzymes and antioxidants. Plants can protect cells and subcellular systems from the toxicity of active oxygen radicals by antioxidant enzymes and low molecular weight antioxidants [133][63]. Therefore, the research on oxidative damage caused by nanoparticles mainly focuses on the determination of antioxidant enzyme activity and ROS content. Some research and analysis, especially when nanoparticles act on plants at high concentrations, results in excessive accumulation of nanoparticles in plants and a large number of ROS that activate the antioxidant system [58,106,134][34][64][65]. As the concentration of plastic NPs increased, the activity of several representative antioxidant enzymes in rice roots increased, suggesting that the plant could stimulate a defensive response and remove the excessive accumulation of ROS [135][66]. Meanwhile, a higher concentration of nanoparticles will affect the level of plant endogenous hormones [58][64]. At present, there are also corresponding studies to evaluate the impact of nanoparticles on plant endogenous hormones [136][67]. For example, iron oxide nanoparticles with a concentration of 100 mg/L reduced the yield of Bt-transgenic cotton and led to an increase in hormone levels. While in the root of Bt-transgenic cotton, the hormone content decreased [137][68]. In addition, metal-based nanoparticles transform and release toxic metal ions in plants, which destroy DNA and protein in plants and inhibit normal cell metabolism [138][69].
In addition to inducing ROS production in plants and triggering hormonal changes in plants, nanoparticles are also genotoxic. Nanoparticles interact with biological macromolecules such as nuclei and lipids and exert genotoxicity, which can affect plant cell division [139][70]. For example, Ag NPs internalized in wheat root tips have been shown to interfere with normal cell division, inhibit DNA synthesis and lead to chromosome aberrations [140][71]. In addition, the mitotic index of onion cells exposed to PS NPs was significantly reduced and chromosomal aberrations and nuclear abnormalities were observed, leading to the destruction of genomic stability, demonstrating the genotoxicity of PS NPs [141][72].
In order to deal with the toxicity caused by nanoparticles, researchers have also developed corresponding solutions, in which the toxic substances released by nanoparticles can be effectively reduced by adding a surface coating. For example, adding an iron coating to the surface of zinc oxide nanoparticles can effectively reduce the release of zinc ions. Fe-coated zinc oxide nanoparticles have no inhibition on plant germination and pigment content in plants [142][73]. The method of reducing toxicity through encapsulation or surface modification of nanoparticles is also applicable to polymer nanoparticles [43][74].
Analyzing the toxicity of different nanoparticles as agricultural chemicals are helpful to determine the optimal concentration of nanoparticles in plant growth. As nontoxic, degradable and biocompatible compound nanoparticles, natural polymer nanoparticles can largely avoid the negative effects of metal-based, silicon-based and organic-based nanoparticles on plants and the environment [143][75]. Chitosan is the only positively-charged polysaccharide in nature, which has been reported as a nanoparticle material due to its antibacterial and antiviral properties. Because of its positive charge, chitosan nanoparticles enhance the affinity with the plant cell membranes and increase the reactivity of the plant systems. When chitosan-based nanoparticles are used as plant growth promoters to treat seeds and seedlings; it has been proven that they can improve plant nutrient absorption, chlorophyll content and photosynthesis rate. Three different sizes of chitosan nanoparticles (420 nm, 750 nm, 970 nm) were applied to plants. The results showed that plant chlorophyll increased by 61%, 81% and 61% and the photosynthetic rate increased by 29%, 59% and 72%, respectively [144][76]. The application of chitosan nanoparticles on wheat and barley has also been proven to promote plant growth [145][77].
References
- Bueno, V.; Gao, X.; Rahim, A.A.; Wang, P.; Bayen, S.; Ghoshal, S. Uptake and Translocation of a Silica Nanocarrier and an Encapsulated Organic Pesticide Following Foliar Application in Tomato Plants. Environ. Sci. Technol. 2022, 56, 6722–6732.
- Shebl, A.; Hassan, A.A.; Salama, D.M.; Abd El-Aziz, M.E.; Abd Elwahed, M.S.A. Green Synthesis of Nanofertilizers and Their Application as a Foliar for Cucurbita pepo L. J. Nanomater. 2019, 2019, 3476347.
- Bala, R.; Kalia, A.; Dhaliwal, S.S. Evaluation of Efficacy of ZnO Nanoparticles as Remedial Zinc Nanofertilizer for Rice. J. Soil Sci. Plant Nutr. 2019, 19, 379–389.
- Vaghar, M.S.; Sayfzadeh, S.; Zakerin, H.R.; Kobraee, S.; Valadabadi, S.A. Foliar application of iron, zinc, and manganese nano-chelates improves physiological indicators and soybean yield under water deficit stress. J. Plant Nutr. 2020, 43, 2740–2756.
- Faizan, M.; Bhat, J.A.; Chen, C.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P.; Yu, F. Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol. Biochem. 2021, 161, 132–140.
- Choukri, M.; Abouabdillah, A.; Bouabid, R.; Abd-Elkader, O.H.; Pacioglu, O.; Boufahja, F.; Bourioug, M. Zn application through seed priming improves productivity and grain nutritional quality of silage corn. Saudi J. Biol. Sci. 2022, 29, 103456.
- Tondey, M.; Kalia, A.; Singh, A.; Dheri, G.S.; Taggar, M.S.; Nepovimova, E.; Krejcar, O.; Kuca, K. Seed Priming and Coating by Nano-Scale Zinc Oxide Particles Improved Vegetative Growth, Yield and Quality of Fodder Maize (Zea mays). Agronomy 2021, 11, 729.
- Armin, M.; Akbari, S.; Mashhadi, S. Effect of time and concentration of nano-Fe foliar application on yield and yield components of wheat. Int. J. Biosci. 2014, 4, 69–75.
- Gao, G.; Tester, M.A.; Julkowska, M.M. The Use of High-Throughput Phenotyping for Assessment of Heat Stress-Induced Changes in Arabidopsis. Plant Phenomics 2020, 2020, 3723916.
- Nadeem, M.; Khan, R.; Afridi, K.; Nadhman, A.; Ullah, S.; Faisal, S.; Ul Mabood, Z.; Hano, C.; Abbasi, B.H. Green Synthesis of Cerium Oxide Nanoparticles (CeO2 NPs) and Their Antimicrobial Applications: A Review. Int. J. Nanomed. 2020, 15, 5951–5961.
- Su, M.; Liu, H.; Liu, C.; Qu, C.; Zheng, L.; Hong, F. Promotion of nano-anatase TiO2 on the spectral responses and photochemical activities of D1/D2/Cyt b559 complex of spinach. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2009, 72, 1112–1116.
- Thabet, S.G.; Sallam, A.; Moursi, Y.S.; Karam, M.A.; Alqudah, A.M. Genetic factors controlling nTiO2 nanoparticles stress tolerance in barley (Hordeum vulgare) during seed germination and seedling development. Funct. Plant Biol. 2021, 48, 1288–1301.
- Elsheery, N.I.; Helaly, M.N.; El-Hoseiny, H.M.; Alam-Eldein, S.M. Zinc Oxide and Silicone Nanoparticles to Improve the Resistance Mechanism and Annual Productivity of Salt-Stressed Mango Trees. Agronomy 2020, 10, 558.
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Exposure to Weathered and Fresh Nanoparticle and Ionic Zn in Soil Promotes Grain Yield and Modulates Nutrient Acquisition in Wheat (Triticum aestivum L.). J. Agric. Food Chem. 2018, 66, 9645–9656.
- Garcia-Gomez, C.; Obrador, A.; Gonzalez, D.; Babin, M.; Dolores Fernandez, M. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24.
- Garcia-Lopez, J.I.; Nino-Medina, G.; Olivares-Saenz, E.; Lira-Saldivar, R.H.; Diaz Barriga-Castro, E.; Vazquez-Alvarado, R.; Rodriguez-Salinas, P.A.; Zavala-Garcia, F. Foliar Application of Zinc Oxide Nanoparticles and Zinc Sulfate Boosts the Content of Bioactive Compounds in Habanero Peppers. Plants 2019, 8, 254.
- Zahra, Z.; Waseem, N.; Zahra, R.; Lee, H.; Badshah, M.A.; Mehmood, A.; Choi, H.-K.; Arshad, M. Growth and Metabolic Responses of Rice (Oryza sativa L.) Cultivated in Phosphorus-Deficient Soil Amended with TiO2 Nanoparticles. J. Agric. Food Chem. 2017, 65, 5598–5606.
- Zhang, P.; Ma, Y.; Xie, C.; Guo, Z.; He, X.; Valsami-Jones, E.; Lynch, I.; Luo, W.; Zheng, L.; Zhang, Z. Plant species-dependent transformation and translocation of ceria nanoparticles. Environ. Sci.-Nano 2019, 6, 60–67.
- Servin, A.D.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Diaz, B.C.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron Micro-XRE and Micro-XANES Confirmation of the Uptake and Translocation of TiO2 Nanoparticles in Cucumber (Cucumis sativus) Plants. Environ. Sci. Technol. 2012, 46, 7637–7643.
- Lopez-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Romenus, K.D.; de la Fuente, M.C.; Benavides-Mendoza, A.; Juarez-Maldonado, A. Foliar Application of Copper Nanoparticles Increases the Fruit Quality and the Content of Bioactive Compounds in Tomatoes. Appl. Sci. 2018, 8, 1020.
- Liu, C.P.; Li, F.B.; Luo, C.L.; Liu, X.M.; Wang, S.H.; Liu, T.X.; Li, X.D. Foliar application of two silica sols reduced cadmium accumulation in rice grains. J. Hazard. Mater. 2009, 161, 1466–1472.
- Khan, Z.; Ansari, M.Y.K. Impact of Engineered Si Nanoparticles on Seed Germination, Vigour Index and Genotoxicity Assessment via DNA Damage of Root Tip Cells in Lens culinaris. J. Plant Biochem. Physiol. 2018, 6.
- Yang, X.; Pan, H.; Wang, P.; Zhao, F.-J. Particle-specific toxicity and bioavailability of cerium oxide (CeO2) nanoparticles to Arabidopsis thaliana. J. Hazard. Mater. 2017, 322, 292–300.
- Sun, L.; Song, F.; Zhu, X.; Liu, S.; Liu, F.; Wang, Y.; Li, X. Nano-ZnO alleviates drought stress via modulating the plant water use and carbohydrate metabolism in maize. Arch. Agron. Soil Sci. 2021, 67, 245–259.
- Miao, Y.; Luo, X.; Gao, X.; Wang, W.; Li, B.; Hou, L. Exogenous salicylic acid alleviates salt stress by improving leaf photosynthesis and root system architecture in cucumber seedlings. Sci. Hortic. 2020, 272, 109577.
- Hussein, M.M.; Abou-Baker, N.H. The contribution of nano-zinc to alleviate salinity stress on cotton plants. R. Soc. Open Sci. 2018, 5, 171809.
- Wang, Z.; Li, H.; Li, X.; Xin, C.; Si, J.; Li, S.; Li, Y.; Zheng, X.; Li, H.; Wei, X.; et al. Nano-ZnO priming induces salt tolerance by promoting photosynthetic carbon assimilation in wheat. Arch. Agron. Soil Sci. 2020, 66, 1259–1273.
- Zhao, P.; Yuan, W.; Xu, C.; Li, F.; Cao, L.; Huang, Q. Enhancement of Spirotetramat Transfer in Cucumber Plant Using Mesoporous Silica Nanoparticles as Carriers. J. Agric. Food Chem. 2018, 66, 11592–11600.
- Lian, J.; Zhao, L.; Wu, J.; Xiong, H.; Bao, Y.; Zeb, A.; Tang, J.; Liu, W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere 2020, 239, 124794.
- Sharifan, H.; Ma, X.; Moore, J.M.; Habib, M.R.; Evans, C. Zinc Oxide Nanoparticles Alleviated the Bioavailability of Cadmium and Lead and Changed the Uptake of Iron in Hydroponically Grown Lettuce (Lactuca sativa L. var. Longifolia). ACS Sustain. Chem. Eng. 2019, 7, 16401–16409.
- Wang, X.; Jiang, J.; Dou, F.; Sun, W.; Ma, X. Simultaneous mitigation of arsenic and cadmium accumulation in rice (Oryza sativa L.) seedlings by silicon oxide nanoparticles under different water management schemes. Paddy Water Environ. 2020, 19, 569–584.
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in transport and toxicity of nanoparticles in plants. J. Nanobiotechnol. 2023, 21, 75.
- Fedorenko, A.G.; Minkina, T.M.; Chernikova, N.P.; Fedorenko, G.M.; Mandzhieva, S.S.; Rajput, V.D.; Burachevskaya, M.V.; Chaplygin, V.A.; Bauer, T.V.; Sushkova, S.N.; et al. The toxic effect of CuO of different dispersion degrees on the structure and ultrastructure of spring barley cells (Hordeum sativum distichum). Environ. Geochem. Health 2021, 43, 1673–1687.
- Tian, L.; Shen, J.; Sun, G.; Wang, B.; Ji, R.; Zhao, L. Foliar Application of SiO2 Nanoparticles Alters Soil Metabolite Profiles and Microbial Community Composition in the Pakchoi (Brassica chinensis L.) Rhizosphere Grown in Contaminated Mine Soil. Environ. Sci. Technol. 2020, 54, 13137–13146.
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781.
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506.
- Giorgetti, L.; Spano, C.; Muccifora, S.; Bottega, S.; Barbieri, F.; Bellani, L.; Castiglione, M.R. Exploring the interaction between polystyrene nanoplastics and Allium cepa during germination: Internalization in root cells, induction of toxicity and oxidative stress. Plant Physiol. Biochem. 2020, 149, 170–177.
- Kalcikova, G.; Gotvajn, A.Z.; Kladnik, A.; Jemec, A. Impact of polyethylene microbeads on the floating freshwater plant duckweed Lemna minor. Environ. Pollut. 2017, 230, 1108–1115.
- Kalcikova, G. Aquatic vascular plants—A forgotten piece of nature in microplastic research. Environ. Pollut. 2020, 262, 114354.
- Sun, X.-D.; Yuan, X.-Z.; Jia, Y.; Feng, L.-J.; Zhu, F.-P.; Dong, S.-S.; Liu, J.; Kong, X.; Tian, H.; Duan, J.-L.; et al. Differentially charged nanoplastics demonstrate distinct accumulation in Arabidopsis thaliana. Nat. Nanotechnol. 2020, 15, 755–760.
- Hossain, Z.; Mustafa, G.; Sakata, K.; Komatsu, S. Insights into the proteomic response of soybean towards Al2O3, ZnO, and Ag nanoparticles stress. J. Hazard. Mater. 2016, 304, 291–305.
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants—A soil microcosm experiment. Chemosphere 2016, 147, 88–97.
- Fellmann, S.; Eichert, T. Acute Effects of Engineered Nanoparticles on the Growth and Gas Exchange of Zea mays L.—What are the Underlying Causes? Water Air Soil Pollut. 2017, 228, 176.
- Bandyopadhyay, S.; Plascencia-Villa, G.; Mukherjee, A.; Rico, C.M.; José-Yacamán, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci. Total Environ. 2015, 515-516, 60–69.
- Jahan, S.; Alias, Y.B.; Bakar, A.F.B.A.; Yusoff, I.B. Toxicity evaluation of ZnO and TiO2 nanomaterials in hydroponic red bean (Vigna angularis) plant: Physiology, biochemistry and kinetic transport. J. Environ. Sci. 2018, 72, 140–152.
- Das, P.; Barua, S.; Sarkar, S.; Chatterjee, S.K.; Mukherjee, S.; Goswami, L.; Das, S.; Bhattacharya, S.; Karak, N.; Bhattacharya, S.S. Mechanism of toxicity and transformation of silver nanoparticles: Inclusive assessment in earthworm-microbe-soil-plant system. Geoderma 2018, 314, 73–84.
- Pittol, M.; Tomacheski, D.; Simões, D.N.; Ribeiro, V.F.; Santana, R.M.C. Macroscopic effects of silver nanoparticles and titanium dioxide on edible plant growth. Environ. Nanotechnol. Monit. Manag. 2017, 8, 127–133.
- Cvjetko, P.; Milošić, A.; Domijan, A.-M.; Vinković Vrček, I.; Tolić, S.; Peharec Štefanić, P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28.
- Al-Huqail, A.A.; Hatata, M.M.; Al-Huqail, A.A.; Ibrahim, M.M. Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J. of Biol. Sci. 2018, 25, 313–319.
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the Phytotoxicity of Metal Oxide Nanoparticles on Two Crop Plants, Maize (Zea mays L.) and Rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 15100–15109.
- Chung, I.-M.; Rekha, K.; Venkidasamy, B.; Thiruvengadam, M. Effect of Copper Oxide Nanoparticles on the Physiology, Bioactive Molecules, and Transcriptional Changes in Brassica rapa ssp. rapa Seedlings. Water Air Soil Pollut. 2019, 230, 48.
- Peng, C.; Xu, C.; Liu, Q.; Sun, L.; Luo, Y.; Shi, J. Fate and Transformation of CuO Nanoparticles in the Soil–Rice System during the Life Cycle of Rice Plants. Environ. Sci. Technol. 2017, 51, 4907–4917.
- Du, W.; Gardea-Torresdey, J.L.; Xie, Y.; Yin, Y.; Zhu, J.; Zhang, X.; Ji, R.; Gu, K.; Peralta-Videa, J.R.; Guo, H. Elevated CO2 levels modify TiO2 nanoparticle effects on rice and soil microbial communities. Sci. Total Environ. 2017, 578, 408–416.
- Andersen, C.P.; King, G.; Plocher, M.; Storm, M.; Pokhrel, L.R.; Johnson, M.G.; Rygiewicz, P.T. Germination and early plant development of ten plant species exposed to titanium dioxide and cerium oxide nanoparticles. Environ. Toxicol. Chem. 2016, 35, 2223–2229.
- Wu, B.; Zhu, L.; Le, X.C. Metabolomics analysis of TiO2 nanoparticles induced toxicological effects on rice (Oryza sativa L.). Environ. Pollut. 2017, 230, 302–310.
- Landa, P.; Cyrusova, T.; Jerabkova, J.; Drabek, O.; Vanek, T.; Podlipna, R. Effect of Metal Oxides on Plant Germination: Phytotoxicity of Nanoparticles, Bulk Materials, and Metal Ions. Water Air Soil Pollut. 2016, 227, 448.
- Yanık, F.; Vardar, F. Toxic Effects of Aluminum Oxide (Al2O3) Nanoparticles on Root Growth and Development in Triticum aestivum. Water Air Soil Pollut. 2015, 226, 296.
- Feichtmeier, N.S.; Walther, P.; Leopold, K. Uptake, effects, and regeneration of barley plants exposed to gold nanoparticles. Environ. Sci. Pollut. Res. 2015, 22, 8549–8558.
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles Alter Secondary Metabolism in Plants via ROS Burst. Front. Plant Sci. 2017, 8, 832.
- Ghosh, I.; Sadhu, A.; Moriyasu, Y.; Bandyopadhyay, M.; Mukherjee, A. Manganese oxide nanoparticles induce genotoxicity and DNA hypomethylation in the moss Physcomitrella patens. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 842, 146–157.
- Mullineaux, P.M.; Exposito-Rodriguez, M.; Laissue, P.P.; Smirnoff, N. ROS-dependent signalling pathways in plants and algae exposed to high light: Comparisons with other eukaryotes. Free. Radic. Biol. Med. 2018, 122, 52–64.
- Biczak, R.; Telesiński, A.; Pawłowska, B. Oxidative stress in spring barley and common radish exposed to quaternary ammonium salts with hexafluorophosphate anion. Plant Physiol. Biochem. 2016, 107, 248–256.
- Qamer, Z.; Chaudhary, M.T.; Du, X.; Hinze, L.; Azhar, M.T. Review of oxidative stress and antioxidative defense mechanisms in Gossypium hirsutum L. in response to extreme abiotic conditions. J. Cotton Res. 2021, 4, 9.
- Xiong, T.; Zhang, T.; Xian, Y.; Kang, Z.; Zhang, S.; Dumat, C.; Shahid, M.; Li, S. Foliar uptake, biotransformation, and impact of CuO nanoparticles in Lactuca sativa L. var.ramosaHort. Environ. Geochem. Health 2021, 43, 423–439.
- Jurkow, R.; Pokluda, R.; Sekara, A.; Kalisz, A. Impact of foliar application of some metal nanoparticles on antioxidant system in oakleaf lettuce seedlings. BMC Plant Biol. 2020, 20, 290.
- Zhou, C.-Q.; Lu, C.-H.; Mai, L.; Bao, L.-J.; Liu, L.-Y.; Zeng, E.Y. Response of rice (Oryza sativa L.) roots to nanoplastic treatment at seedling stage. J. Hazard. Mater. 2021, 401, 123412.
- Vankova, R.; Landa, P.; Podlipna, R.; Dobrev, P.I.; Prerostova, S.; Langhansova, L.; Gaudinova, A.; Motkova, K.; Knirsch, V.; Vanek, T. ZnO nanoparticle effects on hormonal pools in Arabidopsis thaliana. Sci. Total Environ. 2017, 593, 535–542.
- Le Van, N.; Ma, C.; Rui, Y.; Cao, W.; Deng, Y.; Liu, L.; Xing, B. The Effects of Fe2O3 Nanoparticles on Physiology and Insecticide Activity in Non-Transgenic and Bt-Transgenic Cotton. Front. Plant Sci. 2016, 6, 1263.
- Moazzami Farida, S.H.; Karamian, R.; Albrectsen, B.R. Silver nanoparticle pollutants activate oxidative stress responses and rosmarinic acid accumulation in sage. Physiol. Plant. 2020, 170, 415–432.
- Youssef, M.S.; Elamawi, R.M. Evaluation of phytotoxicity, cytotoxicity, and genotoxicity of ZnO nanoparticles in Vicia Faba. Environ. Sci. Pollut. Res. 2020, 27, 18972–18984.
- Abdelsalam, N.R.; Abdel-Megeed, A.; Ali, H.M.; Salem, M.Z.M.; Al-Hayali, M.F.A.; Elshikh, M.S. Genotoxicity effects of silver nanoparticles on wheat (Triticum aestivum L.) root tip cells. Ecotoxicol. Environ. Saf. 2018, 155, 76–85.
- Maity, S.; Chatterjee, A.; Guchhait, R.; De, S.; Pramanick, K. Cytogenotoxic potential of a hazardous material, polystyrene microparticles on Allium cepa L. J. Hazard. Mater. 2020, 385, 121560.
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, zinc nanoparticles and plants. J. Hazard. Mater. 2018, 349, 101–110.
- Rani, S.; Kumari, N.; Sharma, V. Uptake, translocation, transformation and physiological effects of nanoparticles in plants. Arch. Agron. Soil Sci. 2022, 1–21.
- Aranaz, I.; Harris, R.; Heras, A. Chitosan Amphiphilic Derivatives. Chemistry and Applications. Curr. Org. Chem. 2010, 14, 308–330.
- Nguyen Van, S.; Dinh Minh, H.; Nguyen Anh, D. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013, 2, 289–294.
- Behboudi, F.; Sarvestani, Z.T.; Kassaee, M.Z.; Sanavi, S.A.M.M.; Sorooshzadeh, A. Phytotoxicity of Chitosan and SiO2 Nanoparticles to Seed Germination of Wheat (Triticum aestivum L.) and Barley (Hordeum vulgare L.) Plants. Not. Sci. Biol. 2017, 9, 242–249.
More
